Introduction
Differences in the clinicopathological
characteristics between men and women can be observed in a variety
of diseases, including cardiovascular diseases (1-3).
Women of reproductive age tend to be at lower risk of
atherosclerosis, myocardial infarction and coronary artery disease
(CAD) compared with men in the same age bracket and menopausal
women (1). However, the mechanisms
underlying these difference remain unknown.
Endothelial injury or dysfunction is considered to
be a leading factor underlying the progression of atherosclerosis
and CAD (4). Endothelial progenitor
cells (EPCs) serve a key role in vascular re-endothelialization and
angiogenesis, where they can suppress neointima formation after
vascular injury (4). However, the
effects of hyperlipidemia on the population profile of EPCs in
different sexes and the related mechanisms remain poorly
understood.
Oxidized-low-density lipoprotein (ox-LDL) has been
reported to be a pivotal element in the hyperlipidemic status,
where it has been previously observed to contribute to
atherosclerotic plaque formation (5). Patients diagnosed with stable
cardiovascular disease and acute coronary syndrome tend to exhibit
higher levels of ox-LDL in the serum (6). It was also previously suggested that
native LDL can be continuously converted to ox-LDL in the blood
(7). Therefore, the levels of native
LDL are closely associated with the levels of ox-LDL in vivo
(7). Ox-LDL can inhibit the
proliferation and differentiation of EPCs, thereby suppressing EPC
migration, adhesion and vasculogenesis in vitro, and
neovascularization after ischemia in vivo (8-10).
In addition, a previous study suggested that treating wild type
(WT) mice with human ox-LDL confers comparable effects as that of
hyperlipidemia on EPCs in LDL receptor knock out
(LDLR-/-) mice in vivo (11). However, the concentration of ox-LDL
in serum differs between men and women (12). In addition, EPC numbers have also
been reported to be higher in women of reproductive age compared
with those in age-matched men and postmenopausal women (13,14).
These previous observations suggest that EPC numbers may also
differ between men and women with hyperlipidemia due to the
differences in concentrations of ox-LDL and native LDL between the
sexes.
Oxidative stress as a result of reactive oxygen
species (ROS) production is an important mediator of
atherosclerosis (15). It has been
reported that ROS can be induced by elevated ox-LDL levels and a
hyperlipidemic status (11,16). A previous study also showed that ROS
can facilitate the conversion of native LDL to ox-LDL in WT mice in
circulation (7). ROS levels have
been demonstrated to be significantly higher in male rat
cardiomyocytes, male human serum and vascular cells compared with
those in women (17-19).
Furthermore, both experimental and clinical results potentially
suggest a more powerful antioxidant capacity in women compared with
that in men (20). Proinflammatory
cytokines, including TNF-α and IL-1β, were previously found to be
significantly increased in patients with hyperlipidemia (10) or in WT mice following ox-LDL
treatment (21). These cytokines
also promote hematopoietic cell development and function (22). Nevertheless, estrogen may act on
estrogen receptors on EPCs to suppress the expression of genes
related to pro-atherosclerosis, whilst promoting the expression of
anti-atherosclerosis genes to downregulate proinflammatory cytokine
expression (23).
The present study investigated atherosclerotic
plaque formation and the numbers of bone marrow (BM) and
circulating EPCs in female hyperlipidemic mice or following ox-LDL
treatment. The aim was to explore the effects of hyperlipidemia and
ox-LDL on EPCs in different sexes and investigate the underlying
mechanisms.
Materials and methods
Preparation of ox-LDL
All human procedures were performed in accordance
with the Guidelines of the Human Research Ethics Committee of the
Shandong Second Provincial General Hospital Affiliated to Shandong
University (Jinan, China). The Human Research Ethics Committee of
the Shandong Second Provincial General Hospital Affiliated to
Shandong University (Jinan, China) approved the experimental
protocols (approval no. XYK20181224). All participants agreed to
use their samples for scientific research, and informed consent was
obtained. In accordance with the Institutional Review Board under
Food and Drug Administration regulations (24), venous blood was collected via
puncturing the brachiocephalic vein from 10 healthy male donors
aged 21 to 32 years old, after they had provided consent, and the
blood was collected in heparinized tubes on ice. Adults with
diabetes, hyperlipidemia or other diseases that affect blood lipid
levels were excluded. Lipoproteins were isolated from plasma using
sequential ultracentrifugation with a Beckman TL-100 tabletop
ultracentrifuge (Beckman Coulter, Inc.), which was extracted from
blood supernatant by centrifuging at 1,500 x g for 20 min at 4˚C
(25). The lipoproteins were treated
with 0.3 mM EDTA in 1X PBS (pH 7.4) overnight at 4˚C and
subsequently sterilized using a 0.22-µM filter (MilliporeSigma).
The Folin Lowry method was used to calculate the protein
concentration in the lipoproteins. After dialysis using 5 µM copper
sulphate at 4˚C overnight, ox-LDL was sampled from the native LDL
immediately, as previously described (26). Thiobarbituric acid reactive
substances (TBARS; Sigma-Aldrich; Merck KGaA) were used to monitor
the degree of LDL oxidation and to ensure ox-LDL quality and
reproducibility using a microplate reader at a wavelength of 532 nm
(BioTek Instruments, Inc.) (27).
Specifically, the TBARS value was maintained at 40-50 nmol
malondialdehyde/mg protein. There were no detectable TBARS in the
native LDL. All product was then stored at 4˚C and used within 1
month of preparation.
Animal model
All animal procedures were performed in accordance
with the Guidelines of the Animal Care Committee of the Shandong
Second Provincial General Hospital Affiliated to Shandong
University (Jinan, China). The Animal Care Committee of Shandong
Second Provincial General Hospital Affiliated to Shandong
University approved the experimental protocols (approval no.
XYK20181225). All mice were maintained at room temperature with
40-60% humidity and a 12 h light/dark cycle, with ad libitum
access to food and water.
A total of 10 randomized, age-matched wild-type (WT)
male and female C57BL/6 mice (weight, 20±3 g; age, 4-6 weeks;
Jackson Laboratory) were administered 50 µg prepared ox-LDL daily
via tail vein injections for 3 days, as described previously
(7). A total of 10 25±5 g
LDLR-/- C57BL/6 male and female mice (age, 4-6 weeks)
were also obtained from Jackson Laboratory. The genotyping for WT
and LDLR-/- mice were further confirmed by Southern
blots. All mice were fed a normal diet (ND) until 8 weeks of age,
after which they were fed a high-fat diet (HFD; 17% anhydrous milk
fat and 0.2% cholesterol; Harlan Laboratories, Inc.) for 6 months
to induce hyperlipidemia. Age-matched male and female WT C57BL/6
mice on an HFD or ND, and LDLR-/- male and female mice
fed with ND were used as the controls.
After 6 months of HFD treatment, isoflurane was used
to induce (3%) and maintain (1.5%) anesthesia in mice for blood
collection (300-500 µl) via cardiac puncture. Animals were then
immediately euthanized using CO2 (50-70% of the chamber
volume per min) and death was confirmed by ascertaining cardiac and
respiratory arrest or by observing fixed and dilated pupils. Aorta
and BM were collected after confirming death of animals.
Lipid profile measurements and
atherosclerotic plaque ratio calculation
After 6 months of HFD treatment, blood plasma
samples from all mice were collected for lipid profile testing.
Plasma (40 µl) was tested using the Cholestech LDX lipid profile
cassette (Alere 10-989; Central Infusion Alliance, Inc.) for each
test coupled with the Alere Cholestech LDX system (Alere
Cholestech). Total cholesterol (TC), triglyceride (TRG), LDL, high
density lipoprotein (HDL), non-HDL and the TC/HDL ratio were
measured. Mouse aortas were also isolated for the atherosclerotic
plaque formation test. Red oil (MilliporeSigma) was used to stain
the atherosclerotic plaque at room temperature for 5 min, where the
plaque area against the total inner surface of aorta was calculated
as previously described (28).
Analysis of EPCs
BM and blood cells were harvested to observe the
effects of ox-LDL and hyperlipidemia on the population of blood and
BM EPCs in male and female mice. After eliminating red blood cells
(RBCs) with RBC lysis buffer (Thermo Fisher Scientific, Inc.), a
BD™ LSRII system (BD Biosciences) was used to perform multicolor
analysis for BM and blood EPCs.
An endothelial cell marker combined with a stem cell
marker, including CD34+/fetal liver kinase-1
(Flk-1)+, Stem cell antigen-1 (Sca-1)+/Flk-1,
c-Kit+/CD31+ and
CD34+/CD133+, were used to identify EPCs as
previously described (29).
Functional EPCs express the endothelial markers Flk-1 and
CD31(29). BM and blood EPCs with a
total of 50,000 cells in each sample were carefully analyzed and
described (Fig. S1). All cell
populations were carefully compensated (each cell population
percentile was confirmed further using single antibody staining)
and determined using flow cytometry, as previously described
(30-36).
Flk-1 APC-Cy™7 (cat. no. 561252) antibody was obtained from BD
Biosciences and CD34 FITC (cat. no. 11-0341-82) from eBioscience
(Thermo Fisher Scientific, Inc.). Sca-1 AF700 (cat. no. 108142),
c-Kit-APC (cat. no. 105812), CD31-PE-Cy7 (cat. no. 102418) and
CD133-PE (cat. no. 141204) were purchased from BioLegend, Inc. All
antibodies were diluted 1:100.
Intracellular ROS detection
Mouse BM and blood were harvested following
intravenous injection of 50 µg ox-LDL into each mouse for 3 days,
as described previously (7). For
LDLR-/- mice, BM and blood were harvested after 6 months
of HFD feeding. RBC lysis buffer was used to remove all RBCs
(37). A total of four groups of BM
and circulating EPCs were selected for intracellular ROS detection.
The mean of the four groups of ROS levels in EPCs were
statistically analyzed.
Intracellular ROS generation was measured using FITC
conjugated ROS Detection Reagent (cat. no. D399; Invitrogen; Thermo
Fisher Scientific, Inc.) as previously described (38). A total of 1x106 were
incubated at 37˚C for 10 min with 5 µg/ml reagent. All labeled
cells were washed with PBS twice before suspending in warm PBS.
Flow cytometry was used for analysis. BD™ LSRII (BD Biosciences) at
a wavelength of 525 nm was used to calculate the positively
fluorescent cells, as previously described (39).
Measurement of proinflammatory
cytokines
Mouse blood samples were harvested after 6 months of
HFD or ND treatment. The plasma was obtained from the blood samples
after centrifugation at 300 x g for 20 min at 4˚C. The plasma
levels of the proinflammatory cytokines IL-1β (cat. no. 432601) and
TNF-α (cat. no. 430904) were evaluated using ELISA kits from
BioLegend, Inc. according to the manufacturer's protocols.
Statistical analysis
Data are presented as the mean ± standard deviation,
and analyzed using an unpaired Student's t-test (two-sided) for
comparisons between two groups of data, or a two-way ANOVA followed
by a Bonferroni post hoc test for comparing the subgroups of data
between male and female groups to minimize type I errors as
appropriate in GraphPad Prism version 4 (GraphPad Software, Inc.).
A two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Results
Lipid levels and atherosclerosis
formation are lower in female mice with hyperlipidemia
To study the effects of differences in sex on
hyperlipidemia, male and female LDLR-/- mice were fed a
HFD for 6 months. The TC, TRG, LDL and non-HDL lipoprotein levels,
and the TC/HDL ratio were markedly increased in male and female
hyperlipidemic LDLR-/- mice fed a HFD compared with
their respective control groups, confirming that the hyperlipidemic
mouse model was successfully established (Table I). Of note, the lipid levels in
female hyperlipidemic mice was notably lower compared with that in
male hyperlipidemic mice (Table I).
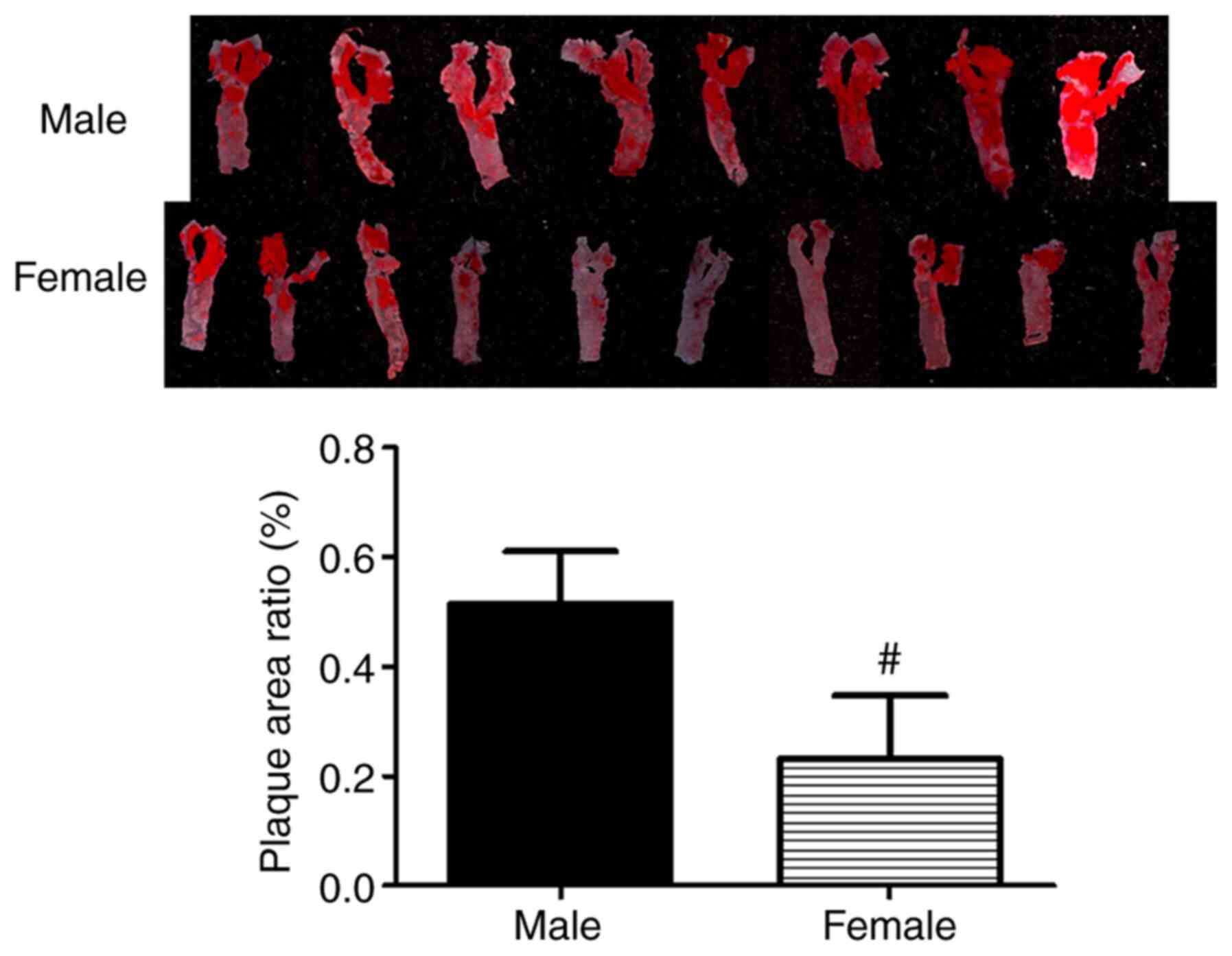
In addition, a number of atherosclerotic plaques were present in
the aorta of the male hyperlipidemic mice, whereas plaque severity
was significantly decreased in the female mouse group (P<0.01;
Fig. 1).
 | Table IMale and female mouse plasma lipid
profiles. |
Table I
Male and female mouse plasma lipid
profiles.
| Male,
n=8d | WT+ND | WT+HFD | KO+ND | KO+HFD |
|---|
| TC, mg±dl | 104.7±3.6 |
207.3±34.2b | 231.5±24.4 |
1,713±215.8a |
| HDL, mg±dl | 56.7±12.9 |
102.1±4.4b | 77±8.3 |
74.4±22.8a |
| TRG, mg±dl | 93±33.3 |
158±12.4b | 102.3±28.7 |
594.3±203.5a |
| LDL, mg±dl | 5.4±4.1 |
12.5±1.3b | 134.8±19.6 |
1,586±90.1a |
| Non-HDL, mg±dl | 19.2±6.7 |
26.5±2.1b | 124.5±68.8 |
1,711±85.6a |
| TC/HDL | 1.2±0.1 | 2±0.4b | 2.8±0.4 |
23.3±4.9a |
| Female,
n=10d | WT+ND | WT+HFD | KO+ND | KO+HFD |
| TC, mg±dl | 101.2±2.4 | 185±24b,c |
113.7±9.3c |
775±94.8a,c |
| HDL, mg±dl | 68.6±6.5 |
76±10.1c | 74.3±2.5 | 73±12.7 |
| TRG, mg±dl | 89.4±10.2 | 49.9±7b,c |
61.7±5.7c |
508±65.1a,c |
| LDL, mg±dl | 4.5±2.1 |
10.4±2.5b |
26.7±7.2c |
596±101.8a,c |
| Non-HDL, mg±dl | 15.6±5.4 |
20.4±3.4b,c |
38.7±7.4c |
697±89.1a,c |
| TC/HDL | 1.5±0.4 |
2.4±1.1b,c |
1.5±0.1c |
10.6±7.5a,c |
BM EPC numbers are increased in female
hyperlipidemic mice
Persistent endothelial cell dysfunction or injury
promotes the progression of atherosclerosis and coronary heart
disease (4). Therefore, the EPC
profiles were examined in the BM of male and female
LDLR-/- mice. Hyperlipidemia did not change the BM
Sca-1+/Flk-1+,
c-Kit+/CD31+ and CD34+/CD133
levels in male mice (Fig. 2B-D),
which only exhibited significantly decreased
CD34+/Flk-1+ levels (P<0.05; Fig. 2A). By contrast, these BM EPC cell
populations, except for those expressing
c-Kit+/CD31+, were found to be significantly
increased in female LDLR-/- mice fed a HFD compared with
female LDLR-/- mice fed a ND (P<0.05) and those in
male LDLR-/- mice fed a HFD (P<0.05; Fig. 2A, B
and D). The
c-Kit+/CD31+ cell population in female
hyperlipidemic mice was lower compared with that in the male mice,
which may be due to the low basal numbers of this cell population
in female mice (Fig. 2C).
 | Figure 2Sustained high level of murine BM and
circulating EPCs in female hyperlipidemic mice. After 6 months of
feeding with a HFD, the peripheral blood cells and BM were
harvested, and the red blood cell were eliminated for flow
cytometry analysis. (A-H) Populations of the cells expressing
CD34+/Flk-1+, or Sca-1+/Flk-1, or
c-Kit+/CD31+ or
CD34+/CD133+ in both BM and blood were
analyzed. The murine BM and circulating EPCs were maintained at a
higher level in female hyperlipidemic mice compared with the males.
*P<0.05 vs. KO+ND; #P<0.05 vs. the
respective male group. BM, bone-marrow; EPC, endothelial progenitor
cell; HFD, high-fat diet; ND, normal diet; Flk-1, fetal liver
kinase-1; Sca-1, stem cell antigen-1; LDLR-/-, low
density lipoprotein receptor knock out; WT+ND, WT C57BL/6 mouse
with ND for 6 months; WT+HFD, WT C57BL/6 mouse with HFD for 6
months; KO+ND, LDLR-/- mice with ND for 6 months;
KO+HFD, LDLR-/- mice with HFD for 6 months. |
Blood EPC numbers are high in female
hyperlipidemic mice
The circulating EPC numbers were also measured in
both LDLR-/- male and female mice. As shown in Fig. 2E-H, the numbers of circulating EPCs
were significantly reduced in both male and female hyperlipidemic
mice (P<0.05) compared with those in their control groups fed a
ND. The numbers of blood EPCs, including those expressing
CD34+/Flk-1+,
Sca-1+/Flk-1+,
c-Kit+/CD31+ and
CD34+/CD133+, were significantly higher
(P<0.05) in the hyperlipidemic female mice compared with those
in the respective male counterparts (Fig. 2E-H).
Lower levels of blood intracellular
ROS, plasma TNF-α and IL-6 are observed in female hyperlipidemic
mice
To investigate the cause of the differences in EPC
numbers between male and female mice with hyperlipidemia, the BM
and blood EPC intracellular ROS levels were determined. Although
there were no intracellular ROS changes in BM EPCs in both male and
female mice, a significantly increased ROS level was observed in
the blood EPCs of male hyperlipidemic mice compared with that in
their corresponding control group fed a ND (P<0.01; Fig. 3A and B). The blood EPC intracellular ROS levels
were significantly decreased in female LDLR-/- mice fed
with HFD compared with that in the female LDLR-/- mice
fed with ND (P<0.05), and was also significantly lower compared
with that in the male LDLR-/- mice fed a HFD (P<0.05;
Fig. 3B).
 | Figure 3Decreased levels of intracellular ROS
formation in blood EPCs in female hyperlipidemic mice. (A) There
were no changes in intracellular ROS formation in the BM in all
groups of mice. (B) Intracellular ROS production is significantly
increased in the blood EPCs in the male hyperlipidemic
LDLR-/- mice, whereas it decreased or was not changed in
the female mice. *P<0.01 vs. KO+ND;
#P<0.01 vs. the respective male group. EPC,
endothelial progenitor cell; ROS, reactive oxygen species; BM, bone
marrow; ND, normal diet; LDLR-/-, low density
lipoprotein receptor knock out; WT+ND, WT C57BL/6 mouse with ND for
6 months; WT+HFD, WT C57BL/6 mouse with HFD for 6 months; KO+ND,
LDLR-/- mice with ND for 6 months; KO+HFD,
LDLR-/- mice with HFD for 6 months. |
The plasma inflammatory factor TNF-α and IL-6 levels
were next measured in both male and female mice. After feeding the
mice with a HFD for 6 months, except for those in the female WT
mice, the TNF-α and IL-6 levels were found to be significantly
increased in both female and male LDLR-/- mice compared
with those in the LDLR-/- mice fed a ND (P<0.05;
Fig. 4A and B). Of note, the TNF-α and IL-6 levels in
all HFD-fed female LDLR-/- mice were significantly lower
compared with those in the male LDLR-/- mice fed a HFD
(P<0.05; Fig. 4A and B).
High numbers of BM and circulating
EPCs coupled with low levels of intracellular ROS are observed in
female mice following ox-LDL treatment
Ox-LDL treatment was used as the primary
hyperlipidemic mediator to treat both male and female WT mice for 3
days prior to measuring their EPC numbers and intracellular ROS
levels. In ox-LDL-treated male mice, the BM
CD34+/Flk-1+,
c-Kit+/CD31+ (Fig.
5A and C) and circulating
Sca-1+/Flk-1+ and
c-Kit+/CD31+ (Fig.
5F and G) cell populations were
significantly decreased (P<0.05) compared with mice without
ox-LDL treatment. However, BM and circulating
CD34+/CD133+ (Fig.
5D and H) and circulating
CD34+/Flk-1+ cell numbers (Fig. 5E) were significantly increased
(P<0.05). There was little to no change in the entire EPC
population (Fig. 5A, B and D-H),
except for the fact that the BM c-Kit+/CD31+
population (Fig. 5C) was
significantly increased (P<0.05) in female mice following ox-LDL
treatment compared with female mice without ox-LDL treatment
(Fig. 5). The female BM and
circulating CD34+/Flk-1+ (Fig. 5A and E), CD34+/CD133+
(Fig. 5D and H) and BM
c-Kit+/CD31+(Fig.
5C) populations were significantly increased (P<0.05)
compared with those in male mice treated with ox-LDL.
Subsequently, the BM and blood EPC intracellular ROS
levels were measured. Similar to that in the hyperlipidemic mice,
there were no changes in ROS levels in the BM in both male and
female mice with or without ox-LDL treatment (Fig. 6A). However, the intracellular blood
ROS levels were significantly elevated in ox-LDL-treated male mice
(P<0.01) compared with that in the ox-LDL-treated female mice
(Fig. 6B).
Discussion
The present study demonstrated that the plasma lipid
levels and atherosclerotic plaque formation were notably reduced in
female hyperlipidemic mice. The BM and circulating EPCs were
maintained in higher numbers in female mice with hyperlipidemia and
following ox-LDL treatment. The potential mechanisms may be
associated with lower levels of intracellular blood EPC ROS
formation, and native LDL, plasma IL-6 and TNF-α levels in female
mice compared with those in their male counterparts. To the best of
our knowledge, the present study was the first to investigate the
effect of differences in sex on the reaction to hyperlipidemia and
ox-LDL in different subgroups of EPCs in the BM and blood.
Ox-LDL is an important mediator of hyperlipidemia
that is closely associated with a number of cardiovascular diseases
(1-3).
Ox-LDL interrupts the activity of EPCs through various mechanisms,
including the downregulation of E-selectin and integrin α(v)β
(5) expression, suppression of
endothelial nitric oxide synthase, acceleration of cell senescence,
suppressing telomerase, promotion of ROS generation and
proinflammatory factor secretion in cardiovascular diseases,
possibly due to the cardioprotective effects of estrogen (1). It has been reported that estrogen can
inhibit EPC apoptosis and senescence, whilst promoting EPC
mobilization (40). In addition,
greater migratory activity and colony-forming capacity in
vitro are also exhibited by EPCs isolated from middle-aged
women compared with those isolated from men, which provides further
support of the protective effects of endogenous estrogen on EPC
function (41). In the present
study, almost all groups of BM and blood EPCs were maintained at
higher numbers in female mice with hyperlipidemia or following
ox-LDL treatment compared with their male counterparts. Apart from
the direct effects on EPC, estrogen may also provide a beneficial
environment for EPCs, including suppression of pro-atherogenic gene
expression, induction of atheroprotective gene expression,
downregulation of IL-6 expression (42), generation of protective growth
factors, including vascular endothelial growth factor and
insulin-like growth factor 1 (43,44), in
addition to the upregulation of suppressor proteins of cytokine
signaling, resulting in resistance to the effects of deleterious
TNF-α signaling in women (45,46). In
support of these previous findings, the present study also
demonstrated that native plasma LDL, IL-6 and TNF-α levels were
considerably lower in female mice compared with those in males.
Although androgen receptors are expressed by EPCs,
there is only limited evidence showing the effects of androgens on
EPCs. Fadini et al (47)
suggested that there is no correlation between androgen stimulation
and late EPC expansion and adhesion in vitro after isolating
both early and late human EPCs. Nevertheless, the number of
circulating EPCs decreased after castration, and this reduction was
irreversible, even with exogenous testosterone administration
(47). In a previous clinical study
of healthy middle-aged men, circulating EPCs were shown to exhibit
a closer correlation with estrogen compared with testosterone
(48). The growth-stimulatory and
pro-survival effects of testosterone may be limited to mature
progenitor cells (48). In addition,
analysis of plasma steroid levels in patients with irritable bowel
syndrome found that EPCs were not correlated with testosterone
levels (49). Therefore,
testosterone may well be less influential than estrogen on EPC
physiology, although conflicting evidence exists concerning the
effects of androgens in this context.
The formation of ROS and the resulting oxidative
stress are important mechanisms underlying the effects of ox-LDL
(7). ROS can disrupt normal EPC
function and is related to a variety of diseases, including CAD,
diabetes, hyperlipidemia and renal ischemia-reperfusion injury
(8-10,15).
It has been reported that the extent of oxidative stress was higher
in males than females in rats (17),
human serum (18) and human vascular
cells (19). In addition, both
previous experimental and clinical studies suggested a potentially
more powerful antioxidant capacity in females over males (20). The present study suggested a
correlation between sex differences and the levels of oxidative
stress, where females are less prone to ROS damage. A previous
study on mice suggested that the stronger antioxidant protection
from ROS in females may be related to the higher levels of
pulmonary and brain superoxide dismutase (SOD) activity (50). Additionally, it has been demonstrated
that estradiol can activate the MAP kinase signaling pathway, to
upregulate manganese-SOD gene expression (51). Another study reported that estrogen
can act as an antioxidant scavenger to eliminate free radicals due
to the presence of the phenolic hydroxyl group (17). ROS levels were also shown to be
higher in spayed female rats compared with corresponding female
controls, but no significant difference was found in male rats
after castration (17). By contrast,
differences in the expression of NADPH-oxidase subunits were also
observed between the two sexes, with higher expression of Nox1 and
Nox4 in males compared with that in females (52). The higher expression of Nox1 and Nox4
in men could partially explain why males are more susceptible to
oxidative stress than females. The present study showed that
intracellular blood EPC ROS formation was reduced in female mice
with hyperlipidemia or after ox-LDL treatment compared with that in
their male counterparts.
There remain a number of questions on the
mechanisms underlying the effects of hyperlipidemia or ox-LDL on
EPCs in different sexes that need to be addressed. It is
well-established that the identification and characterization of
EPCs is a challenging and complex process as shown in previous
studies (53-57).
There are no uniform criteria for the identification of EPCs as of
yet. However, combinations of a variety of cell markers are
frequently used to characterize EPCs in the literature.
Specifically, CD34+/Flk-1+,
Sca-1+/Flk-1+,
c-Kit+/CD31+ and the
CD34+/CD133+ cell populations are primarily
distributed in the blood and BM, and confer protective effects on
the cardiovascular system (58-61).
To provide a broader picture on the sex-specific reaction of EPCs
under conditions of hyperlipidemia or after ox-LDL treatment, the
specific EPC population responsible for the protective effects
against atherosclerosis must be investigated in a future study. In
addition, other potential mechanisms mediating the high numbers of
BM and circulating EPCs in female mice warrants further study. The
effect of estrogen on the modulation of blood lipid levels and
estrogen receptors on EPCs upstream of plaque formation is a line
of research worthy of further investigation. Furthermore, the
application of these findings in clinical studies and the
respective outcomes would be the ultimate desired outcomes of
research into this field.
In conclusion, the present study demonstrated that
the decreased atherosclerotic plaque formation in female mice with
hyperlipidemia compared with male mice may be due to the sustained
high numbers of BM and circulating EPCs in association with lower
levels of intracellular blood EPC ROS formation, plasma TNF-α and
IL-6 levels, and plasma native LDL levels in female mice compared
with those in male mice.
Supplementary Material
FACS for EPC analysis. A total of four
groups of EPCs, including CD34+/Flk-1+,
CD34+/CD133+,
c-Kit+/CD31+ and
Sca-1+/Flk-1+ from BM and blood, were
carefully scaled and analyzed using FACS. FACS, fluorescence
activated cell sorting; EPC, endothelial progenitor cell; BM, bone
marrow; Flk-1, fetal liver kinase-1; Sca-1, stem cell antigen-1;
FSC, forward scatter; SSC, side scatter; Sca-1, stem cell
antigen-1; Flk-1, fetal liver kinase-1; FITC, fluorescein
isothiocyanate; APC, allophycocyanin; PE, phycoerythrin.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Nature Science
Foundation of China (grant nos. 81600222 and 81800255), Young
experts of Taishan Scholar Program of Shandong Province (grant no.
tsqn201812142), Academic Promotion Programme of Shandong First
Medical University (grant nos. 2019RC017), The Natural Science
Foundation of Shandong Province (grant nos. ZR2016HM22 and
ZR2018BH002) and Clinical Medical Science and Technology Innovation
Development Plan Project of Jinan in China (grant nos.
201704106).
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YC and LC designed the experiments. XZ, HB, YJ, XM,
LY, ST, QZ, YX and YC performed the experiments. ZS, KH, LC, PZ and
HS collected and analyzed the data. YC and ST wrote the manuscript.
All authors have read and approved the final manuscript. YC, HS, XZ
and HB confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All human procedures were performed in accordance
with the Guidelines of the Human Research Ethics Committee of the
Shandong Second Provincial General Hospital Affiliated to Shandong
University (Jinan, China). The Human Research Ethics Committee of
the Shandong Second Provincial General Hospital Affiliated to
Shandong University (Jinan, China) approved the experimental
protocols (approval no. XYK20181224). All participants agreed to
use their samples for scientific research, and informed consent was
obtained. All animal procedures were performed in accordance with
the Guidelines of the Animal Care Committee of the Shandong Second
Provincial General Hospital Affiliated to Shandong University
(Jinan, China). The Animal Care Committee of Shandong Second
Provincial General Hospital Affiliated to Shandong University
approved the experimental protocols (approval no. XYK20181225).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo Y, Yin F, Fan C and Wang Z: Gender
difference in clinical outcomes of the patients with coronary
artery disease after percutaneous coronary intervention: A
systematic review and meta-analysis. Medicine (Baltimore).
97(e11644)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shufelt CL, Pacheco C, Tweet MS and Miller
VM: Sex-specific physiology and cardiovascular disease. Adv Exp Med
Biol. 1065:433–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Woodward M: Cardiovascular disease and the
female disadvantage. Int J Environ Res Public Health.
16(1165)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mudyanadzo TA: Endothelial progenitor
cells and cardiovascular correlates. Cureus.
10(e3342)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kattoor AJ, Kanuri SH and Mehta JL: Role
of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 26:1693–1700.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hartley A, Haskard D and Khamis R:
Oxidized LDL and anti-oxidized LDL antibodies in
atherosclerosis-Novel insights and future directions in diagnosis
and therapy. Trends Cardiovasc Med. 29:22–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cui Y, Narasimhulu CA, Liu L, Zhang Q, Liu
PZ, Li X, Xiao Y, Zhang J, Hao H, Xie X, et al: N-acetylcysteine
inhibits in vivo oxidation of native low-density lipoprotein. Sci
Rep. 5(16339)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hamed S, Brenner B and Roguin A: Nitric
oxide: A key factor behind the dysfunctionality of endothelial
progenitor cells in diabetes mellitus type-2. Cardiovasc Res.
91:9–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thum T and Bauersachs J: Spotlight on
endothelial progenitor cell inhibitors: Short review. Vasc Med. 10
(Suppl 1):S59–S64. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Q, Chen L, Si Z, Bu H, Narasimhulu
CA, Song X, Cui MY, Liu H, Lu T, He G, et al: Probucol protects
endothelial progenitor cells against oxidized low-density
lipoprotein via suppression of reactive oxygen species formation in
vivo. Cell Physiol Biochem. 39:89–101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cui Y, Narasimhulu CA, Liu L, Li X, Xiao
Y, Zhang J, Xie X, Hao H, Liu JZ, He G, et al: Oxidized low-density
lipoprotein alters endothelial progenitor cell populations. Front
Biosci (Landmark Ed). 20:975–988. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Hermsdorff HH, Barbosa KB, Volp AC, Puchau
B, Bressan J, Zulet MÁ and Martínez JA: Gender-specific
relationships between plasma oxidized low-density lipoprotein
cholesterol, total antioxidant capacity, and central adiposity
indicators. Eur J Prev Cardiol. 21:884–891. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lemieux C, Cloutier I and Tanguay JF:
Menstrual cycle influences endothelial progenitor cell regulation:
A link to gender differences in vascular protection? Int J Cardiol.
136:200–210. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fadini GP, de Kreutzenberg S, Albiero M,
Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M,
Nardelli GB, et al: Gender differences in endothelial progenitor
cells and cardiovascular risk profile: The role of female
estrogens. Arterioscler Thromb Vasc Biol. 28:997–1004.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Forrester SJ, Kikuchi DS, Hernandes MS, Xu
Q and Griendling KK: Reactive oxygen species in metabolic and
inflammatory signaling. Circ Res. 122:877–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Botham KM and Wheeler-Jones CP:
Postprandial lipoproteins and the molecular regulation of vascular
homeostasis. Prog Lipid Res. 52:446–464. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Barp J, Araujo AS, Fernandes TR, Rigatto
KV, Llesuy S, Belló-Klein A and Singal P: Myocardial antioxidant
and oxidative stress changes due to sex hormones. Braz J Med Biol
Res. 35:1075–1081. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ide T, Tsutsui H, Ohashi N, Hayashidani S,
Suematsu N, Tsuchihashi M, Tamai H and Takeshita A: Greater
oxidative stress in healthy young men compared with premenopausal
women. Arterioscl Throm Vas. 22:438–442. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matarrese P, Colasanti T, Ascione B,
Margutti P, Franconi F, Alessandri C, Conti F, Riccieri V, Rosano
G, Ortona E and Malorni W: Gender disparity in susceptibility to
oxidative stress and apoptosis induced by autoantibodies specific
to RLIP76 in vascular cells. Antioxid Redox Signal. 15:2825–2836.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kander MC, Cui Y and Liu Z: Gender
difference in oxidative stress: A new look at the mechanisms for
cardiovascular diseases. J Cell Mol Med. 21:1024–1032.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ji KT, Chai JD, Xing C, Nan JL, Yang PL
and Tang JF: Danshen protects endothelial progenitor cells from
oxidized low-density lipoprotein induced impairment. J Zhejiang
Univ Sci B. 11:618–626. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Santee SM and OwenSchaub LB: Human tumor
necrosis factor receptor p75/80 (CD120b) gene structure and
promoter characterization. J Biol Chem. 271:21151–21159.
1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ali ES, Mangold C and Peiris AN: Estriol:
Emerging clinical benefits. Menopause. 24:1081–1085.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Administration CFaD: Food and Drug
Administration regulations, 2021 (In Chinese). http://www.nhc.gov.cn/wjw/s9492/202004/31b4fa14ee174bb1999142525ceba608/files/fd630f2e64cd4060aae826e07d00f562.pdf.
|
|
25
|
Chung BH, Wilkinson T, Geer JC and Segrest
JP: Preparative and quantitative isolation of plasma lipoproteins:
Rapid, single discontinuous density gradient ultracentrifugation in
a vertical rotor. J Lipid Res. 21:284–291. 1980.PubMed/NCBI
|
|
26
|
Chandrakala AN, Sukul D, Selvarajan K,
Sai-Sudhakar C, Sun B and Parthasarathy S: Induction of brain
natriuretic peptide and monocyte chemotactic protein-1 gene
expression by oxidized low-density lipoprotein: Relevance to
ischemic heart failure. Am J Physiol Cell Physiol. 302:C165–C177.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li X, Jiang M, Tan T, Narasimhulu CA, Xiao
Y, Hao H, Cui Y, Zhang J, Liu L, Yang C, et al: N-acetylcysteine
prevents oxidized low-density lipoprotein-induced reduction of MG53
and enhances MG53 protective effect on bone marrow stem cells. J
Cell Mol Med. 24:886–898. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chang PC, Wu HL, Lin HC, Wang KC and Shi
GY: Human plasminogen kringle 1-5 reduces atherosclerosis and
neointima formation in mice by suppressing the inflammatory
signaling pathway. J Thromb Haemost. 8:194–201. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cui Y, Liu L, Xiao Y, Li X, Zhang J, Xie
X, Tian J, Sen CK, He X, Hao H and Liu Z: N-acetylcysteine
differentially regulates the populations of bone marrow and
circulating endothelial progenitor cells in mice with limb
ischemia. Eur J Pharmacol. 881(173233)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Traverse JH, Henry TD, Pepine CJ,
Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD,
Hatzopoulos AK, Penn MS, et al: Effect of the use and timing of
bone marrow mononuclear cell delivery on left ventricular function
after acute myocardial infarction: The TIME randomized trial. JAMA.
308:2380–2389. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Perin EC, Willerson JT, Pepine CJ, Henry
TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW,
et al: Effect of transendocardial delivery of autologous bone
marrow mononuclear cells on functional capacity, left ventricular
function, and perfusion in chronic heart failure: The FOCUS-CCTRN
Trial. JAMA. 307:1717–1726. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao Y, Yu P, Wu R, Ge Y, Wu J, Zhu J and
Jia R: Renal cell carcinoma-adjacent tissues enhance mobilization
and recruitment of endothelial progenitor cells to promote the
invasion of the neoplasm. Biomed Pharmacother. 67:643–649.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang JJ, Ii M, Kamei N, Alev C, Kwon SM,
Kawamoto A, Akimaru H, Masuda H, Sawa Y and Asahara T: CD34+ Cells
represent highly functional endothelial progenitor cells in murine
bone marrow. PLoS One. 6(e20219)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu LP, Kakiuchi-Kiyota S, Arnold LL,
Johansson SL, Wert D and Cohen SM: Pathogenesis of human
hemangiosarcomas and hemangiomas. Hum Pathol. 44:2302–2311.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng YM, Schouteden S, Geenens R, Van
Duppen V, Herijgers P, Holvoet P, Van Veldhoven PP and Verfaillie
CM: Hematopoietic Stem/Progenitor cell proliferation and
differentiation is differentially regulated by high-density and
low-density lipoproteins in mice. PLoS One.
7(e47286)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Westerweel PE, Teraa M, Rafii S, Jaspers
JE, White IA, Hooper AT, Doevendans PA and Verhaar MC: Impaired
endothelial progenitor cell mobilization and dysfunctional bone
marrow stroma in diabetes mellitus. PLoS One.
8(e60357)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Houlihan DD, Mabuchi Y, Morikawa S, Niibe
K, Araki D, Suzuki S, Okano H and Matsuzaki Y: Isolation of mouse
mesenchymal stem cells on the basis of expression of Sca-1 and
PDGFR-α. Nat Protoc. 7:2103–2111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bilski P, Belanger AG and Chignell CF:
Photosensitized oxidation of 2',7'-dichlorofluorescin: Singlet
oxygen does not contribute to the formation of fluorescent
oxidation product 2',7'-dichlorofluorescein. Free Radical Bio Med.
33:938–946. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Robinson JP, Bruner LH, Bassoe CF, Hudson
JL, Ward PA and Phan SH: Measurement of intracellular fluorescence
of human-monocytes relative to oxidative-metabolism. J Leukocyte
Biol. 43:304–310. 1988.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cai JJ, Wen J, Jiang WH, Lin J, Hong Y and
Zhu YS: Androgen actions on endothelium functions and
cardiovascular diseases. J Geriatr Cardiol. 13:183–196.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hoetzer GL, MacEneaney OJ, Irmiger HM,
Keith R, Van Guilder GP, Stauffer BL and DeSouza CA: Gender
differences in circulating endothelial progenitor cell
colony-forming capacity and migratory activity in middle-aged
adults. Am J Cardiol. 99:46–48. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu H, Liu K and Bodenner DL: Estrogen
receptor inhibits interleukin-6 gene expression by disruption of
nuclear factor kappaB transactivation. Cytokine. 31:251–257.
2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hamada H, Kim MK, Iwakura A, Ii M, Thorne
T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, et al:
Estrogen receptors alpha and beta mediate contribution of bone
marrow-derived endothelial progenitor cells to functional recovery
after myocardial infarction. Circulation. 114:2261–2270.
2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shao R, Egecioglu E, Weijdegård B,
Kopchick JJ, Fernandez-Rodriguez J, Andersson N and Billig H:
Dynamic regulation of estrogen receptor-alpha isoform expression in
the mouse fallopian tube: Mechanistic insight into
estrogen-dependent production and secretion of insulin-like growth
factors. Am J Physiol Endocrinol Metab. 293:E1430–E1442.
2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Matthews J, Almlof T, Kietz S, Leers J and
Gustafsson JA: Estrogen receptor-alpha regulates SOCS-3 expression
in human breast cancer cells. Biochem Biophys Res Commun.
335:168–174. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Roggia C, Gao YH, Cenci S, Weitzmann MN,
Toraldo G, Isaia G and Pacifici R: Up-regulation of TNF-producing T
cells in the bone marrow: A key mechanism by which estrogen
deficiency induces bone loss in vivo. Proc Natl Acad Sci USA.
98:13960–13965. 2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fadini GP, Albiero M, Cignarella A, Bolego
C, Pinna C, Boscaro E, Pagnin E, De Toni R, de Kreutzenberg S,
Agostini C and Avogaro A: Effects of androgens on endothelial
progenitor cells in vitro and in vivo. Clin Sci (Lond).
117:355–364. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim SW, Hwang JH, Cheon JM, Park NS, Park
SE, Park SJ, Yun HJ, Kim S and Jo DY: Direct and indirect effects
of androgens on survival of hematopoietic progenitor cells in
vitro. J Korean Med Sci. 20:409–416. 2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Garolla A, D'Inca R, Checchin D, Biagioli
A, De Toni L, Nicoletti V, Scarpa M, Bolzonello E, Sturniolo GC and
Foresta C: Reduced endothelial progenitor cell number and function
in inflammatory bowel disease: A possible link to the pathogenesis.
Am J Gastroenterol. 104:2500–2507. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen Y, Ji LL, Liu TY and Wang ZT:
Evaluation of gender-related differences in various oxidative
stress enzymes in mice. Chinese J Physiol. 54:385–390.
2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Vina J, Gambini J, Lopez-Grueso R,
Abdelaziz KM, Jove M and Borras C: Females live longer than males:
Role of oxidative stress. Curr Pharm Design. 17:3959–3965.
2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Miller AA, Drummond GR, Mast AE, Schmidt
HH and Sobey CG: Effect of gender on NADPH-oxidase activity,
expression, and function in the cerebral circulation: Role of
estrogen. Stroke. 38:2142–2149. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Leal V, Ribeiro CF, Oliveiros B, Antonio N
and Silva S: Intrinsic vascular repair by endothelial progenitor
cells in acute coronary syndromes: An Update overview. Stem Cell
Rev. 15:35–47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zaccone V, Flore R, Santoro L, De Matteis
G, Giupponi B, Li Puma DD and Santoliquido A: Focus on biological
identity of endothelial progenitors cells. Eur Rev Med Pharmacol
Sci. 19:4047–4063. 2015.PubMed/NCBI
|
|
55
|
Kawakami Y, Matsumoto T, Mifune Y, Fukui
T, Patel KG, Walker GN, Kurosaka M and Kuroda R: Therapeutic
potential of endothelial progenitor cells in the field of
orthopaedics. Curr Stem Cell Res Ther. 12:3–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Testa U, Saulle E, Castelli G and Pelosi
E: Endothelial progenitor cells in hematologic malignancies. Stem
Cell Investig. 3(26)2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sandhu K, Mamas M and Butler R:
Endothelial progenitor cells: Exploring the pleiotropic effects of
statins. World J Cardiol. 9:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kawamoto A and Asahara T: Role of
progenitor endothelial cells in cardiovascular disease and upcoming
therapies. Catheter Cardiovasc Interv. 70:477–484. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Torsney E and Xu Q: Resident vascular
progenitor cells. J Mol Cell Cardiol. 50:304–311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Heissig B, Werb Z, Rafii S and Hattori K:
Role of c-kit/Kit ligand signaling in regulating vasculogenesis.
Thromb Haemost. 90:570–576. 2003.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Beneventi F, De Maggio I, Cavagnoli C,
Bellingeri C, Ruspini B, Riceputi G, Viarengo G, Ramoni V and
Spinillo A: Endothelial progenitor cell CD34+ and
CD133+ concentrations and soluble HLA-G concentrations
during pregnancy and in cord blood in undifferentiated connective
tissue diseases compared to controls. Reprod Sci. 28:1382–1389.
2021.PubMed/NCBI View Article : Google Scholar
|