Introduction
Disseminated intravascular coagulation (DIC) is a
serious condition in which coagulation and fibrinolysis in the
circulating blood are markedly increased. The characteristic
clinical manifestations of DIC are organ failure and an increased
tendency of bleeding. DIC can result in multi-organ failure (MOF)
due to multiple microthrombi in the microvasculature of multiple
organs (1,2).
Lipopolysaccharide (LPS) is frequently used to
induce DIC in experimental animal models. The pathophysiology of
LPS-induced DIC is well documented, and mimics the type of DIC
observed in patients with sepsis (3,4). The
profibrinolytic response is almost immediately followed by the
suppression of fibrinolytic activity. This suppression is induced
by a sustained increase in the plasma levels of plasminogen
activator inhibitor in the LPS-induced DIC model (5,6). The
activation of coagulation and impairment of fibrinolysis has been
shown to be mediated by cytokines, such as TNF, IL-1 and IL-6 in
the LPS-induced DIC model (7,8).
Erythropoietin (EPO) is a 30,400-dalton glycoprotein
that regulates red cell production. In humans, EPO is produced by
peritubular cells in the kidneys, and causes maturation and
proliferation of erythroid progenitor cells (9). Recombinant human EPO is licensed
worldwide for the treatment of anemia in patients with chronic
renal failure (10,11). EPO protects several organs,
including the brain, heart, kidneys and liver against injury caused
by ischemia/reperfusion and excessive inflammation, and these
beneficial effects of EPO are associated with reductions in tissue
apoptosis secondary to prevention of the activation of caspase-3,
-8 and -9 (12-14).
The tissue-protective effects of EPO following its anti-apoptotic
properties have also been reported in rodent models of sepsis,
using human recombinant EPO and its analogue (15,16).
Numerous studies have shown that endothelial cells can undergo
apoptosis in response to polymicrobial endotoxic shock (17,18).
Endothelial apoptosis enhances exposure of negatively charged
phospholipids, which are involved in factor VIIIa- and factor
IXa-dependent activation of factor X on endothelial cells (19,20).
Encouraged by these studies, it was hypothesized that EPO may
abrogate LPS-induced DIC with MOF.
The aim of the present study was to clarify the role
of apoptosis in LPS-induced DIC by investigating the effects of
EPO. Specifically, whether EPO attenuated organ dysfunction and
apoptosis in the LPS-induced rat DIC model was investigated. The
effects of EPO on LPS-induced DIC were also compared with that of
low molecular weight heparin (LMWH). This is the first report to
investigate the effects of EPO on an animal model of DIC, to the
best of our knowledge.
Materials and methods
Animals
Animals were maintained according to the Standards
of Animal Care and Experimentation published by Kanazawa
University. All animal experiments were approved by the Committee
on Animal Experimentation at Kanazawa University (Kanazawa, Japan;
approval no. AP-183920). Male Wistar rats (aged, 6-7 weeks; body
weight, 160-170 g; Nippon SLC) were acclimatized for at least 3
days in the animal quarters before experimentation, and maintained
with a 12-h light/dark cycle (lights on from between 8:45 a.m. and
8:45 p.m.) at a temperature of 24-26˚C and humidity of 45-65%, with
free access to water and a regular diet (CRF-1; Charles River
Laboratories, Inc.). A total of 126 rats were used in the present
study.
Experimental procedure
Rats were anesthetized with isoflurane (3%, 0.3
l/min, inhaled). Blood was withdrawn from the abdominal aorta into
plastic syringes at 4, 8 and 12 h after the start of LPS
administration. All samples were diluted (1:9 v/v) with 4% sodium
citrate. Rats were sacrificed by death from exsanguination due to
blood sampling from the abdominal aorta under deep anesthesia with
isoflurane (3%, 0.3 l/min, inhaled).
The control groups were administered a sustained
infusion of 10 ml physiological saline for 4 h via the tail vein in
the first experiment. Blood was withdrawn 4, 8 and 12 h after this
infusion (n=7 for each control group).
LPS (Escherichia coli 055: B5
lipopolysaccharide; Sigma-Aldrich; Merck KGaA) was freshly
dissolved in physiological saline before each experiment.
Experimental DIC was induced by sustained infusion of 5 mg/kg LPS
diluted in 10 ml saline into the tail vein over 4 h (n=7) (LPS
group). Blood was withdrawn 4, 8 and 12 h after the start of LPS
administration.
EPO (Epoetin α, 10,000 IU/kg; Kyowa Hakko Kirin Co.)
was administered to rats from 0.5 h before LPS infusion until LPS
infusion was started (n=7 per group) (LPS+EPO groups). EPO diluted
in 1.25 ml saline was infused over the first 0.5 h, after which LPS
diluted in 10 ml saline was infused over the next 4 h. Blood was
withdrawn 4, 8 and 12 h after the start of LPS administration. The
above administration schedule of EPO was used as pretreatment as it
was the primary form of EPO administration used in previous studies
investigating the effects of EPO on animal models of sepsis
(15,16), and because plasma concentrations of
EPO were reportedly highly maintained 12 h after the intravenous
administration of EPO in healthy rats (21). In the EPO-only groups (without LPS),
1.25 ml physiological saline with EPO was infused over the first
0.5 h, after which 10 ml physiological saline was infused over the
next 4 h via the tail vein. Blood was withdrawn 4, 8 and 12 h after
the start of physiological saline.
Infusion of LMWH (400 IU/kg) (Kissei Pharmaceutical
Co.) was started from 0.5 h prior to LPS infusion. LMWH was
administered for 4.5 h until LPS infusion was finished (n=7 per
group) (LPS+LMWH group). LMWH diluted in 1.25 ml saline was infused
over the first 0.5 h, after which LPS and LMWH diluted in 10 ml
saline were simultaneously infused over the next 4 h. Blood was
withdrawn 4, 8 and 12 h after starting LPS administration. The
above administration schedule of LMWH was used as plasma levels of
D-dimer, fibrinogen, and thrombin-antithrombin complex were
significantly suppressed by this administration schedule of LMWH in
previous investigations on LPS-induced DIC in rats (22,23).
In the LMWH groups (without LPS), 1.25 ml physiological saline with
LMWH were simultaneously infused over the first 0.5 h, after which
10 ml physiological saline with LMWH was infused over the next 4 h
via the tail vein. Blood was withdrawn 4, 8 and 12 h after the
start of physiological saline administration.
Parameters
Platelets were counted using an automated device for
animals (cat. no. MEK-6558; Celltac α; Nihon Kohden Co.) within 1 h
of sampling. Citrated plasma samples obtained by whole-blood
centrifugation were stored at -80˚C until required. Fibrinogen
concentration and prothrombin time (PT) were determined using a
clotting assays according to the manufacturer's protocol
(Fibrinogen determination and Thromborel S; Sysmex Co.). D-dimer
levels were determined using a quantitative latex agglutination
test according to the manufacturer's protocol (ELPIA ACE DD dimer;
LSI Medience). Plasma levels of TNF were measured using a rat ELISA
kit (cat. no. MBS2507393; BioSource). To determine the extent of
organ dysfunction in rats, the plasma levels of creatinine and
alanine aminotransferase (ALT) were determined using enzymatic
(PureautoS CRE-L; cat. no. 20800AMZ10178000; Sekisui Medical Co.)
and ultraviolet (Ltypewako ALT J2; FUJIFILM Wako Pure Chemical
Corporation) determinations according to the manufacturer's
protocol, respectively.
Pathological examination
Renal tissue specimens were obtained from animals
sacrificed immediately after blood sampling, only at 8 h after
starting LPS or saline infusion, as in our previous study it was
shown that fibrin deposition in glomeruli could be assessed 8 h
after starting LPS administration (22), and then fixed in formalin. The ratio
(as a percentage) of glomerular fibrin deposition (GFD) was
determined by microscopy. After staining specimens with
phosphotungstic acid hematoxylin (12-24 h), each sample was
histologically examined by a pathologist who was blinded to sample
group allocations. A total of 100 glomeruli were examined in each
sample, and the numbers of thrombi containing fibrin was expressed
as a percentage.
Hepatic tissue specimens were fixed in formalin. The
apoptotic index (AI) was determined by microscopy. After staining
of hepatic tissue specimens using a TUNEL assay according to the
manufacturer's protocol (in situ Apoptosis Detection kit;
Takara Bio Inc.), each sample was histologically examined by a
pathologist blinded to the sample groups. Apoptotic cells were
countered in 10 random fields of view using a light microscope
(magnification, x200) (24). The AI
per sample was calculated as follows: AI=(number of apoptotic
cells/total number of cells) x100 (%) (25). AI was evaluated 12 h after LPS
infusion, as no significant hepatocellular apoptosis was observed
until at least 8 h in the pilot experiments (data not shown),
although hepatocellular apoptosis was only slightly even then, and
therefore evaluation of hepatocyte AI was most appropriate after 12
h, when marked hepatocellular apoptosis was observed in rat models
of LPS-induced DIC.
Statistical analysis
All data are presented as the mean ± the standard
error of the mean. Results were statistically analyzed using a
Student's t-test. For multiple comparisons, P-values were adjusted
using the Holm's method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in hemostatic parameters, TNF levels, organ
function and AI in hepatocytes are shown in Fig. 1, Fig.
2, Fig. 3 and Fig. 4. No significant changes were
observed in any of the parameters examined in rats treated with
physiological saline and EPO or LMWH at 4, 8 and 12 h after the
start of physiological saline administration (data not shown).
 | Figure 1Changes in plasma levels of (A)
platelets, (B) fibrinogen, (C) PT, (D) D-dimer and (E) TNF 4, 8 and
12 h after the start of LPS administration in the LPS-induced DIC
model. n=7. *P<0.05, **P<0.01 vs. LPS
group. LPS, lipopolysaccharide (5 mg/kg/4 h); LPS+EPO, LPS +
erythropoietin (10,000 IU/kg/0.5 h); LPS+LMWH, LPS + low molecular
weight heparin, (400 IU/kg/4.5 h); PT, prothrombin time; DIC,
disseminated intravascular coagulation. |
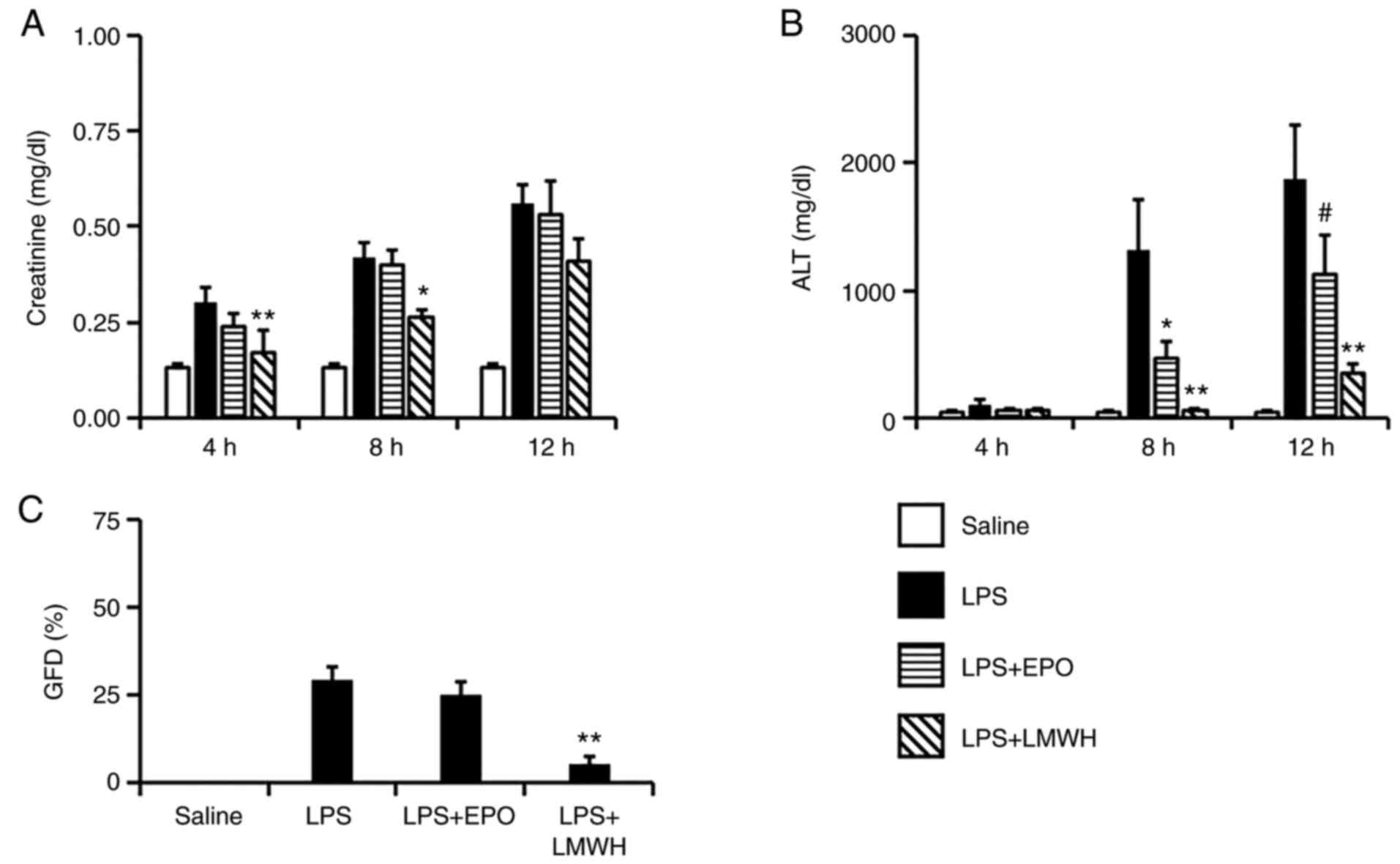 | Figure 2Changes in the plasma levels of (A)
creatinine and (B) ALT 4, 8 and 12 h after the start of LPS
administration, and the percentage of (C) glomerular fibrin
deposition after 8 h in the LPS-induced DIC model. n=7.
*P<0.05, **P<0.01 vs. LPS group. LPS;
#P=0.062 vs. LPS group. ALT, alanine aminotransferase;
DIC, disseminated intravascular coagulation; LPS,
lipopolysaccharide (5 mg/kg/4 h); LPS+EPO, LPS + erythropoietin
(10,000 IU/kg/0.5 h); LPS+LMWH, LPS + low molecular weight heparin,
(400 IU/kg/4.5 h). |
Hemostatic parameters
Platelet counts fell in the LPS groups, and were
unimproved in the LPS+EPO groups, while a significant increase was
observed in the LPS+LMWH group at 4 h (P<0.01; Fig. 1A). Plasma levels of fibrinogen
decreased markedly to the point of being undetectable in the LPS
and LPS+EPO groups, and this decrease was attenuated in the
LPS+LMWH groups at 4 and 8 h (P<0.05 and P<0.01,
respectively; Fig. 1B). PT was
prolonged in the LPS and LPS+EPO groups, and this prolongation was
attenuated in the LPS+LMWH groups at all three timepoints (all
P<0.01; Fig. 1C). Significant
suppression of LPS-induced D-dimer elevation was observed in the
LPS+EPO group at 4 h (P<0.01; Fig.
1D), and in the LPS+LMWH groups after 4 and 8 h (both
P<0.01; Fig. 1D). Plasma levels
of TNF were markedly increased after 4 h in the LPS group, followed
by an early decline. The elevation of plasma TNF was not attenuated
in the LPS+EPO groups, but was significantly suppressed in the
LPS+LMWH groups at 4 and 8 h (P<0.01 each; Fig. 1E).
Organ function
Plasma levels of creatinine, as an indicator of
renal dysfunction, were increased during LPS infusion and increased
further after infusion in the LPS groups. This elevation of plasma
creatinine was not modulated in the LPS+EPO groups, but was
significantly suppressed in the LPS+LMWH groups after 4 and 8 h
(P<0.01 and P<0.05, respectively; Fig. 2A). Plasma ALT levels, as an
indicator of liver injury, were increased in the LPS groups after 8
and 12 h, but were significantly suppressed in the LPS+EPO group
after 8 h (P<0.05; Fig. 2B) and
in the LPS+LMWH group after 8 and 12 h (P<0.05 and P<0.01,
respectively; Fig. 2B). In the
LPS+EPO group, elevation of ALT levels was not significantly
attenuated after 12 h, although a suppressive tendency was still
observed (P=0.062; Fig. 2B). GFD,
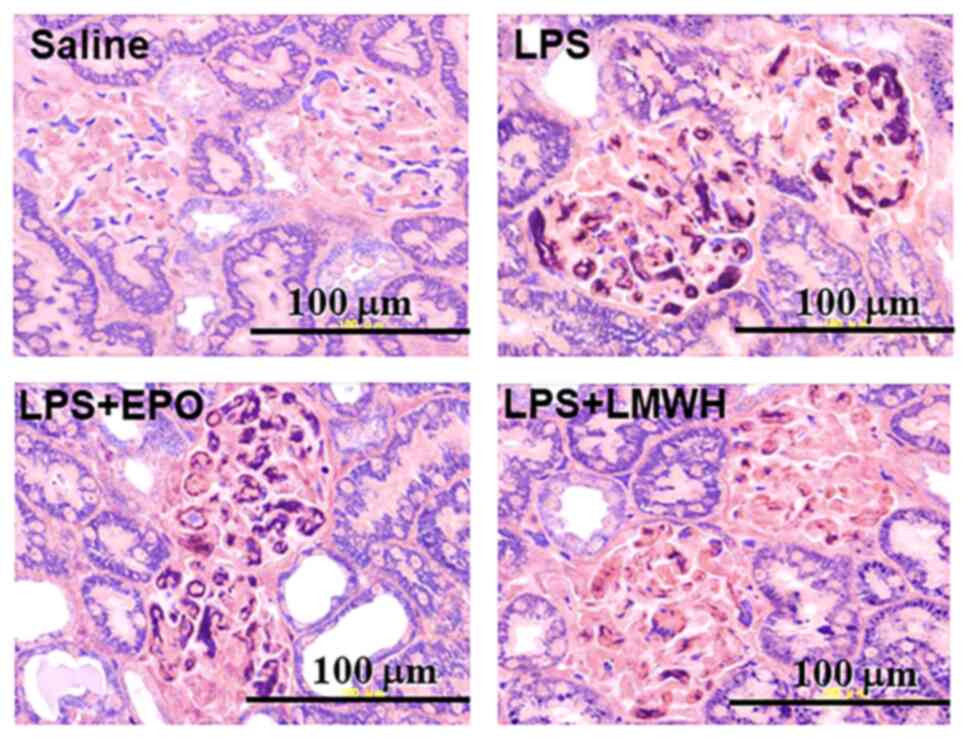
presented as % GFD, after 8 h is shown in Figs. 2C and 3. Significant fibrin deposition was found
in the LPS groups, and was not suppressed in the LPS+EPO groups,
but a significant suppression of % GFD was observed in the LPS+LMWH
groups (P<0.01; Fig. 2C). The AI
in hepatocytes is shown in Fig. 4.
Marked hepatocellular apoptosis was observed in the LPS groups. In
the LPS+EPO and LPS+LMWH groups, AI was significantly reduced
(P<0.05 each; Fig. 4).
Discussion
Recombinant human EPO and its analogue exert
beneficial effects on organ dysfunction in several rodent models of
sepsis (14-16).
As severe sepsis often causes MOF due to DIC (26), the organ-protective effect of EPO
apparent in animal models of sepsis has been hypothesized to be
attributable, at least in part, to improvement of sepsis-induced
DIC. The primary purpose of the present study was to investigate
the effects of anti-apoptotic therapy on the pathophysiology of
sepsis-induced DIC, using human recombinant EPO in the LPS-induced
DIC model.
Reduced platelet counts and fibrinogen levels were
unimproved by EPO, while elevations of plasma D-dimer levels were
significantly suppressed. Although EPO administration did not
notably improve reduced platelet counts and fibrinogen levels, a
decrease in plasma D-dimer levels may suggest that thrombus caused
by LPS stimulation was improved by EPO administration. In addition,
as discussed in greater detail later, the liver dysfunction
developed by thrombus in the LPS model was significantly improved
by EPO administration (significantly lower ALT in the LPS+EPO
groups). This result also suggested that EPO treatment improved the
thrombus formation that caused liver dysfunction, resulting in
decreased levels of D-dimer in the LPS+EPO groups. Inflammatory
cytokines, such as TNF and IL-6 are systemically upregulated, and
play an important role in initiating and accelerating hemostatic
activation in the rat DIC model (27,28).
In the present study, plasma TNF elevation remained unattenuated by
EPO. The anti-inflammatory effects of EPO on sepsis-induced
systemic inflammation have been examined previously, but the
results have varied according to the methods of sepsis induction
(15). The LPS-induced DIC model
used in the present study seemed to present a severe septic
pathophysiology, as the rat model of sepsis was caused by
intravenous administration of high doses of LPS (5 mg/kg).
Accordingly, EPO failed to exert any appreciable anti-inflammatory
(anti-cytokine) effect on LPS-induced systemic inflammation in the
present study.
In the LPS+LMWH groups, abnormalities in hemostatic
parameters, such as platelet counts, fibrinogen levels, PT and
plasma D-dimer levels were significantly improved. Elevation of
plasma TNF was also notably suppressed by the administration of
LMWH. In our previous studies it was reported that LMWH had several
beneficial effects, such as improvement of hemostatic markers and
organ dysfunction, against LPS-induced DIC (22,23).
The improvement of renal and hepatic dysfunction at 8 h was
hypothesized to be due to the strong inhibition of thrombus
formation by LMWH at an early stage. Since single administration of
EPO did not improve LPS-induced hemostatic abnormalities, except in
the D-dimer levels, modification of hemostatic abnormalities and
suppression of plasma TNF elevation in the LPS+LMWH group was
attributed to the anticoagulant and anti-inflammatory effects of
LMWH (22).
Protective effects of EPO against sepsis-induced
renal dysfunction have been reported previously, with EPO
significantly attenuating renal dysfunction and ameliorating kidney
histopathological changes in experimental mice (29). The difference in results between the
previous and the present study may be due to the different animals
used, and the severity of septic pathophysiology resulting from
differences in the methods of inducing sepsis. On the other hand,
hepatic dysfunction was significantly attenuated in the LPS+EPO
group, as indicated by the suppression of plasma ALT elevations
caused by LPS, accompanied by significant suppression of
LPS-induced hepatocellular apoptosis. Although hepatoprotective
effects of darbepoetin-α, a long-acting analogue of EPO, were
reported in another study using a different mouse model of septic
acute liver injury (30), the
results of the present study clarified that EPO was effective
against hepatic dysfunction induced by high doses of LPS, further
mimicking the hepatic pathophysiology of sepsis-induced DIC. In the
LPS+LMWH groups, plasma ALT elevation, PT prolongation and the AI
were suppressed compared with those in the LPS groups. Previously,
the LMWH derivative certoparin was reported to suppress
Adriamycin-induced DNA fragmentation, a significant biochemical
indicator of apoptotic cell death, in heart and kidney tissues from
a rat model of Adriamycin-induced oxidative cytotoxicity (31). The results of the present study
suggested that LMWH may also be protective against sepsis-induced
apoptosis, at least in hepatocytes, indicating a novel effect of
heparinoids against LPS-induced DIC, in addition to the well-known
anti-coagulant effects. From the results of the present study, a
single administration of EPO was considered to improve hepatic
dysfunction primarily through the exertion of anti-apoptotic
effects. Administration of EPO with co-administration of LMWH may
thus be more effective against LPS-induced organ dysfunctions, as
both anticoagulant and anti-inflammatory effects are offered by
co-administration of LMWH, in addition to the potent anti-apoptotic
effects of EPO.
Several limitations should be considered when
examining the results of the present study. First, TNF was the only
measure of inflammatory status used. Although the results for TNF
after 12 h in the LPS+LMWH group suggested that inflammation had
disappeared, inflammatory status cannot be considered to have been
accurately assessed, since other proinflammatory cytokines,
including IL-1 and IL-6, were not measured. This issue thus needs
to be clarified in future studies. Similarly, apoptosis requires
multidimensional evaluations (32,33),
and evaluations using cell viability, DRAQ7 or caspase expression
should be performed in the future. Finally, creatinine and GFD were
used as markers of renal function in the present study, but
measurement of urinary protein and pathological examinations may
also be warranted to evaluate renal function more accurately.
The present study is the first to examine the
effects of EPO on the pathophysiology of DIC, to the best of our
knowledge. Although administration of EPO was ineffective against
hemostatic abnormalities and systemic inflammation in LPS-induced
DIC, hepatic dysfunction was significantly improved following
suppression of hepatocellular apoptosis. This study also clarified
that LMWH exhibited anti-apoptotic properties in LPS-induced DIC.
Anti-apoptotic therapy may thus be beneficial in patients with
sepsis accompanied by DIC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and/or analyzed during the
present study are included in the published article.
Authors' contributions
YS and HA contributed to the experimental design,
analysis of data and wrote the manuscript. FA contributed to the
experiments. SY assisted with the experiments and analysis of data.
EM contributed to drafting/editing of the manuscript and assisted
with the analysis of data. All authors have read and approved the
final manuscript. YS and HA confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Committee on
Animal Experimentation of Kanazawa University (Kanazawa, Japan;
approval no. AP-183920).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levi M and Ten Cate H: Disseminated
intravascular coagulation. N Engl J Med. 341:586–592.
1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adelborg K, Larsen JB and Hvas AM:
Disseminated intravascular coagulation: Epidemiology, biomarkers,
and management. Br J Haematol. 192:803–818. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Asakura H: Classifying types of
disseminated intravascular coagulation: Clinical and animal models.
J Intensive Care. 2(20)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brooks MB, Turk JR, Guerrero A, Narayanan
PK, Nolan JP, Besteman EG, Wilson DW, Thomas RA, Fishman CE,
Thompson KL, et al: Non-lethal endotoxin injection: A rat model of
hypercoagulability. PLoS One. 12(e0169976)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schöchl H, Solomon C, Schulz A, Voelcke W,
Hanke A, Van Griensven M, Redl H and Bahrami S: Thromboelastometry
(TEM) findings in disseminated intravascular coagulation in a pig
model of endotoxinemia. Mol Med. 17:266–272. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schoergenhofer C, Schwameis M, Gelbenegger
G, Buchtele N, Thaler B, Mussbacher M, Schabbauer G, Wojta J,
Jilma-Stohlawetz P and Jilma B: Inhibition of Protease-activated
receptor (PAR1) reduces activation of the endothelium, coagulation,
fibrinolysis and inflammation during human endotoxemia. Thromb
Haemost. 118:1176–1184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Levi M and van der Poll T: Coagulation and
sepsis. Thromb Res. 149:38–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suresh S, Rajvanshi PK and Noguchi CT: The
Many Facets of erythropoietin physiologic and metabolic response.
Front Physiol. 10(1534)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bonomini M, Del Vecchio L, Sirolli V and
Locatelli F: New Treatment approaches for the anemia of CKD. Am J
Kidney Dis. 67:133–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh AK, Szczech L, Tang KL, Barnhart H,
Sapp S, Wolfson M and Reddan D: CHOIR Investigators. Correction of
anemia with epoetin alfa in chronic kidney disease. N Engl J Med.
355:2085–2098. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang J, Zhou J, Wang X, Wang X, Ji L, Wang
S, Chen X and Yang L: Erythropoietin attenuates experimental
contrast-induced nephrology: A role for the janus Kinase 2/Signal
transducer and activator of transcription 3 signaling pathway.
Front Med (Lausanne). 8(634882)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Golmohammadi MG, Banaei S, Nejati K and
Chinifroush-Asl MM: Vitamin D3 and erythropoietin protect against
renal ischemia-reperfusion injury via heat shock protein 70 and
microRNA-21 expression. Sci Rep. 10(20906)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thiemermann C: Beneficial effects of
erythropoietin in preclinical models of shock and organ failure.
Crit Care. 11(132)2007.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Silva I, Alipio C, Pinto R and Mateus V:
Potential anti-inflammatory effect of erythropoietin in
non-clinical studies in vivo: A systematic review. Biomed
Pharmacother. 139(111558)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li K, Liu TX, Li JF, Ma YR, Liu ML, Wang
YQ, Wu R, Li B, Shi LZ and Chen C: rhEPO inhibited cell apoptosis
to alleviate acute kidney injury in sepsis by AMPK/SIRT1 activated
autophagy. Biochem Biophys Res Commun. 517:557–565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tu Q, Zhu Y, Yuan Y, Guo L, Liu L, Yao L,
Zou Y, Li J and Chen F: Gypenosides inhibit inflammatory response
and apoptosis of endothelial and epithelial cells in LPS-Induced
ALI: A study based on bioinformatic analysis and in vivo/vitro
experiments. Drug Des Devel Ther. 15:289–303. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duan H, Zhang Q, Liu J, Li R, Wang D, Peng
W and Wu C: Suppression of apoptosis in vascular endothelial cell,
the promising way for natural medicines to treat atherosclerosis.
Pharmacol Res. 168(105599)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bombeli T, Karsan A, Tait JF and Harlan
JM: Apoptotic vascular endothelial cells become procoagulant.
Blood. 89:2429–2442. 1997.PubMed/NCBI
|
|
20
|
Toltl LJ, Swystun LL, Pepler L and Liaw
PC: Protective effects of activated protein C in sepsis. Thromb
Haemost. 100:582–592. 2008.PubMed/NCBI
|
|
21
|
Woo S, Krzyzanski W and Jusko WJ:
Pharmacokinetic and pharmacodynamic modeling of recombinant human
erythropoietin after intravenous and subcutaneous administration in
rats. J Pharmacol Exp Ther. 319:1297–1306. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Asakura H, Sano Y, Yoshida T, Omote M,
Ontachi Y, Mizutani T, Yamazaki M, Morishita E, Takami A, Miyamoto
K and Nakao S: Beneficial effect of low-molecular-weight heparin
against lipopolysaccharide-induced disseminated intravascular
coagulation in rats is abolished by coadministration of tranexamic
acid. Intensive Care Med. 30:1950–1955. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Asakura H, Sano Y, Omote M, Yoshida T,
Ontachi Y, Mizutani T, Kaneda M, Yamazaki M, Morishita E, Takami A,
et al: Significance of decreased plasma D-dimer levels following
lipopolysaccharide-induced disseminated intravascular coagulation
in rats. Int J Hematol. 79:394–399. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koroglu TF, Yilmaz O, Ozer E, Baskin H,
Gokmen N, Kumral A, Duman M and Ozkan H: Erythropoietin attenuates
lipopolysaccharide-induced splenic and thymic apoptosis in rats.
Physiol Res. 55:309–316. 2006.PubMed/NCBI
|
|
25
|
Zhou HB, Chen JM, Cai JT, Du Q and Wu CN:
Anticancer activity of genistein on implanted tumor of human SG7901
cells in nude mice. World J Gastroenterol. 14:627–631.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Colantuoni A, Martini R, Caprari P,
Ballestri M, Capecchi PL, Gnasso A, Lo Presti R, Marcoccia A, Rossi
M and Caimi G: COVID-19 Sepsis and microcirculation dysfunction.
Front Physiol. 11(747)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Okajima K: Regulation of inflammatory
responses by natural anticoagulants. Immunol Rev. 184:258–274.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suga Y, Kubo A, Katsura H, Staub Y,
Tashiro K, Yamada S, Morishita E and Asakura H: Detailed
exploration of pathophysiology involving inflammatory status and
bleeding symptoms between lipopolysaccharide- and tissue
factor-induced disseminated intravascular coagulation in rats. Int
J Hematol. 114:172–178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stoyanoff TR, Todaro JS, Aguirre MV,
Zimmermann MC and Brandan NC: Amelioration of
lipopolysaccharide-induced acute kidney injury by erythropoietin:
Involvement of mitochondria-regulated apoptosis. Toxicology.
318:13–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Le Minh K, Klemm K, Abshagen K, Eipel C,
Menger MD and Vollmar B: Attenuation of inflammation and apoptosis
by pre- and posttreatment of darbepoetin-alpha in acute liver
failure of mice. Am J Pathol. 170:1954–1963. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Deepa PR and Varalakshmi P: Influence of a
low-molecular-weight heparin derivative on the nitric oxide levels
and apoptotic DNA damage in adriamycin-induced cardiac and renal
toxicity. Toxicology. 217:176–183. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang W, Tian Y, Gao Q, Li X, Li Y, Zhang
J, Yao C, Wang Y, Wang H, Zhao Y, et al: Inhibition of apoptosis
reduces diploidization of haploid mouse embryonic stem cells during
differentiation. Stem Cell Reports. 15:185–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang H, Zhang W, Gao Q, Cao X, Li Y, Li X,
Min Z, Yu Y, Guo Y and Shuai L: Extractive from Hypericum ascyron L
promotes serotonergic neuronal differentiation in vitro. Stem Cell
Res. 31:42–50. 2018.PubMed/NCBI View Article : Google Scholar
|