Introduction
Diabetic macula edema (DME) is a serious
complication of diabetic retinopathy (DR) occurring in ~3.7% of DR
patients, and, based on the estimated worldwide prevalence of
diabetes of 5.4% by 2025 an exponential growth of DME and
associated loss of vision is expected (1,2). In
addition, retinal vein occlusion (RVO) results in hypoxia of the
retina and eventually in the development of macular edema (3). Elevated permeability of the retinal
endothelium and a decreased re-uptake of fluid by Mueller cells,
which express multiple ion- and water-channels (such as
Na+-, K+-, and aquaporin channels) leads to
the accumulation of fluid in the macula (4). Decreased expression of ion channels
results in deregulated trans-glial water transport and as a
consequence in the swelling of retinal glial cells (4). Vascular endothelial growth factor A
(VEGF-A), higher levels of which are present in the vitreous of
patients with DR, DME, or RVO, elevates the permeability of the
retinal endothelium, playing a key role in the pathophysiology of
macular edema (3-7).
Higher concentrations in the vitreous or aqueous humor of
inflammatory cytokines, such as interleukin (IL)-6 and IL-1β are
not only associated with the pathogenesis of RVO, respectively, but
also with the development of DME and proliferative DR (8-12).
Additionally, the ocular renin-angiotensin aldosterone-system
(RAAS) also regulates the retinal blood and fluid balance; several
studies point to its major role in the development of DME (13-15).
Members of the RAAS, including angiotensinogen (AGT),
angiotensin-converting enzyme (ACE), and ACE2 as well as the
receptors of angiotensin II, AT1, and AT2,
encoded by the genes AGTR1 and AGTR2, respectively, and the
G-protein-coupled receptor MAS1 of angiotensin (1-7), are expressed
by retinal tissues including Mueller cells and retinal vessels
(16-25).
AGT is cleaved by the protease renin to give rise to angiotensin I
and cleavage of the decapeptide by ACE in turn results in the
vasoconstrictive octapeptide angiotensin II. This peptide hormone
not only induces pro-inflammatory responses but may also be
involved in angiogenesis, and these processes are mediated by its
receptor AT1 (13,25).
Through its weakly expressed alternative receptor AT2,
the actions of angiotensin II can be counteracted (25,26).
The G-protein-coupled receptor MAS1 induces anti-angiogenic and
anti-inflammatory processes not only systemically but also in the
retina; its ligand, the vasodilator angiotensin (1-7), is formed by
the proteolytic removal of the C-terminal phenylalanine of
angiotensin II by ACE2 (24,27).
Taken together, upregulation of AGT can lead to
increased production of angiotensin II resulting in higher local
RAAS activity in general; however, the consequences of its actions
on Mueller cells and the retina in its entirety are not fully
understood. Thus, the influence of angiotensin II or aldosterone on
the expression of different mediators of DME or RVO pathogenesis,
as well as the components of the RAAS under hypoxic and
hyperglycemic conditions in MIO-M1 cells, a model of human Mueller
cells, were investigated (28,29).
Materials and methods
Culture and treatment of MIO-M1
The spontaneously immortalized human Mueller glial
cell line MIO-M1 (RRID: CVCL_0433) was purchased from University
College London (28). Cells were
cultured in DMEM (cat. no. 21885025, Thermo Fisher Scientific,
Inc.) containing 5 mM glucose, and supplemented with 10% FBS,
glutamax II, and penicillin/streptomycin (all purchased from Thermo
Fisher Scientific, Inc.) at 37˚C and 5% CO2. To confirm
absence of mycoplasma, fixed MIO-M1 cells were regularly stained
with DAPI (λexcitation/λemmission=359 nm/461
nm) and evaluated using fluorescence microscopy, which would have
enabled the detection of non-nuclear DNA, which would have
indicated the possible presence of mycoplasma. To study the changes
induced by 30 mM glucose (Carl Roth), 10 nM angiotensin II (cat.
no. 05-23-0125, MilliporeSigma), 10 nM aldosterone (cat. no. A9477,
MilliporeSigma), and hypoxia (0.1% O2) or their
combinations, 4x104 MIO-M1 cells were seeded per well of
a 12-well cell culture plate (Greiner Bio-One) in 1 ml DMEM
supplemented with 10% FBS, glutamax II, and
penicillin/streptomycin. When ~90% of the cell culture surface was
covered by a monolayer of cells, the cell culture medium was
replaced with serum-free DMEM. After further culture for 16 h,
glucose, angiotensin II or aldosterone were added in a volume of 10
µl DMEM, and cells were incubated for an additional 6 h before cell
culture supernatants and cells were harvested.
RNA isolation and cDNA synthesis
The InviTrap Spin Universal RNA Mini Kit (cat. no.
1060100200 Stratec Molecular) was used to isolate total RNA. The
quality of the RNA samples was analyzed using a NanoDrop 1000
spectrophotometer (Peqlab). The A260/A280
ratio was between 1.95 and 2.05 demonstrating a sufficiently good
quality of the RNA samples. Possible contamination with DNA was
removed with recombinant RNAse-free DNase I (cat. no. 4716728001,
MilliporeSigma). cDNA synthesis was performed using 0.6 µg total
RNA and a RevertAid H Minus First Strand cDNA Synthesis Kit,
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc.).
Quantitative (q)PCR
Semi-quantitative PCR was performed using a CFX
Connect Real-Time PCR System (Bio-Rad Laboratories, Inc.). The
sequences of the primers used in the present study are listed in
Table I. The amplification mixture
(10 µl in total) contained 5 µl iQ™ SYBR® Green Supermix
(cat. no. 170888x, Bio-Rad Laboratories, Inc.), specific primers
(0.2 µM each), and 1 µl (~0.1 µg) of cDNA. The PCR amplification
conditions were: Initial denaturation and enzyme activation at
95˚C, 3 min; followed by 45 cycles of denaturation at 95˚C for 30
sec, annealing at 58˚C for 20 sec, and extension at 72˚C for 45
sec. Each measurement included a melting curve analysis in the
range of 65-95˚C with an increment of 0.5 K, and the length of the
PCR products was determined by standard agarose gel
electrophoresis. mRNA expression of actin (ACTB) was used for
normalization and relative mRNA levels were calculated using the
2-ΔΔCq method: ΔCq=Cqtarget
gene-CqACTB and
ΔΔCq=ΔCqtreatment-ΔCqcontrol (30).
 | Table ISequences of the primers used for
quantitative PCR. |
Table I
Sequences of the primers used for
quantitative PCR.
| Gene | Accession
number | Forward primer,
5'-3' | Reverse primer,
5'-3' | Product size,
bp |
|---|
| ACTB | NM_001101.5 |
ATGGCCACGGCTGCTTCCAGC |
CATGGTGGTGCCGCCGAGCAG | 237 |
| IL6 | NM_000600.4 |
TACCCCCAGGAGAAGATTCC |
TTTTCTGCCAGTGCCTCTTTT | 175 |
| ACE | NM_001178057.1 |
TCAGCTACCTCGTCGATCAGT |
TGTAAGGCACGCTAGAAGGAA | 183 |
| ACE2 | NM_021804.3 |
GGGATCAGAGATCGGAAGAAGAAA |
GGAGGTCTGAACATCATCAGTG | 123 |
| AGT | NM_000029.3 |
CTTTCAACACCTACGTCCACTTC |
AGAAGTTGTCCTGGATGTCACTC | 159 |
| AGTR1 | NM_032049.3 |
CAGTTTGCCAGCTATAATCCATC |
TTCTTTAGGGCCTTCCAAATAAG | 195 |
| AGTR2 | NM_000686.5 |
CTCTTCCTCTATGGGCAACCTAT |
CAACACTCATGCAGGTGATAAAAA | 134 |
| MAS1 | NM_002377.4 |
ACGTGACATCATTTGTTGTTGAG |
AGTGAAGGGATTTCTTCTCATCC | 188 |
| VEGFAa | NM_001025370.3,
NM_001287044.2, NM_003376.6 |
CCTGGTGGACATCTTCCAGGAGTA |
CTCACCGCCTCGGCTTGTCACA | 275, 407, 479 |
| VEGFR2 | NM_001110000.3 |
CTTCGAAGCATCAGCATAAGAAACT |
TGGTCATCAGCCCACTGGAT | 156 |
Determination of secreted VEGF-A or
IL-6
The concentration of VEGF-A or IL-6 in cell culture
supernatants of the treated MIO-M1 cells were determined using a
Quantikine human VEGF-A ELISA kit (cat. no. DVE00, Bio-Techne) and
a Quantikine Human IL-6 ELISA kit (cat. no. DVE6050, Bio-Techne),
respectively. For measuring IL-6 levels, samples were diluted 1:10
in PBS without Ca2+- and Mg2+-ions (cat. no.
14190-169, Thermo Fisher Scientific, Inc.), and undiluted samples
were used to determine the concentration of VEGF-A. Duplicate
samples were processed according to the manufacturer's instructions
and the analyte-dependent absorbance at 450 nm (reference
wavelength: 570 nm) was measured 15 min after the addition of the
stop solution with an Infinite 200Pro M Nano spectrophotometer
controlled by Tecan I control software (version 2.0.10.0; Tecan
Group, Ltd.). Standard curves (16 pg/ml to 1 ng/ml VEGF-A or 3-300
pg/ml IL-6) were always generated in parallel to the analysis of
samples.
Statistical analysis
All experiments were performed at least three times.
A one-way ANOVA followed by a Tukey's post-hoc test was used to
compare the RT-qPCR signals from the treated cells and the ELISA
results. For comparison of RT-qPCR signals from treated cells to
the hypothetical value of 1 (normalized to the signal of control
cells), a one-sample t-test was used, as in this type of
statistical analysis, the variability of the values obtained from
control cell signals is taken into consideration, although they
appear without standard deviations (SD=0). P#x003C;0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed in GraphPad Prism version 9
(GraphPad Software, Inc.); means and standard deviations are
provided as numbers or as scatter plots.
Results
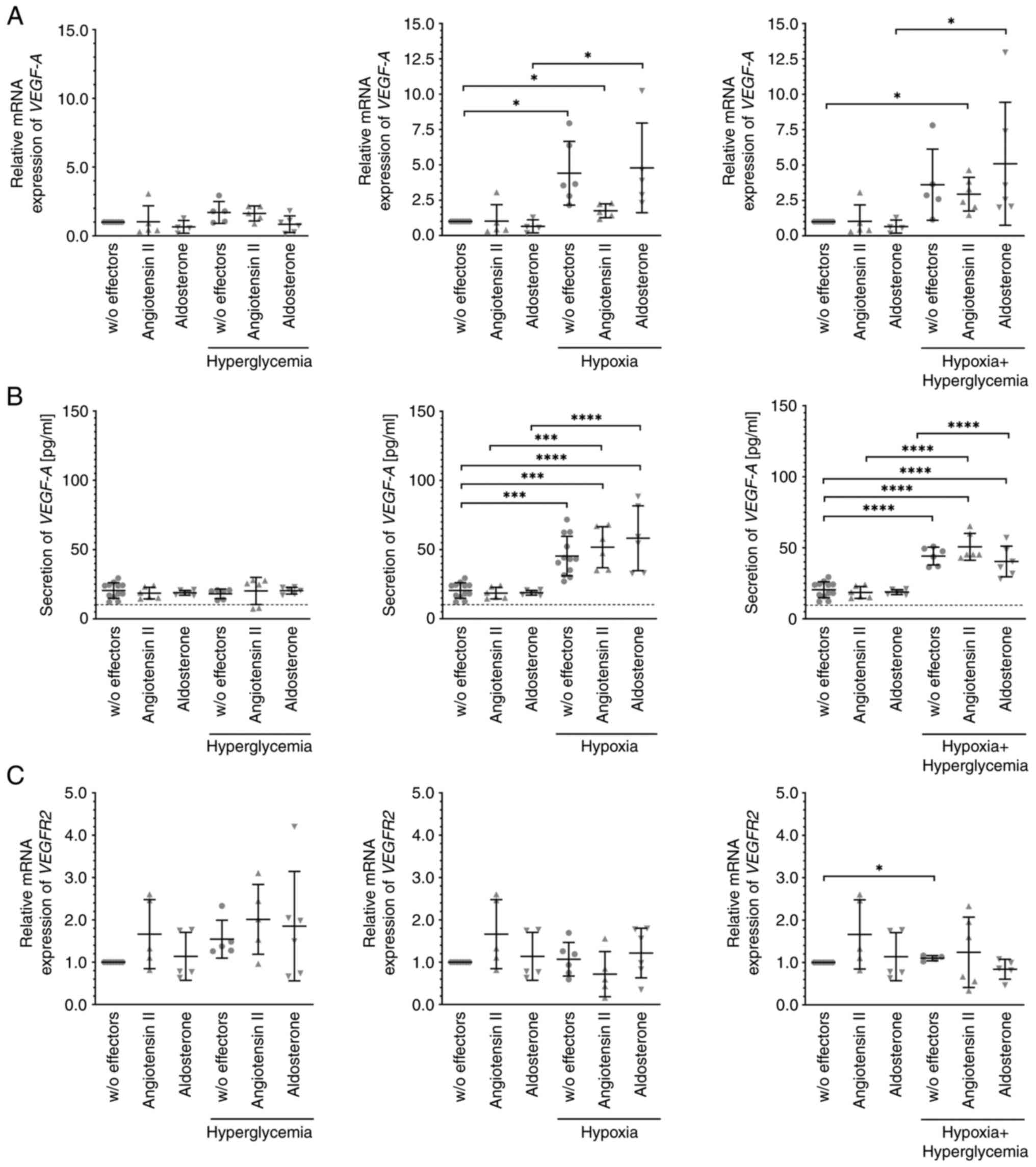
Angiotensin II and aldosterone do not
alter the expression of VEGF-A or VEGFR2 in MIO-M1 cells
Unchallenged MIO-M1 cells expressed or secreted
considerable quantities of VEGF-A mRNA (Fig. 1A) or protein (Fig. 1B), respectively, and this was not
significantly altered after treatment of the cells with 30 mM
glucose, 10 nM angiotensin II, 10 nM aldosterone, or combinations
thereof. Expression of VEGF-A mRNA and, accordingly, secretion of
this growth factor was substantially and significantly higher after
exposure of the cells to hypoxic or hypoxic plus hyperglycemic
conditions. Again, these levels were not significantly altered by
additional exposure of the cells to angiotensin II or aldosterone.
Similarly, expression of VEGFR2 mRNA (Fig. 1C) remained stable. Only after
culture of the cells under hypoxic plus hyperglycemic conditions,
was its expression weakly but significantly higher.
Angiotensin II and aldosterone
treatment alters the expression of RAAS members
When cells were cultured under standard conditions,
the mRNA levels of members of the RAAS, AGT (Fig. 2A), ACE (Fig. 2B), the receptors of angiotensin II
AT1 (Fig. 2D) and
AT2 (Fig. 2E), and the
receptor of angiotensin (1-7) MAS1 (Fig. 2F) were not significantly changed by
additional treatment with angiotensin II or aldosterone. Exposure
of the cells to 30 mM glucose did not alter their expression.
However, the mRNA levels of AT1 (Fig. 2D), AT2 (Fig. 2E), and MAS1 (Fig. 2F) were elevated after treatment with
angiotensin II under hyperglycemic conditions. These conditions
also resulted in significantly enhanced expression of ACE2
(Fig. 2C), which also tended to be
higher after angiotensin II exposure under normal conditions.
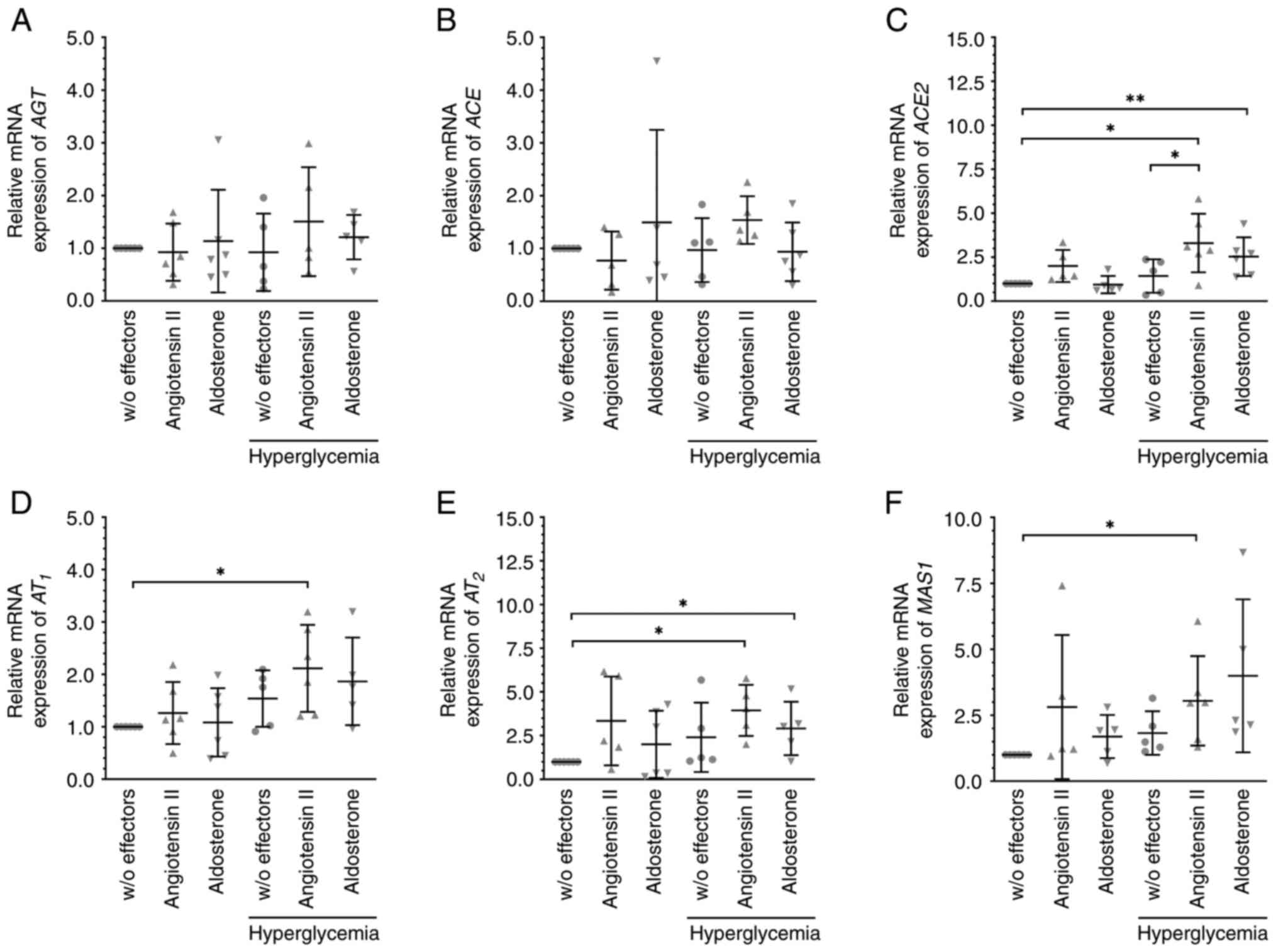 | Figure 2Angiotensin II and aldosterone
altered the expression of members of the RAAS under hyperglycemic
conditions. Cells were treated with 10 nM angiotensin II, 10 nM
aldosterone or glucose (final concentration of 30 mM) to induce
hyperglycemic conditions for 6 h. The mRNA expression levels of (A)
AGT, (B) ACE, or (C) ACE2, (D) AT1 and (E)
AT2, and (F) the G-protein-coupled receptor MAS1 were
analyzed. Angiotensin II increased the mRNA expression levels of
(C) ACE2, (D) AT1, (E) AT2, and (F) MAS1, and
aldosterone those of (C) ACE2 and (E) AT2. Data are
presented as scatter plots depicting the mean ± standard deviation.
*P#x003C;0.05, **P#x003C;0.01. RAAS,
renin-angiotensin aldosterone system; AGT, angiotensinogen; ACE,
angiotensin-converting enzyme; AT1, angiotensin II
receptor 1; AT2, angiotensin II receptor 2; w/o,
without. |
Cells were cultured under hypoxic conditions (at
0.1% O2), which by itself did not affect the mRNA
expression levels of the members of the RAAS (Fig. 3); however, additional treatment with
angiotensin II significantly enhanced the expression of ACE2 mRNA
(Fig. 3C) under these conditions.
Angiotensin II-induced significantly higher mRNA expression of
AT2 when MIO-M1 cells were cultured under hypoxic plus
hyperglycemic but not under hypoxic conditions (normalized
expression levels were: 1.36±0.92 for hypoxia + angiotensin II
compared to 6.14±3.47 for hypoxia plus hyperglycemia + angiotensin
II, P=0.0029; n=6 per condition; Figs.
3E and 4E). The levels of
AT2 mRNA did not differ from the elevated levels
observed when MIO-M1 cells were cultured in the presence of 30 mM
glucose (normalized expression levels were: 3.94±1.47 for
hyperglycemia + angiotensin II, n=5; compared to 6.14±3.47 for
hypoxia plus hyperglycemia + angiotensin II, n=6 per condition;
P>0.05; Figs. 2E and 4E). Under hypoxic plus hyperglycemic
conditions, angiotensin II treatment also resulted in significantly
reduced expression of ACE mRNA (Fig.
4B). Similar to angiotensin II, aldosterone-induced
significantly higher levels of ACE2 mRNA (Fig. 2C) as well as of angiotensin II
receptor AT2 mRNA (Fig.
2E) levels under hyperglycemic conditions. Slightly, but
significantly elevated levels of ACE mRNA (Fig. 3B) were observed after exposure of
MIO-M1 cells to aldosterone under hypoxic conditions; the
expression levels of the other targets remained unchanged
throughout.
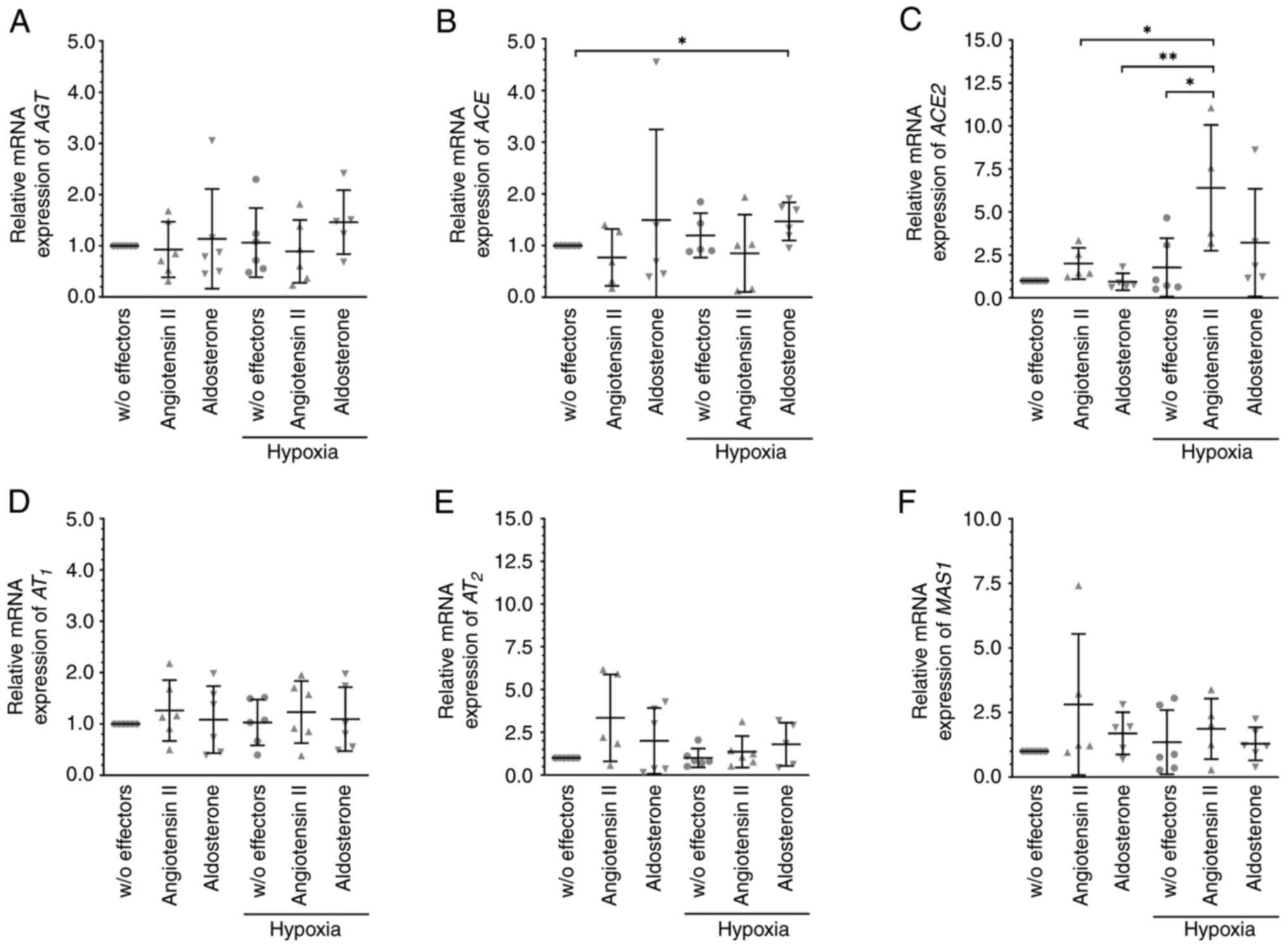 | Figure 3Angiotensin II and aldosterone
treatment altered the expression levels of members of the RAAS
under hypoxic conditions. To induce a hypoxic environment, MIO-M1
cells were cultured at 0.1% O2 with 10 nM angiotensin II
or 10 nM aldosterone for 6 h, and the mRNA expression levels of (A)
AGT, (B) ACE, (C) ACE2, (D) AT1, (E) AT2 and
(F) MAS1 were analyzed. Aldosterone slightly increased the
expression of ACE, and angiotensin II increased the expression of
ACE2. Data are presented as scatter plots depicting the mean ±
standard deviation. *P#x003C;0.05,
**P#x003C;0.01. RAAS, renin-angiotensin aldosterone
system; AGT, angiotensinogen; ACE, angiotensin-converting enzyme;
AT1, angiotensin II receptor 1; AT2,
angiotensin II receptor 2; w/o, without. |
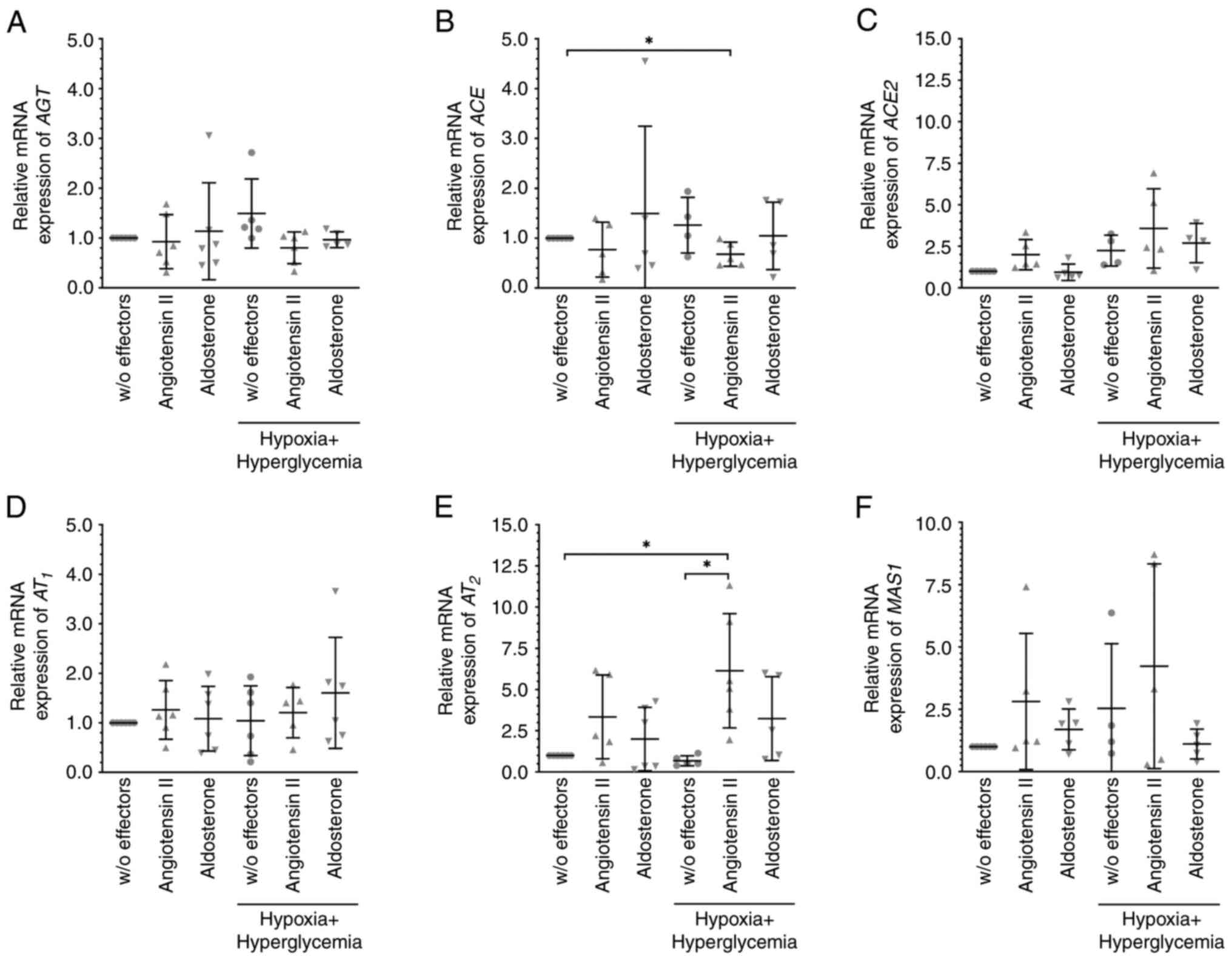 | Figure 4Angiotensin II, but not aldosterone
altered the expression levels of members of the RAAS under hypoxic
plus hyperglycemic conditions. MIO-M1 cells were cultured at 0.1%
O2 plus glucose (final concentration of 30 mM) with 10
nM angiotensin II or 10 nM aldosterone for 6 h, and the mRNA
expression levels of (A) AGT, (B) ACE, (C) ACE2, (D)
AT1, (E) AT2, and (F) MAS1 were analyzed.
Angiotensin II lowered the levels of (B) ACE but increased those of
(E) AT2. Data are presented as scatter plots depicting
the means ± standard deviation. *P#x003C;0.05. RAAS,
renin-angiotensin aldosterone system; AGT, angiotensinogen; ACE,
angiotensin-converting enzyme; AT1, angiotensin II
receptor 1; AT2, angiotensin II receptor 2; w/o,
without. |
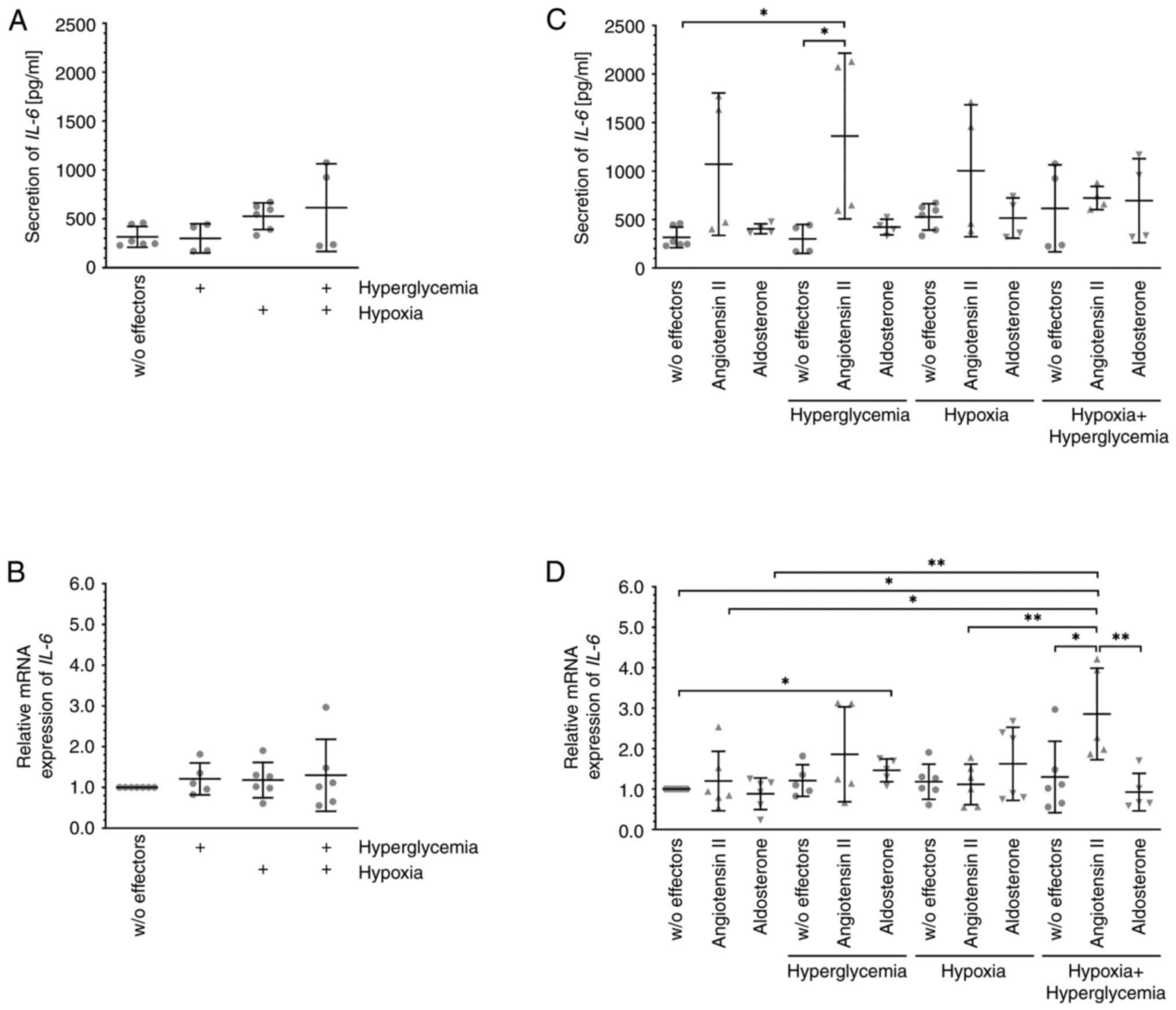
Angiotensin II alters the expression
and secretion of IL-6 depending on the environment
As previously reported by others, unchallenged
MIO-M1 cells secrete substantial amounts of the pro-inflammatory
cytokine IL-6 (Fig. 5A) (31). However, neither its secretion
(Fig. 5A) nor the expression of the
corresponding mRNA (Fig. 5B) was
altered after exposure of the cells to hyperglycemia, hypoxia, or a
combination of both. Both hormones also did not change IL-6 mRNA
expression (Fig. 5D) in MIO-M1
cells cultured under normoxic or hypoxic conditions, and secretion
(Fig. 5C) of the encoded protein
also remained relatively unchanged. During exposure to angiotensin
II under hyperglycemic conditions, MIO-M1 cells secreted
significantly more IL-6 (Fig. 5C),
and the mRNA expression (Fig. 5D)
of the cytokine also tended to be higher. Under hypoxic plus
hyperglycemic conditions, substantially and significantly more IL-6
mRNA (Fig. 5D) was expressed after
additional exposure of the cells to angiotensin II and cells also
secreted more IL-6 (Fig. 5C),
although the difference was not significant. Aldosterone treatment
of MIO-M1 cells under hyperglycemic conditions resulted in
slightly, but significantly enhanced expression of IL-6 mRNA
(Fig. 5D), but secretion of the
cytokine (Fig. 5C) remained
unchanged.
Discussion
Confirming previously published data, it was shown
that the human cell line MIO-M1, an accepted model of human Mueller
cells, expresses AGT, ACE, ACE2, angiotensin II receptors
AT1 and AT2, as well as the receptor of
angiotensin (1-7) MAS1 (17-19,21-24,28).
The mRNA expression levels of these were largely unchanged when
cells were exposed to hyperglycemic or hypoxic conditions or both.
Exposure of cells to these conditions seemed to not result in
induction of cellular stress, which may adversely affect the
outcome of the investigations, since expression of IL-6 mRNA, a
marker of cellular stress, remained stable. It could be suggested
that an incubation time of 6 h is too short, but due to the short
half-life of angiotensin II, longer exposure times would likely not
result in more relevant data. However, expression and secretion of
VEGF-A were substantially increased by hypoxia alone or in
combination with hyperglycemia confirming the expected strong
response of the cells to their altered environment within the
studied time span. Angiotensin II and aldosterone did not modulate
VEGF-A levels, proving the dominant role of hypoxia in the
regulation of the growth factors' expression and secretion. It was
to be expected that hyperglycemia alone did not modulate VEGF-A
expression and secretion within 6 h, as possible changes likely
manifest only after extended exposure (32). VEGF receptors are expressed in
various retinal tissues including the retinal vasculature, Mueller
cells, and the retinal pigment epithelium (33,34).
Upregulation of VEGFR2 in the retinal vasculature is associated
with the development of DR and its activation by the ligand
VEGF-A165 results in elevated permeability of retinal
endothelial cells or increased expression of pro-inflammatory
mediators in Mueller cells in vitro (29,34,35).
Inhibitors of ACE and/or AT1, at least in part, prevent
VEGF-A165-induced permeability of retinal endothelial
cells in vitro and in vivo as well as retinal
neovascularization, thereby proving an interaction between both
signaling pathways (36-39).
However, expression and secretion of VEGF-A by MIO-M1 cells were
not altered by angiotensin II likely reflecting the different
behaviors of both cell types.
mRNAs coding for proteins of the RAAS indeed
exhibited differential expression patterns in the presence of
either angiotensin II or aldosterone when cells were cultured under
hyperglycemic and/or hypoxic conditions. It is of interest, that
angiotensin II did not alter the expression of its precursor AGT
under any of the tested conditions, which may indicate that the
peptide hormone cannot, directly or indirectly, induce its own
expression in Mueller cells. To assess a possible pro-inflammatory
response of Mueller cells, the changes in the expression of mRNA as
well as secretion of the pro-inflammatory cytokine IL-6, which is
constitutively expressed by this cell type (including MIO-M1
cells), was assessed (31).
However, under normoxic or hypoxic conditions, the amounts of the
secreted cytokine and its mRNA expression levels were not
significantly altered by the treatment with angiotensin II or
aldosterone, suggesting that the hormones do not induce a
pro-inflammatory response of the cells under these circumstances.
Interestingly, aldosterone significantly enhanced the expression of
ACE mRNA under hypoxic conditions, thus not resulting in an
inflammatory response, that is enhanced expression or secretion of
IL-6 via the angiotensin II/AT1-axis. Angiotensin II, on
the other hand, increased the expression of ACE2, resulting in its
own inactivation by the formation of angiotensin (1-7), which does
not activate AT1. Similar to the behavior of endothelial
cells from the human umbilical cord, the mRNA expression levels of
IL-6 were increased in MIO-M1 cells exposed to hyperglycemia and
aldosterone, although the amount of the secreted cytokine remained
unchanged from control cells (40).
However, increased IL-6 expression is likely independent of the
angiotensin II/AT1-axis, as possibly endogenously
produced peptide hormone is inactivated by high levels of ACE2.
Although angiotensin II did not significantly
increase IL-6 mRNA expression in MIO-M1 cells cultured under
hyperglycemic conditions, more IL-6 was secreted under these
conditions. Higher expression of AT1 mRNA could lead to
stronger activation of the pro-inflammatory angiotensin
II/AT1 signaling cascade, similar to that observed for
angiotensin II-activated retinal microglia, which express higher
quantities of various pro-inflammatory cytokines and chemokines
including IL-6, a process that is mediated by AT1
(41). Elevated permeability of
retinal endothelial cells due to IL-6-mediated trans-signaling
in vitro likely contributes to the breakdown of the inner
blood-retina barrier in vivo (42). The protective ACE2/angiotensin
(1-7)/MAS1 signaling cascade is upregulated during acute and
chronic diseases of the heart or kidney to counteract detrimental
processes (43,44). This signaling cascade seems to also
be activated by Mueller cells exposed to hyperglycemia and
angiotensin II, as expression of ACE2 and MAS1 RNA was elevated.
Thus, the concentrations of the vasodilator angiotensin (1-7)
formed by protease ACE2 may be higher, and through its interaction
with receptor MAS1, anti-angiogenic and anti-inflammatory processes
can be induced (24-27).
However, as MIO-M1 cells secreted increased quantities of IL-6 when
exposed to hyperglycemia and angiotensin II, the pro-inflammatory
axis seems to exceed the anti-inflammatory response. A similar
inflammatory response to angiotensin II was also observed after
additional exposure of the cells to hyperglycemia plus hypoxia, as
the expression of IL-6 mRNA was substantially upregulated. However,
the observed lower expression of ACE mRNA is in line with an
assumed capacity of MIO-M1 cells to counteract angiotensin
II-induced pro-inflammatory signaling.
In vivo, Mueller cells, retinal endothelial
cells, and retinal pericytes form the so-called neurovascular unit,
which tightly regulates vascular homeostasis in the retina
(45). Whether the cellular
interactions change their individual responses to angiotensin II
could not be evaluated in the present study. However, inhibitors of
ACE or AT1 were found to, at least in part, improve the
outcomes of DME in diabetic patients, which supports the findings
of the present study that Mueller cells likely contribute to
angiotensin II-mediated inflammatory responses present in the early
development of this disease (46,47).
In contrast, the impact of Mueller cells on angiotensin II-mediated
inflammatory responses observed in the early development of RVO is
likely low when hypoxia plays a dominant role accompanied by
induction of expression and secretion of the angiogenic and
permeability-inducing growth factor VEGF-A (9).
In conclusion, the results of the present in
vitro study provide evidence that the responses of Mueller
cells to activation of the RAAS by angiotensin II depend on the
environment: A pro-inflammatory response is observed under
hyperglycemic (plus hypoxic) conditions, whereas changes induced by
hypoxia are not modulated by angiotensin II.
Acknowledgements
We would like to thank Mr Julian S. Pottier
(Leipzig, Germany) for their editorial support and linguistic
revision of the manuscript. We would also like to thank Mrs Ute
Weinbrecht (Department of Ophthalmology, University of Leipzig,
Germany) and Ms Stefanie Wölfel (Department of Ophthalmology,
Justus Liebig University, Giessen, Germany) for their excellent
technical assistance.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CB and MR designed the general concept of the study;
specific experimental conditions were established by AB, MH, HLD,
and CB. AB and MH performed the experiments. AB, CB, MH, HLD, JDU
curated and analyzed the data. AB, CB, MH, and HLD wrote the
original draft manuscript. All authors reviewed and edited the
manuscript. JDU and MH provided resources. All authors have read
and approved the final manuscript. CB and HLD confirm the
authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Li JQ, Welchowski T, Schmid M, Letow J,
Wolpers C, Pascual-Camps I, Holz FG and Finger RP: Prevalence,
incidence and future projection of diabetic eye disease in Europe:
A systematic review and meta-analysis. Eur J Epidemiol. 35:11–23.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
King H, Aubert RE and Herman WH: Global
burden of diabetes, 1995-2025: Prevalence, numerical estimates, and
projections. Diabetes Care. 21:1414–1431. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ozaki H, Yu AY, Della N, Ozaki K, Luna JD,
Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL and
Campochiaro PA: Hypoxia inducible factor-1alpha is increased in
ischemic retina: Temporal and spatial correlation with VEGF
expression. Invest Ophthalmol Vis Sci. 40:182–189. 1999.PubMed/NCBI
|
|
4
|
Bringmann A, Reichenbach A and Wiedemann
P: Pathomechanisms of cystoid macular edema. Ophthalmic Res.
36:241–249. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Campochiaro PA: Seeing the light: New
insights into the molecular pathogenesis of retinal diseases. J
Cell Physiol. 213:348–354. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampe HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial frowth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin
W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD
and Adamis AP: VEGF-initiated blood-retinal barrier breakdown in
early diabetes. Invest Ophthalmol Vis Sci. 42:2408–2413.
2001.PubMed/NCBI
|
|
8
|
Park SP and Ahn JK: Changes of aqueous
vascular endothelial growth factor and interleukin-6 after
intravitreal triamcinolone for branch retinal vein occlusion. Clin
Exp Ophthalmol. 36:831–835. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Noma H, Funatsu H, Mimura T, Harino S and
Hori S: Vitreous levels of interleukin-6 and vascular endothelial
growth factor in macular edema with central retinal vein occlusion.
Ophthalmology. 116:87–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ghodasra DH, Fante R, Gardner TW, Langue
M, Niziol LM, Besirli C, Cohen SR, Dedania VS, Demirci H, Jain N,
et al: Safety and feasibility of quantitative multiplexed cytokine
analysis from office-based vitreous aspiration. Invest Ophthalmol
Vis Sci. 57:3017–3023. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsai T, Kuehn S, Tsiampalis N, Vu MK,
Kakkassery V, Stute G, Dick HB and Joachim SC: Anti-inflammatory
cytokine and angiogenic factors levels in vitreous samples of
diabetic retinopathy patients. PLoS One.
13(e0194603)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Klaassen I, Avery P, Schlingemann RO and
Steel DHW: Vitreous protein networks around ANG2 and VEGF in
proliferative diabetic retinopathy and the differential effects of
aflibercept versus bevacizumab pre-treatment. Sci Rep.
12(21062)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Danser AH, van den Dorpel MA, Deinum J,
Derkx FH, Franken AA, Peperkam E, de Jong PT and Schalekamp MA:
Renin, prorenin, and immunoreactive renin in vitreous fluid from
eyes with and without diabetic retinopathy. J Clin Endocrinol
Metab. 68:160–167. 1989.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Funatsu H, Yamashita H, Nakanishi Y and
Hori S: hypox II and vascular endothelial growth factor in the
vitreous fluid of patients with proliferative diabetic retinopathy.
Br J Ophthalmol. 86:311–315. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Funatsu H, Yamashita H, Ikeda T, Nakanishi
Y, Kitano S and Hori S: Angiotensin II and vascular endothelial
growth factor in the vitreous fluid of patients with diabetic
macular edema and other retinal disorders. Am J Ophthalmol.
133:537–543. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Berka JL, Stubbs AJ, Wang DZ,
DiNicolantonio R, Alcorn D, Campbell DJ and Skinner SL:
Renin-containing Mueller cells of the retina display endocrine
features. Invest Ophthalmol Vis Sci. 36:1450–1458. 1995.PubMed/NCBI
|
|
17
|
Gerhardinger C, Costa MB, Coulombe MC,
Toth I, Hoehn T and Grosu P: Expression of acute-phase response
proteins in retinal Mueller cells in diabetes. Invest Ophthalmol
Vis Sci. 46:349–357. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Senanayake P, Drazba J, Shadrach K,
Milsted A, Rungger-Brandle E, Nishiyama K, Miura S, Karnik S, Sears
JE and Hollyfield JG: Angiotensin II and its receptor subtypes in
the human retina. Invest Ophthalmol Vis Sci. 48:3301–3311.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Downie LE, Vessey K, Miller A, Ward MM,
Pianta MJ, Vingrys AJ, Wilkinson-Berka JL and Fletcher EL: Neuronal
and glial cell expression of angiotensin II type 1 (AT1) and type 2
(AT2) receptors in the rat retina. Neuroscience. 161:195–213.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ferrari-Dileo G, Davis EB and Anderson DR:
Angiotensin binding sites in bovine and human retinal blood
vessels. Invest Ophthalmol Vis Sci. 28:1747–1751. 1987.PubMed/NCBI
|
|
21
|
Wheeler-Schilling TH, Sautter M, Guenther
E and Kohler K: Expression of angiotensin-converting enzyme (ACE)
in the developing chicken retina. Exp Eye Res. 72:173–182.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tikellis C, Johnston CI, Forbes JM, Burns
WC, Thomas MC, Lew RA, Yarski M, Smith AI and Cooper ME:
Identification of angiotensin converting enzyme 2 in the rodent
retina. Curr Eye Res. 29:419–427. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prasad T, Verma A and Li Q: Expression and
cellular localization of the Mas receptor in the adult and
developing mouse retina. Mol Vis. 20:1443–1455, eCollection 2014.
2014.PubMed/NCBI
|
|
24
|
Zhu P, Verma A, Prasad T and Li Q:
Expression and function of Mas-related G protein-coupled receptor D
and its ligand alamandine in retina. Mol Neurobiol. 57:513–527.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wilkinson-Berka JL, Suphapimol V, Jerome
JR, Deliyanti D and Allingham MJ: Angiotensin II and aldosterone in
retinal vasculopathy and inflammation. Exp Eye Res.
187(107766)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chung O, Kühl H, Stoll M and Unger T:
Physiological and pharmacological implications of AT1 versus AT2
receptors. Kidney Int. 54 (Suppl):S95–SS99. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patel VB, Zhong JC, Grant MB and Oudit GY:
Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin
system in heart failure. Circ Res. 118:1313–1326. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Limb GA, Salt TE, Munro PM, Moss SE and
Khaw PT: In vitro characterization of a spontaneously immortalized
human Mueller cell line (MIO-M1). Invest Ophthalmol Vis Sci.
43:864–869. 2002.PubMed/NCBI
|
|
29
|
Schmalen A, Lorenz L, Grosche A, Pauly D,
Deeg CA and Hauck SM: Proteomic phenotyping of stimulated Mueller
cells uncovers profound pro-inflammatory signaling and
antigen-presenting capacity. Front Pharmacol.
12(771571)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Eastlake K, Banerjee PJ, Angbohang A,
Charteris DG, Khaw PT and Limb GA: Mueller glia as an important
source of cytokines and inflammatory factors present in the gliotic
retina during proliferative vitreoretinopathy. Glia. 64:495–506.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mohammad G, AlSharif HM, Siddiquei MM,
Ahmad A, Alam K and Abu El-Asrar AM: Rho-associated protein
kinase-1 mediates the regulation of inflammatory markers in
diabetic retina and in retinal Mueller cells. Ann Clin Lab Sci.
48:137–145. 2018.PubMed/NCBI
|
|
33
|
Gilbert RE, Vranes D, Berka JL, Kelly DJ,
Cox A, Wu LL, Stacker SA and Cooper ME: Vascular endothelial growth
factor and its receptors in control and diabetic rat eyes. Lab
Invest. 78:1017–1027. 1998.PubMed/NCBI
|
|
34
|
Witmer AN, Blaauwgeers HG, Weich HA,
Alitalo K, Vrensen GF and Schlingemann RO: Altered expression
patterns of VEGF receptors in human diabetic retina and in
experimental VEGF-induced retinopathy in monkey. Invest Ophthalmol
Vis Sci. 43:849–857. 2002.PubMed/NCBI
|
|
35
|
Deissler HL, Stutzer JN, Lang GK, Grisanti
S, Lang GE and Ranjbar M: VEGF receptor 2 inhibitor nintedanib
completely reverts VEGF-A165-induced disturbances of
barriers formed by retinal endothelial cells or long-term
cultivated ARPE-19 cells. Exp Eye Res. 194(108004)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Y, Yan Z, Chaudhry K and Kazlauskas A:
The renin-angiotensin-aldosterone system (RAAS) is one of the
effectors by which vascular endothelial growth factor
(VEGF)/anti-VEGF controls the endothelial cell barrier. Am J
Pathol. 190:1971–1981. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sano H, Hosokawa K, Kidoya H and Takakura
N: Negative regulation of VEGF-induced vascular leakage by blockade
of angiotensin II type 1 receptor. Arterioscler Thromb Vasc Biol.
26:2673–2680. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim JH, Kim JH, Yu YS, Cho CS and Kim KW:
Blockade of angiotensin II attenuates VEGF-mediated blood-retinal
barrier breakdown in diabetic retinopathy. J Cereb Blood Flow
Metab. 29:621–628. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Moravski CJ, Kelly DJ, Cooper ME, Gilbert
RE, Bertram JF, Shahinfar S, Skinner SL and Wilkinson-Berka JL:
Retinal neovascularization is prevented by blockade of the
renin-angiotensin system. Hypertension. 36:1099–1104.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chou CH, Hung CS, Liao CW, Wei LH, Chen
CW, Shun CT, Wen WF, Wan CH, Wu XM, Chang YY, et al: IL-6
trans-signalling contributes to aldosterone-induced cardiac
fibrosis. Cardiovasc Res. 114:690–702. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Phipps JA, Vessey KA, Brandli A, Na N,
Tran MX, Jobling AI and Fletcher E: The role of angiotensin II/AT1
receptor signaling in regulating retinal microglial activation.
Invest Ophthalmol Vis Sci. 59:487–498. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Glass J, Robinson R, Lee TJ, Sharma A and
Sharma S: Interleukin-6 trans-signaling mediated regulation of
paracellular permeability in human retinal endothelial cells. Inter
J Transl Med. 1:137–153. 2021.
|
|
43
|
Kaschina E, Namsolleck P and Unger T: AT2
receptors in cardiovascular and renal diseases. Pharmacol Res.
125(Pt A):39–47. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rodrigues Prestes TR, Rocha NP, Miranda
AS, Teixeira AL and Simoes-E-Silva AC: The anti-inflammatory
potential of ACE2/angiotensin-(1-7)/Mas receptor axis: Evidence
from basic and clinical research. Curr Drug Targets. 18:1301–1313.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Klaassen I, Van Noorden CJ and
Schlingemann RO: Molecular basis of the inner blood-retinal barrier
and its breakdown in diabetic macular edema and other pathological
conditions. Prog Retin Eye Res. 34:19–48. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mauer M, Zinman B, Gardiner R, Suissa S,
Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC
and Klein R: Renal and retinal effects of enalapril and losartan in
type 1 diabetes. N Engl J Med. 361:40–51. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cernes R, Mashavi M and Zimlichman R:
Differential clinical profile of candesartan compared to other
angiotensin receptor blockers. Vasc Health Risk Manag. 7:749–759.
2011.PubMed/NCBI View Article : Google Scholar
|