Introduction
Sexually transmitted diseases (STDs) occur worldwide
and are an important public health problem. In developing
countries, STDs rank among the five most frequent reasons for
health services being sought (1).
The term STDs refers to the various clinical symptoms generated by
pathogenic microorganisms that are transmitted through sexual
intercourse. Among the causative agents of STDs, Chlamydia
trachomatis (C. trachomatis) is associated particularly
with urethritis and cervicitis. Mycoplasma hominis (M.
hominis) and Ureaplasma species are frequently found in
the commensal microorganisms of the lower genital tract; however,
their role in other sexually transmitted infections remains
unclear. Ureaplasma urealyticum (U. urealyticum) was
originally considered to have two biovars, biovar 1 and biovar 2,
which were subsequently found to be separate species by polymerase
chain reaction (PCR) and named U. parvum and U.
urealyticum, respectively (2).
Ureaplasma species are the most widely investigated
pathogens associated with non-gonococcal urethritis, and the
results are conflicting (3). U.
urealyticum has been reported to cause infections in the lower
genital tract, being a pathogen of male urethritis and a likely
cause of bacterial vaginosis (2,3).
Ureaplasma species, M. hominis and C.
trachomatis can cause infertility in both men and women
(4).
Mollicutes (U. urealyticum and M.
hominis) and Chlamydia, when localized and colonized
within certain anatomical sites, may cause pathological disorders,
including urethritis in males and females, prostatitis and
epididymitis in males, and vaginitis, endometriosis and salpingitis
in females. Urethritis and vaginitis are characterized by discharge
and/or dysuria, although they may also be entirely asymptomatic
(5). C. trachomatis is the
most common cause of non-gonococcal urethritis, with a prevalence
in the general population of between 1 and 10% (6). The prevalence of Ureaplasma
species and M. hominis has been reported to be 21 and 3%,
respectively (7). These
microorganisms can also cause sexually transmitted reactive
arthritis (Reiter's Syndrome) (8).
Regarding female patients, it is worthy of note that their role in
the etiology of pregnancy complications has been suggested,
including the induction of preterm labor, infertility, spontaneous
abortion, puerperal fever and pelvic inflammatory disease (5,8).
Moreover, the transmission of U. urealyticum to the fetus or
newborn may cause severe bronchopulmonary dysplasia and central
nervous system (CNS) infections (5).
The objective of the present study was to estimate
the prevalence of U. urealyticum, M. hominis and
C. trachomatis in a Romanian population taking into
consideration the presence or absence of genital symptoms.
Materials and methods
Patients
The present study is a retrospective, observational
study, conducted from January 2021 to December 2021. The study was
conducted at ‘Ponderas’ Academic Hospital (Bucharest, Romania) in
the Dermato-venerology Department. Data on all 266 patients who
provided urogenital samples for Ureaplasma and
Mycoplasma detection by culture were collected and analyzed,
as well as urogenital samples for Chlamydia detection by
PCR. Specimens were obtained from two different groups of patients:
Symptomatic subjects who reported urogenital symptoms and were
categorized in four subgroups, namely urethritis, prostatitis,
vaginitis, and urethritis with prostatitis; and completely
asymptomatic subjects who came for microbiological screening for
STDs, a number of whom reported sexual contact with infected
individuals. All patients were sexually active. Only samples
collected during the first visit were considered in the study, and
specimens obtained during follow-ups of the same patient were
excluded. All procedures performed in the study were in accordance
with the ethical standards of the institutional and/or national
research committee and with the 1964 Declaration of Helsinki and
its later amendments or comparable ethical standards. Written
informed consent was obtained from all individual participants
included in the study.
Urethral/vaginal swabs
Male patients were placed in the gynecological
position and asked to retract the foreskin of the penis and keep it
retracted throughout the procedure. The doctor used sterile cotton
or gauze to clean the opening of the urethra at the tip of the
penis. To facilitate sample collection and stimulate prostatic
gland secretion, prostatic massage was performed prior to
collection of the sample. Then, a first cotton swab was gently
inserted ~2 cm into the urethra and rotated. To obtain a good
sample, the test was performed ≥3 days from the last sexual
intercourse and 2 h after urination. The swabs were placed in R1
broth from a Mycoplasma IST 2 kit (bioMérieux) to initiate the
isolation of mycoplasmas. For female patients, the vaginal sample
was taken by placing the patient in a gynecological position and
carefully introducing a cotton swab into the vaginal canal. The use
of commercial lubricants or antiseptics was avoided. The swabs were
placed in R1 broth to initiate the isolation of Mollicutes. The
liquid medium for U. urealyticum and M. hominis was a
transport medium used for inoculation of a test strip. In order to
perform the phenotypic identification of U. urealyticum,
urea broth was used, which contained medium base
(pleuropneumonia-like organism broth), yeast extract, horse serum
and urea. To determine the growth of this microorganism, phenol red
was added to the culture medium, as it changes from red to intense
raspberry red in the presence of urease and ammonium production.
The culture medium specific for M. hominis included arginine
which, when metabolized, produces an alkaline compound that changes
phenol red to a raspberry red color. The culture media were
incubated at 37˚C until the phenol red indicator changed color. The
Mycoplasma IST 2 kit was used according to the manufacturer's
instructions as follows. As aforementioned, the sample-bearing swab
was placed in the transport medium R1 broth (3 ml). The broth was
mixed with the contents of the lyophilised R2 vial provided with
the kit, which contained the substrates necessary for the
development of microorganisms. A volume of 55 ml was added to each
of the 22 domes in the test strip. Firstly, the phenotypic
detection of M. hominis and U. urealyticum was
performed. Secondly, the microorganisms were quantified, to
determine whether the sample concentration was >1x104
change color-changing units (CCU), as this indicated an important
presence of these microorganisms (positive result) (9).
A second swab was inserted in the urethra of male
patients and the vaginal canal of women to collect urogenital
samples for the detection of C. trachomatis by PCR. The
samples were collected using a DNA collection device, comprising a
cytobrush and DNA holder buffer (Specimen Transport Medium; Digene;
Qiagen, Inc.), for the investigation of bacterial infections.
Bacterial DNA samples were extracted from samples collected from
the urogenital tract using an RTP®-Bacteria DNA kit
(Invitek Diagnostics), according to the manufacturer's procedures
and amplified using a 5TD6 ACE Detection kit (Allplex STI Essential
Assay; Seegene, Inc.) for the detection of C. trachomatis by
PCR. The PCR primer sequences are not disclosed by the
manufacturer. The PCR conditions were as follows: 1 cycle of 94˚C
for 15 min, 40 cycles of 94˚C for 30 sec, 63˚C for 90 sec and 72˚C
for 90 sec, and 1 cycle of 72˚C for 10 min. The amplification of
plasmidial DNA as an internal control occurred in the same
reaction. The PCR product was subjected to electrophoresis on a gel
containing 2% agarose stained with ethidium bromide. Amplification
of the target was only observed when the respective bacterial DNA
was present in the clinical sample (10). This method was applied to 30
samples. For the remaining 236 the testing method was changed due
to financial issues at the laboratory. Images of the agarose gel
are not available.
The subsequent method used for the detection of
C. trachomatis involved the insertion of a swab in the
urethra for male patients and the vaginal canal for women to
collect urogenital samples for analysis by an alternative PCR
method. The urethral sample was transferred into an Aptima Swab
Specimen Transfer Tube (Hologic, Inc.). The samples were
transported at a temperature of between 2 and 30˚C. Determination
of C. trachomatis rRNA in the genital secretions was
performed using the Panther® System analyzer (Hologic,
Inc.), which is based on nucleic acid amplification testing with
transcription-mediated amplification (TMA) and dual kinetic
detection. TMA is an isothermal amplification method that uses RNA
polymerase and reverse transcriptase. Since the amplification
temperature is 37-42˚C, the technique does not require a
thermocycler and can be performed using a thermoblock. TMA uses two
primers that flank the region to be amplified: A promoter primer
and a non-promoter primer with the same sense as the target. The 3'
end of the promoter primer is complementary to that of the target
RNA and the 5' end is recognized by RNA polymerase. Amplification
is initiated via the binding of the promoter primer to the target
RNA, which is then reverse transcribed to generate cDNA. The
DNA-RNA duplex is degraded, and the RNA released through the RNase
H activity of the reverse transcriptase. The second primer binds to
the cDNA and generates double-stranded molecules. Hundreds of
copies of the RNA amplicons are thus transcribed by means of this
DNA and each copy can be converted into new double-stranded DNA
molecules. The amplification products are analyzed by hybridization
with oligonucleotide probes labeled with chemiluminescent
substances. Assay results were automatically interpreted by APTIMA
Assay software (Panther System®; Hologic, Inc.) using
the APTIMA Combo 2 protocol, and presented as individual CT test
results. Based on the kinetic type and total relative light units
(RLU) in the detection step, the test results were assigned as
negative (RLU <25), equivocal (RLU <100), positive (RLU
>100) or invalid (11).
Statistical analysis
Categorical variables are expressed as counts and
percentages. Tests of association were performed using Chi-square
or Fisher's exact tests, as appropriate. P<0.05 was considered
to indicate a statistically significant result, at which the null
hypothesis could be rejected. Data were analyzed with R Statistical
Software version 4.1.1 (https://www.r-project.org).
Results
The study population consisted of 266 patients (225
males and 41 females) aged between 18 and 80 years, and 89% of the
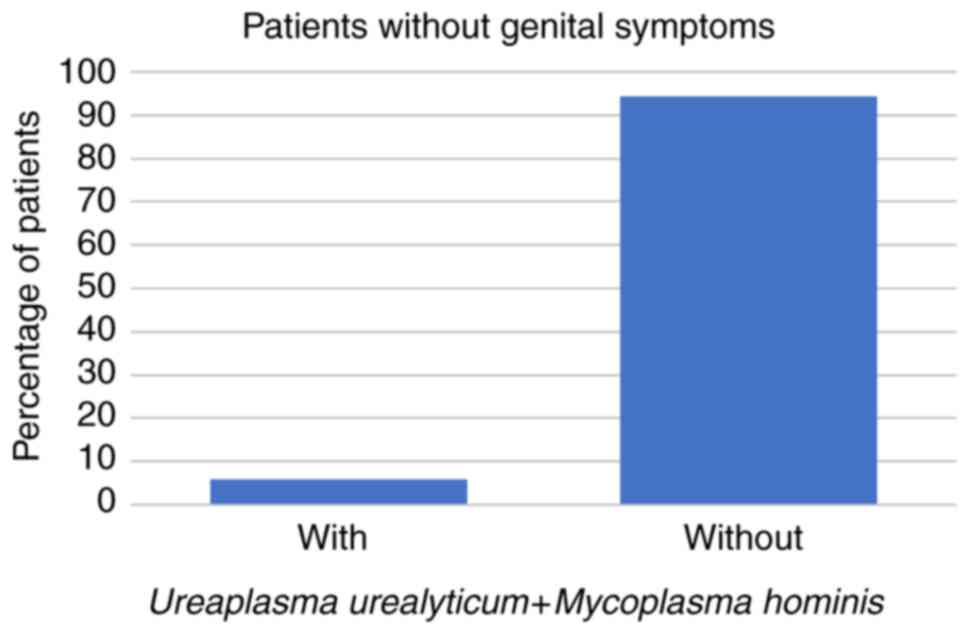
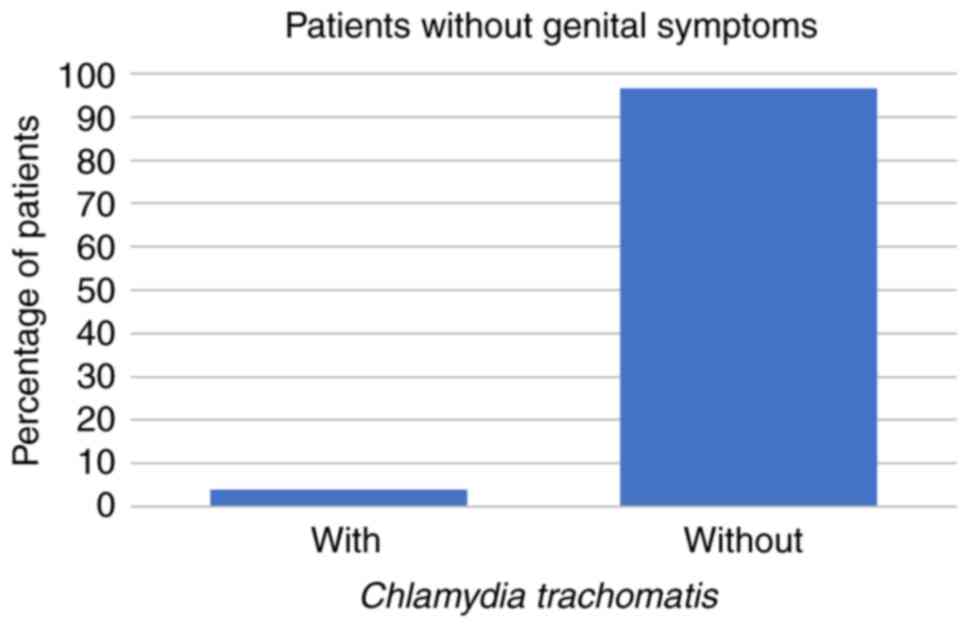
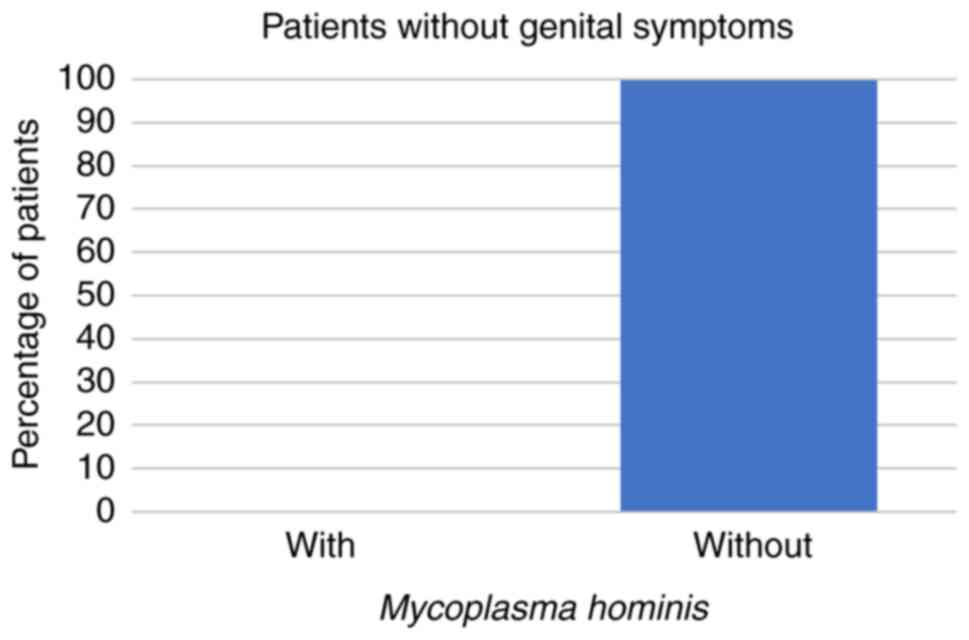
patients were aged 31-50 years. Among these 266 patients, 59 (22%)
had STD symptoms while the other 207 patients (78%) had no STD
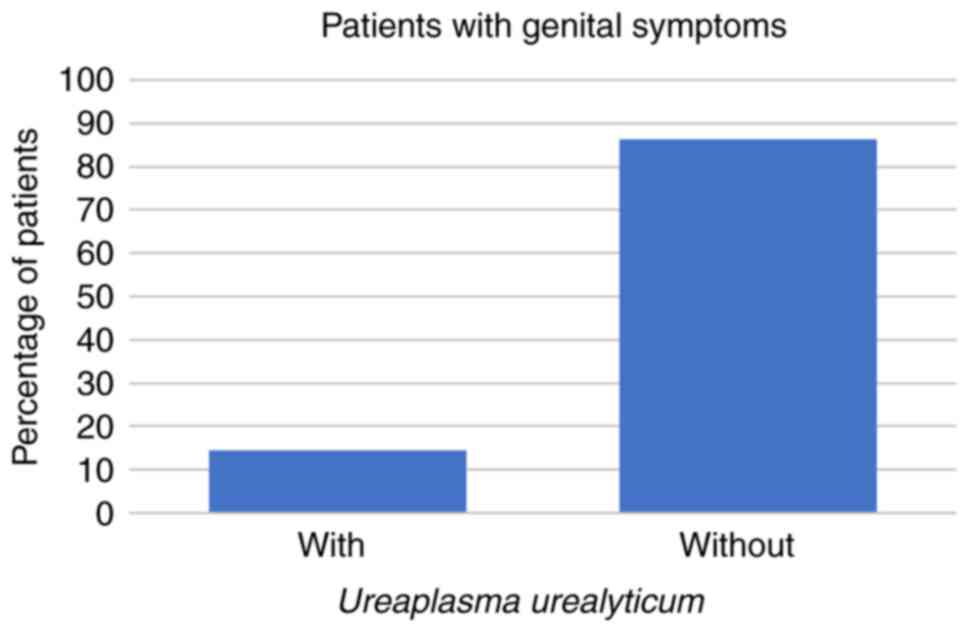
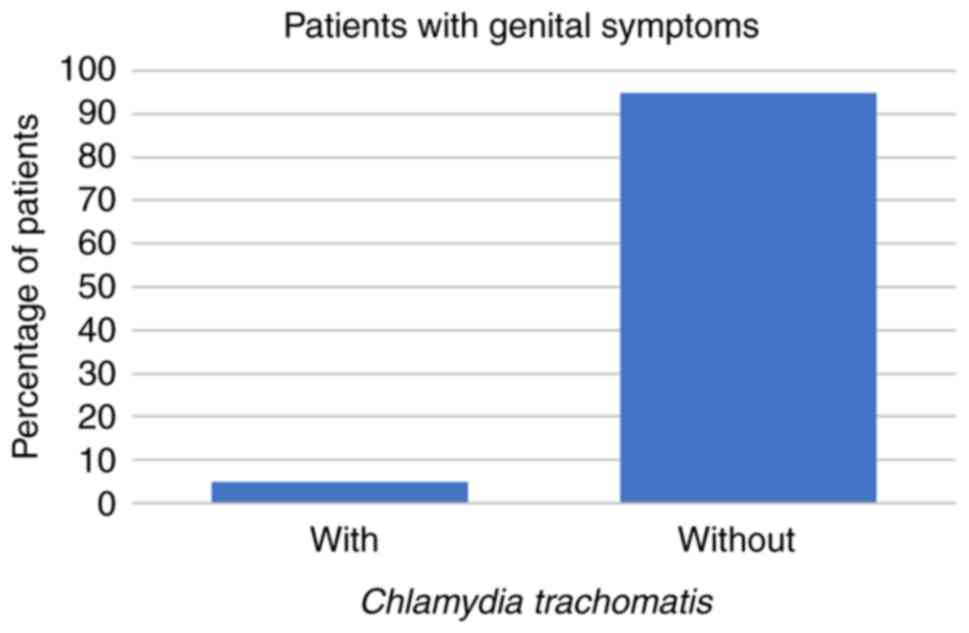
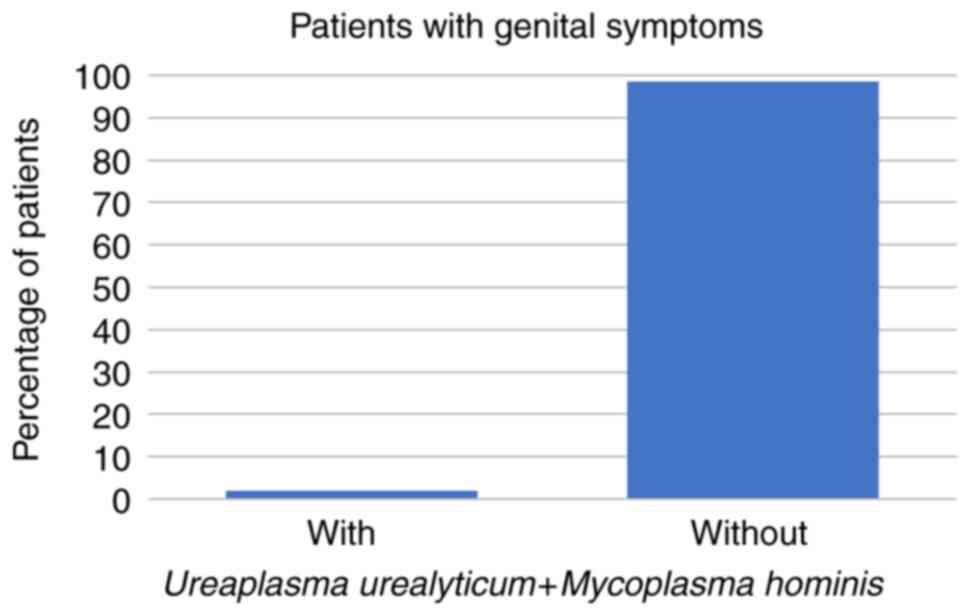
symptoms at all. Regarding the symptomatic patients, 8 (14%) had
U. urealyticum, 1 (2%) had U. urealyticum and M.
hominis coinfection, 3 (5%) had C. trachomatis, and no
symptomatic patients were infected with M. hominis alone
(all P<0.001; Fig. 1, Fig. 2 and Fig.
3; Table I).
 | Table IDistribution of patients according to
the presence of symptoms and pathogens. |
Table I
Distribution of patients according to
the presence of symptoms and pathogens.
| | Symptomatic, n
(%) | Asymptomatic, n
(%) |
|---|
| Pathogens | Males | Females | Males | Females |
|---|
| U.
urealyticum | 7(88) | 1(13) | 21(72) | 8(28) |
| M.
hominis | 0 (0) | 0 (0) | 1(100) | 0 (0) |
| Coinfection | 1(100) | 0 (0) | 5(38) | 8(62) |
| C.
trachomatis | 3(100) | 0 (0) | 2(50) | 2(50) |
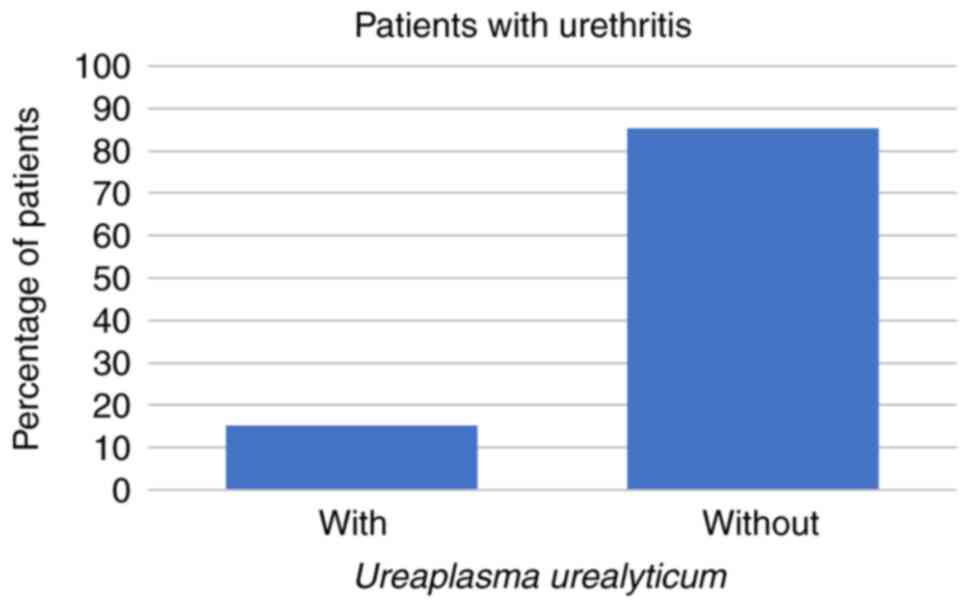
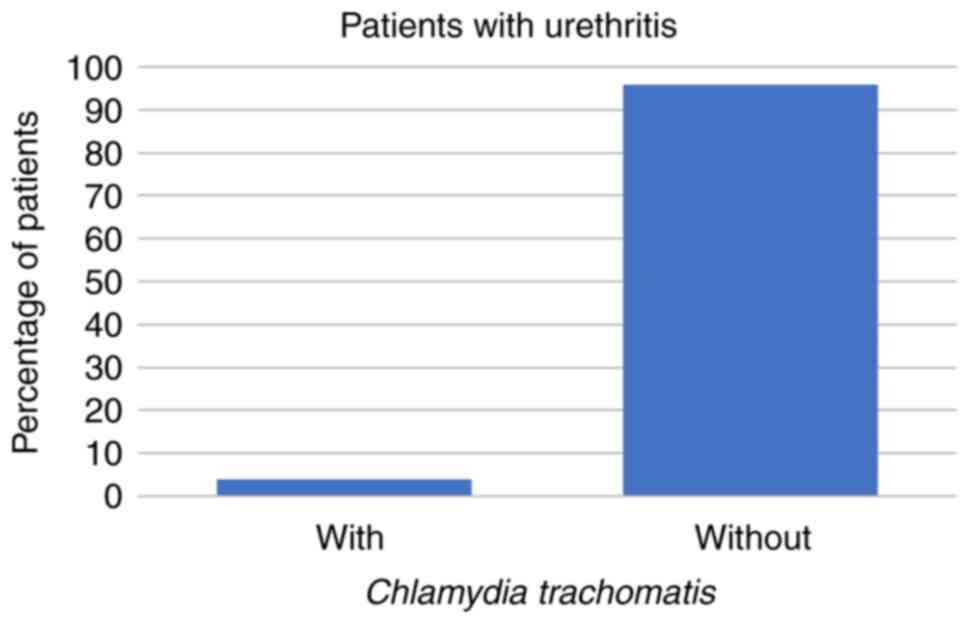
Symptoms of urethritis were reported by 27/266 (10%)
of the patients. Of those 27 patients, 4 (15%) had U.
urealyticum and 1 (4%) had C. trachomatis, but none of
the patients with symptoms of urethritis had U. urealyticum
and M. hominis coinfection or were infected with M.
hominis alone (all P<0.001; Figs. 4 and 5; Table
II).
 | Table IIDistribution of patients according to
specific symptoms and pathogens. |
Table II
Distribution of patients according to
specific symptoms and pathogens.
| | Symptoms, n
(%) |
|---|
| Pathogens | Urethritis | Prostatitis | Urethritis +
prostatitis | Asymptomatic |
|---|
| U.
urealyticum | 4(15)a | 3(13)a | 0 (0)a | 29(14)a |
| M.
hominis | 0 (0)a | 0 (0)a | 0 (0)a | 1 (0)a |
| Coinfection | 0 (0)a | 0 (0)a | 0 (0)a | 13(6)a |
| C.
trachomatis | 1(4)a | 1(4)a | 1(17)b | 4(2)a |
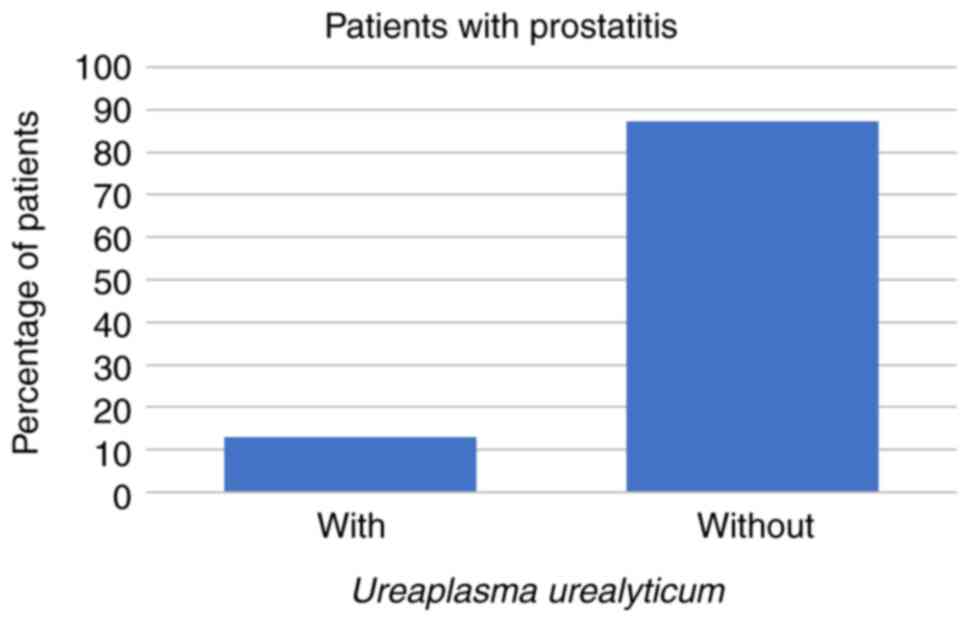
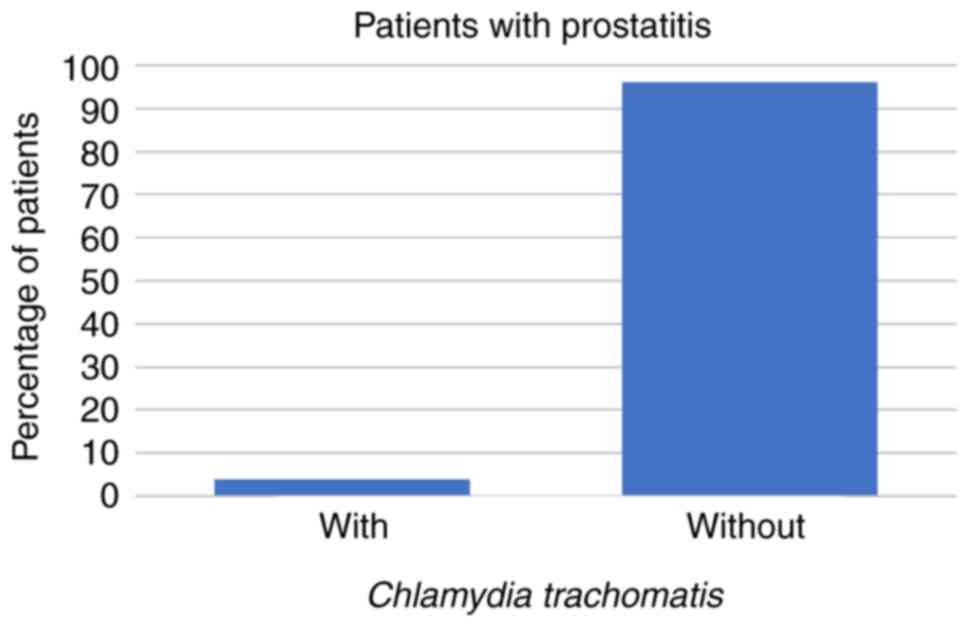
In the present study, 23/266 (9%) patients presented
symptoms characteristic of prostatitis. These comprised 3 patients
(13%) whose prostatitis was caused by U. urealyticum and 1
(4%) in which the prostatitis was caused by C. trachomatis,
while prostatitis was caused neither by U. urealyticum and
M. hominis coinfection, nor by M. hominis alone in
these patients (all P<0.001; Figs.
6 and 7; Table II).
Symptoms of both urethritis and prostatitis were
present in 6/266 (2%) of the patients. Only 1/6 patients with these
symptoms (17%) was infected with C. trachomatis (P=0.1025;
Table II). Neither of the
Mollicutes was detected in patients with urethritis and prostatitis
(P=0.003892).
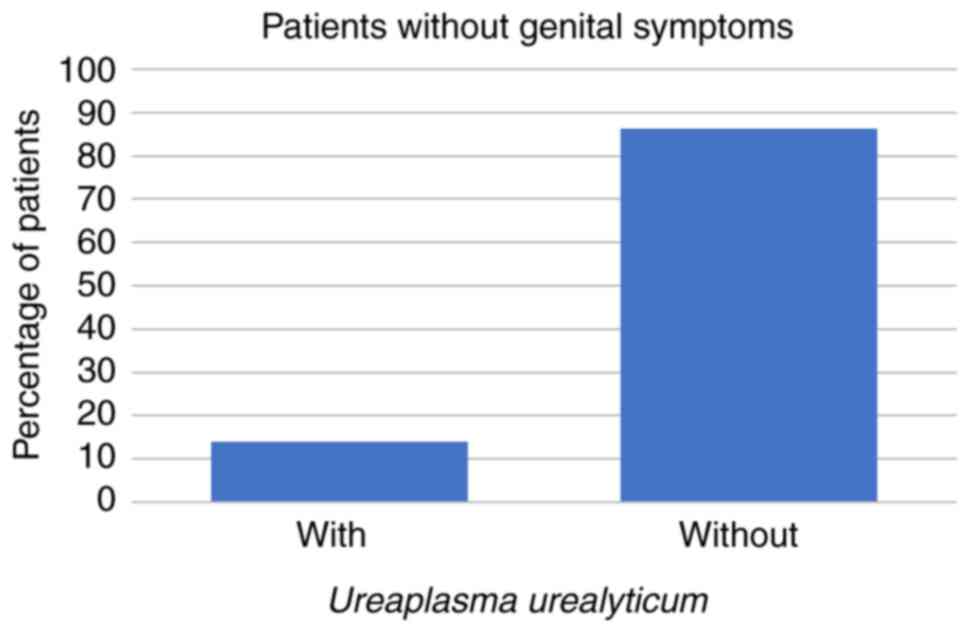
The study population included 207/266 (78%) patients
who were completely asymptomatic. Among these 207 patients, 29
(14%) were discovered to have U. urealyticum, 13 (6%) were
coinfected with both Mollicutes, 1 (0%) was infected with M.
hominis alone and 4 (2%) were infected with C.
trachomatis (all P<0.001; Fig.
8, Fig. 9, Fig. 10 and Fig. 11; Tables I and II).
Regarding the female patients, there were only 3
cases of vaginitis, one of which was a symptomatic coinfection with
Mollicutes. The remaining 38 women had asymptomatic genital
infections.
The association between the Mollicutes and C.
trachomatis was also evaluated. It was found that 2 patients
(14%) with U. urealyticum and M. hominis coinfection
were also infected with C. trachomatis, while none of the
patients who were infected with U. Urealyticum or M.
hominis alone were also positive for C. trachomatis
(P=0.1071).
Discussion
The genital tract is a propitious area for the
growth of numerous microorganisms, some of which may cause
pathologies, including urethritis, endometriosis, epididymitis and
salpingitis. Urethritis is characterized by discharge and/or
dysuria, although it can also occur without any symptoms.
Urethritis may be either gonococcal, when Neisseria
gonorrhoeae is detected, or non-gonococcal (5). M. hominis and U.
urealyticum are commonly found in the genitourinary tract as
causative agents for several STDs. In men, U. urealyticum is
a major cause of non-gonococcal urethritis, which may also be
caused by M. hominis to a lesser extent (12). These microorganisms can cause
sexually transmitted reactive arthritis (Reiter's syndrome),
epididymitis and chronic prostatitis, and are suggested to play a
role in pregnancy complications. In addition, U. urealyticum
transmission to the fetus may cause bronchopulmonary dysplasia and
CNS infections (5).
Although previous studies showed a greater
prevalence of the Mollicutes and/or Chlamydia in women
(13,14), mainly male patients are seen in the
Dermato-venerology Department at ‘Ponderas’ Academic Hospital as
women are usually referred to the Gynecology Department. Therefore,
225 of the 266 patients enrolled in the study were male. Similar to
other studies (15,16), 207/266 (78%) were completely
asymptomatic. This emphasizes the requirement for STD screening in
sexually active individuals, since many modern STDs can be
clinically silent, while their outcomes could be serious.
The most common symptom in all male patients was
urethritis (10%), the main causes of which were U.
urealyticum (15%) and C. trachomatis (4%). In addition,
9% of the male patients had symptoms suggestive of prostatitis.
Similar to urethritis, the causative agents of prostatitis were
found to be U. urealyticum (13%) and C. trachomatis
(4%). Both urethritis and prostatitis were present in 6 (2%) of the
male patients, which was caused by C. trachomatis in 1 case.
Regarding the asymptomatic patients, the main infective agent
detected in the present study was U. urealyticum (14%),
followed by coinfection (6%), C. trachomatis (2%), and M.
hominis in a single patient (0%), which is similar to the
pattern of infection in symptomatic patients: 14% U.
urealyticum, 5% C. trachomatis and 2% coinfection. The
small proportion of cases with M. hominis, only one
asymptomatic male, may be attributed to the small number of women
included, although it is consistent with literature data (17).
Recent studies have shown that the prevalence of
Ureaplasma species and M. hominis is ~21 and 3%,
respectively (7) and the prevalence
of C. trachomatis is ~2.9% (18). In the present study, the percentages
are consistent with the worldwide trend regarding Mollicutes, but
differ slightly regarding infection with C. trachomatis,
with a prevalence of 2% for asymptomatic patients and 5% for
individuals who presented with genital symptoms. A similar
percentage to that in the present study has been identified in
Latin America and regions of Africa, at 6.7 and 3.8%, respectively
(18). Regional variations may be
associated with social, cultural and economic conditions,
differences in control policy and gender inequality, but those
require examination in further studies (18). Moreover, it should be taken into
account that, generally, women are more affected by these
infections than men. Since the individuals enrolled in the present
study were predominantly males, further studies are required in
order to obtain an accurate percentage. In the future, statistics
from the Dermato-venerology Department will be compared from those
in the Gynecology Department to determine if there is an important
difference regarding infection with Mollicutes and C.
trachomatis between the sexes in Romania. However, many
gynecologists in Romania do not include testing for Mollicutes and
C. trachomatis in the basic screening process, unless the
patients report urogenital symptoms. A study from Spain illustrated
that the prevalence of U. urealyticum was 17.73%, and the
prevalence of M. hominis and C. trachomatis was 10.64
and 26.95%, respectively, in men with and without symptoms of
urethritis (19). In addition,
studies of Chinese patients indicated that the overall prevalence
of total Ureaplasma species and/or M. hominis was
38.1% from 2013 to 2019. Ureaplasma species were the most
frequently isolated (overall prevalence, 31.3%), followed by
Ureaplasma species/M. hominis coinfection (6.0%) and
single M. hominis infection (0.8%) (20,21).
These marked differences between countries and
regions could be due to the lack of sexual education in young
individuals in developing countries, insufficient screening tests
or poor technique during sample collecting. In addition, numerous
physicians do not routinely perform tests for Mollicutes and/or
C. trachomatis.
Notably, it is recommended that sampling should be
performed by the physician, not a nurse, since the nurse may not
have undergone adequate training in the collection of urethral
swabs, resulting in false negative results. In the
Dermato-venerology Department of ‘Ponderas’ Academic Hospital, the
dermatovenerologists perform these maneuvers to enhance the
validity of the tests. Moreover, urethral swabs are collected from
male patients to search for C. trachomatis, instead
of urine samples. This is because it is more convenient to collect
all samples at once when performing a complete STD screen. However,
this procedure is more uncomfortable for the patient.
Finally, it must be emphasised that
Ureaplasma and Mycoplasma are opportunistic
pathogens, frequently found in the commensal flora of the lower
genital tract. The Mycoplasma kit used in the present study
determined whether the sample concentration was
>1x104 CCU in order to make a diagnosis of Mollicute
infection. However, previously reported studies describe different
techniques for Mollicute and Chlamydia detection. Some of
these evaluated the microscopy of Gram-stained urethral smears in
the diagnosis of non-gonococcal urethritis, and reported a
threshold of ≥2 polymorphonuclear leukocytes/high power field as
being indicative of a positive result (22-24).
A comparison of the two diagnostic methods will be made in a future
study.
In conclusion, the most prevalent pathogen
populating the genital tract in both males and females is U.
urealyticum, followed by U. urealyticum and M.
hominis coinfection, and C. trachomatis. Numerous
infections are asymptomatic, but should be screened for, since they
can cause serious complications, most importantly infertility in
men and women. Furthermore, the present study raises awareness of
the importance of complete STD screening, regardless of the
presence of symptoms.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AC performed the statistical analysis, critically
reviewed literature findings and revised the manuscript. DB
collected the urethral and vaginal swabs from the patients and sent
them to the laboratory, and conceived and designed the study. AC
and DB confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Ponderas’ Academic Hospital (approval no. 509/16.02.2022). Written
informed consent was obtained from all patients prior to
publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campos GB, Lobão TN, Selis NN, Amorim AT,
Martins HB, Barbosa MS, Oliveira TH, dos Santos DB, Figueiredo TB,
Miranda Marques L and Timenetsky J: Prevalence of Mycoplasma
genitalium and Mycoplasma hominis in urogenital tract of Brazilian
women. BMC Infect Dis. 15(60)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Choe HS, Lee DS, Lee SJ, Hong SH, Park DC,
Lee MK, Kim TH and Cho YH: Performance of AbyplexsTM II
multiplex real-time PCR for the diagnosis of seven sexually
transmitted infections: Comparison with currently available
methods. Int J Infect Dis. 17:e1134–e1140. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wetmore CM, Manhart LE, Lowens MS, Golden
MR, Whittington WL, Xet-Mull AM, Astete SG, McFarland NL, McDougal
SJ and Totten PA: Demographic, behavioral, and clinical
characteristics of men with nongonococcal urethritis differ by
etiology: A case-comparison study. Sex Transm Dis. 38:180–186.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Esen B, Gozalan A, Sevindi DF, Demirbas A,
Onde U, Erkayran U, Karakoc AE, Hasçiçek AM, Ergün Y and Adiloglu
AK: Ureaplasma urealyticum: Presence among Sexually transmitted
diseases. Jpn J Infect Dis. 70:75–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Salari MH and Karimi A: Prevalence of
Ureaplasma urealyticum and Mycoplasma genitalium in men with
non-gonococcal urethritis. East Mediterr Health J. 9:291–295.
2003.PubMed/NCBI
|
|
6
|
Dielissen PW, Teunissen DA and
Lagro-Janssen AL: Chlamydia prevalence in the general population:
Is there a sex difference? a systematic review. BMC Infect Dis.
13(534)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee JY and Yang JS: Prevalence and
antimicrobial susceptibility of mycoplasma hominis and ureaplasma
species in nonpregnant female patients in South Korea indicate an
increasing trend of pristinamycin-resistant isolates. Antimicrob
Agents Chemother. 64:e01065–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cutoiu A and Boda D: Antimicrobial
Resistance of Ureaplasma Urealyticum and Mycoplasma Hominis in the
Romanian population. Farmacia. 71(1)2023.
|
|
9
|
D'Inzeo T, De Angelis G, Fiori B,
Menchinelli G, Liotti FM, Morandotti GA, De Maio F, Nagel D,
Antonaci M, Sanguinetti M and Spanu T: Comparison of Mycoplasma
IES, Mycofast Revolution and Mycoplasma IST2 to detect genital
mycoplasmas in clinical samples. J Infect Dev Ctries. 11:98–101.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Christofolini DM, Leuzzi L, Mafra FA,
Rodart I, Kayaki EA, Bianco B and Barbosa CP: Prevalence of cases
of Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma
urealyticum and Chlamydia trachomatis in women with no gynecologic
complaints. Reprod Med Biol. 11:201–215. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stary A, Schuh E, Kerschbaumer M, Gotz B
and Lee H: Performance of transcription-mediated amplification and
Ligase chain reaction assays for detection of chlamydial infection
in urogenital samples obtained by invasive and noninvasive methods.
J Clin Microbiol. 36:2666–2670. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cordova CM and Cunha RA: Relevant
prevalence of Mycoplasma hominis and Ureaplasma urealyticum
serogroups in HIV-1 infected men without urethritis symptoms. Rev
Inst Med Trop Sao Paulo. 42:185–188. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Foschi C, Salvo M, Galli S, Moroni A,
Cevenini R and Marangoni A: Prevalence and antimicrobial resistance
of genital Mollicutes in Italy over a two-year period. New
Microbiol. 41:153–158. 2018.PubMed/NCBI
|
|
14
|
Moridi K, Hemmaty M, Azimian A, Fallah MH,
Khaneghahi Abyaneh H and Ghazvini K: Epidemiology of genital
infections caused by Mycoplasma hominis, M. genitalium and
Ureaplasma urealyticum in Iran; a systematic review and
meta-analysis study (2000-2019). BMC Public Health.
20(1020)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moi H, Blee K and Horner PJ: Management of
non-gonococcal urethritis. BMC Infect Dis. 15(294)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park JJ, Seo YB, Jeong S and Lee J:
Prevalence of and risk factors for sexually transmitted infections
among Korean adolescents under probation. J Korean Med Sci.
32:1771–1778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grad AI, Vica ML, Ungureanu L, Siserman
CV, Tătaru AD and Matei HV: Assessment of STI screening in Romania
using a multiplex PCR technique. J Infect Dev Ctries. 14:341–348.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huai P, Li F, Chu T, Liu D, Liu J and
Zhang F: Prevalence of genital Chlamydia trachomatis infection in
the general population: A meta-analysis. BMC Infect Dis.
20(589)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Foronda-García-Hidalgo C, Liébana-Martos
C, Gutiérrez-Soto B, Expósito-Ruiz M, Navarro-Marí JM and
Gutiérrez-Fernández J: Prevalence among males from the general
population of agents responsible of not ulcerative genital tract
infections, assisted in specialized care. Rev Esp Quimioter.
32:545–550. 2019.PubMed/NCBI(In Spanish).
|
|
20
|
Song J, Wu X, Kong Y, Jin H, Yang T, Xie X
and Zhang J: Prevalence and antibiotics resistance of Ureaplasma
species and Mycoplasma hominis in Hangzhou, China, from 2013 to
2019. Front Microbiol. 13(982429)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu X, Li M, Cao H, Yang X and Zhang C:
Epidemiology of Ureaplasma urealyticum and Mycoplasma hominis in
the semen of male outpatients with reproductive disorders. Exp Ther
Med. 12:1165–1170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sarier M, Sepin N, Duman I, Demir M, Hizel
A, Göktaş Ş, Emek M, Kukul E and Soylu A: Microscopy of
Gram-stained urethral smear in the diagnosis of urethritis: Which
threshold value should be selected? Andrologia.
50(e13143)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sarier M, Sepin N, Emek M, Germen AT,
Hoscan MB, Konuk E and Turgut H: Evaluating the utility of the A.F.
Genital System test for pathogen diagnosis in acute male
urethritis. Andrologia. 54(e14377)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sarier M, Demir M, Turgut H, Hizel A, Emek
M, Kukul E and Sepin N: New approach to microscopy of gram-stained
urethral smear: The kissing slide method. Sex Transm Dis.
47:712–715. 2020.PubMed/NCBI View Article : Google Scholar
|