Introduction
H. pylori (H. pylori) is a
gram-negative, microaerophilic bacterium that can survive in the
highly acid environment of the human stomach. Most infected
individuals are asymptomatic; however, for a significant number of
individuals, infection with H. pylori causes the development
of gastritis, gastric-duodenal ulcers, and even cancer. For this
reason, H. pylori is listed as a Class I carcinogen
(1). The first virulence factor of
H. pylori to be identified cagA, is an oncoprotein
encoded by the cagA gene localized on the Cag pathogenicity
island (2). cagA protein is
delivered into host cells by a type-4 secretory system (T4SS) and
then induces cellular alterations that can lead to pathological
changes, via activation of signaling pathways leading to gene
expression (3). The presence of
cagA+ H. pylori is associated with the infiltration
of neutrophils and peripheral mononuclear cells (PBMCs) with the
secretion of pro-inflammatory cytokines such as IL-1b, IL-8, IL-6,
and TNF-a in the gastric mucosa (4). cagA+ H. pylori induces
gastric epithelial cells to secrete IL-8, which attracts
neutrophils and causes mucosal tissue damage (5). Therefore, cagA+ H.
pylori strains are associated with strong inflammatory
responses and severe clinical outcomes (6).
Neutrophils are the most abundant circulating immune
cells. They play a crucial role in innate immune responses through
the secretion of toxic molecules, such as reactive oxygen species
(ROS) to kill invading bacteria. To generate ROS, the nicotinamide
adenine dinucleotide phosphate oxidase (NADPH) oxidase becomes
activated and provides electrons to oxygen (O2) to
generate superoxide (O2-). Then superoxide dismutase
catalyzes O2- to hydrogen peroxide
(H2O2) which is a substrate for
myeloperoxidase to generate hypohalous acids (7). Although ROS is generated as part of
the mechanisms used to kill invading pathogens, if unregulated,
bystander effects of ROS can cause tissue injury including cellular
DNA damage to host tissues (8,9).
Hence, neutrophil functions are usually highly regulated by
serum/plasma factors such as complement proteins and
immunoglobulins (10).
The majority of studies on neutrophil H.
pylori interactions involve experiments using live bacteria and
purified neutrophils (11,12). While these experiments can shed
light on live pathogen: immune cell interactions, they may fail to
give insights into the full range of effects of pathogenicity
factors as the immune cells may not be exposed to intra-bacterial
molecules. In the present study, novel activation of neutrophils by
H. pylori extracts that was only observed when neutrophils
were co-incubated with plasma was identified. When PBMCs were
co-incubated with neutrophils and extracts, this activity was not
seen. It was also shown that extracts containing cagA
generated significantly higher levels of ROS compared to extracts
devoid of this protein. These novel data identify a new and
pathologically important process whereby cagA can stimulate
adverse immune processes that contribute to tissue damage.
Materials and methods
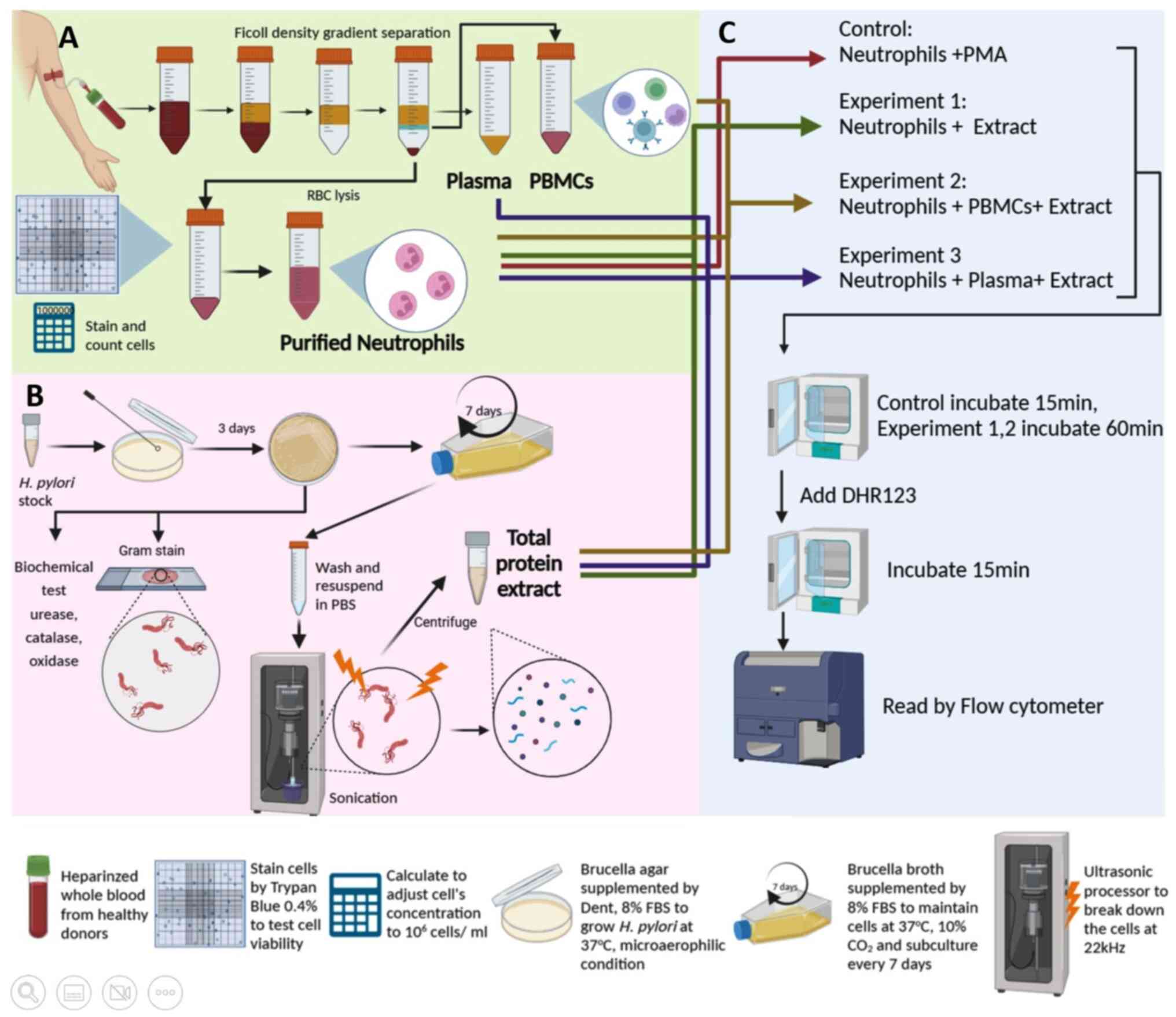
Study workflow
Neutrophils, PBMCs, and plasma were separated from
whole blood using the Ficoll density gradient separation method
(Fig. 1A). Stocks of cagA+
and cagA- H. pylori strains were grown on
Brucella agar plates for 3 days before being expanded into
broth media for 7 days. Bacterial cells were disrupted using an
ultra-sonicator and centrifuged to collect total protein extract
(Fig. 1B). As a positive control
for ROS production, purified neutrophils were activated by PMA.
Experiment 1 was designed to investigate whether H. pylori
extract could stimulate ROS of neutrophils in the absence of
plasma. Experiment 2 was designed to investigate whether
cell-to-cell contact between purified neutrophils and PBMCs could
induce ROS production by extracts in the absence of plasma. In this
experiment, PBMCs were added to purified neutrophils in ratios of
1:1 and 5:1. These mixtures were then co-cultured with extracts
from cagA+ and cagA- H. pylori. Experiment 3
was designed to determine whether H. pylori extract could
trigger neutrophils to produce ROS in the presence of plasma. In
this experiment, neutrophils were mixed with autologous plasma (1:1
v/v) and co-incubated with H. pylori extract at 37˚C for 60
min. All experiments were performed in technical duplicates, n=3
donors.
Participants
Blood was provided by healthy blood donors from the
Blood Bank of Srinagarind University Hospital. The present study
was approved by the Ethics Committee of Khon Kaen University,
Faculty of Medicine, Khon Kaen, Thailand (approval no. HE651442).
The 6 donors were 30-65 years old, with a male: female ratio of
1:1. Patients did not disclose any underlying infections or
inflammatory conditions.
H. pylori strains and extract
preparation
cagA + and cagA- H. pylori
strains were provided by the Tropical Disease Research Center, Khon
Kaen University. CagA+ and CagA- H. pylori
isogenic strains of P12(13) were
generously provided by Professor R. Haas (Max von
Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie,
Ludwig-Maximilians-Universität, München, Germany) and grown on
Brucella solid agar plates containing H. pylori selective
supplement (Dent) (cat. no. SR0147, Oxoid Limited), at 37˚C in
microaerophilic conditions for 7 days. Colonies were then
inoculated into Dent-supplemented Brucella broth-culture flasks and
incubated at 37˚C, 10% CO2.
From broth media, bacterial cells were pelleted and
washed twice by centrifugation at 800 x g, 18˚C, for 5 min, then
re-suspended in 1 ml PBS. The bacterial cells were fragmented by an
ultrasonic processor at 22 kHz for 3 min on ice. The sonicated
suspension was centrifuged at 7,000 x g, 4˚C, for 10 min. The
supernatant containing total protein extract was removed and the
protein concentration was measured using a NanoDrop®
ND-1000 UV-Vis Spectrophotometer (Thermo Fisher Scientific, Inc.;
OD A280-A310). The extracts were stored at
-20˚C until required.
Neutrophil isolation
Neutrophils were isolated from heparinized whole
blood using the density gradient separation method. HetaSep™ (cat.
no. 7906; Stem Cell Technologies, Inc.) was added to whole blood at
a ratio of 1:5. The mixture was incubated at 37˚C for 30 min until
the RBC interface was 50% of the total volume. The leucocyte-rich
plasma was removed and overlaid gently onto Ficoll-Hypaque (cat.
no. 17144002; Cytiva), at a ratio of 1:1, and centrifuged at 500 x
g for 30 min at 25˚C. The upper layer (containing platelets and
plasma) and the second interface (containing PBMCs) were collected
and retained, while the Ficoll suspension above the cell pellet was
discarded. To the pellet, 1 ml RPMI 1640 (cat. no. SH303555.02;
Cytiva) was added, and gently re-suspended before adding 9 ml
ammonium chloride lysis buffer (13.4 mM KHCO3, 155 mM
NH4Cl, 96.7 µM EDTA) and then incubated for 3 min at
25˚C to disrupt the red blood cells. This mixture was centrifuged
at 500 x g, 25˚C for 3 min. The supernatant was discarded, and
neutrophils were resuspended in 2 ml RPMI 1640. An aliquot of the
purified neutrophils was stained with Trypan Blue (0.4%, w/v) for 1
min at 25˚C (cat. no. 15250061, Thermo Fisher Scientific, Inc.) and
counted on a hemocytometer slide before adjusting the neutrophil
concentration to 2x106 cells/ml with RPMI 1640 medium.
Purity was determined using Wright's staining and morphological
staining (14) and was routinely
>95% neutrophils.
ROS measurement
A total of 250 µl plasma and/or 250 µl RPMI 1640
were added sequentially to 250 µl purified neutrophils containing
5x105 cells, then co-incubated with 200 µg/ml
cagA+ and cagA- H. pylori extracts for 1 h at
37˚C. A total of 2 µg/ml dihydrodichlorofluorescein (cat. no.
309825, MilliporeSigma), used to detect H2O2,
was added and incubated for a further 15 min at 37˚C. Phorbol
myristate acetate (PMA, final concentration 0.1 µg/ml) (cat. no.
P1585-1MG; MilliporeSigma) was used as a positive control. ROS
production was detected using flow cytometry on a BD FACSCanto™ II
flow cytometer (BD Biosciences). The flow cytometry results were
analyzed using FlowJo™ v10.8 Software (BD Biosciences).
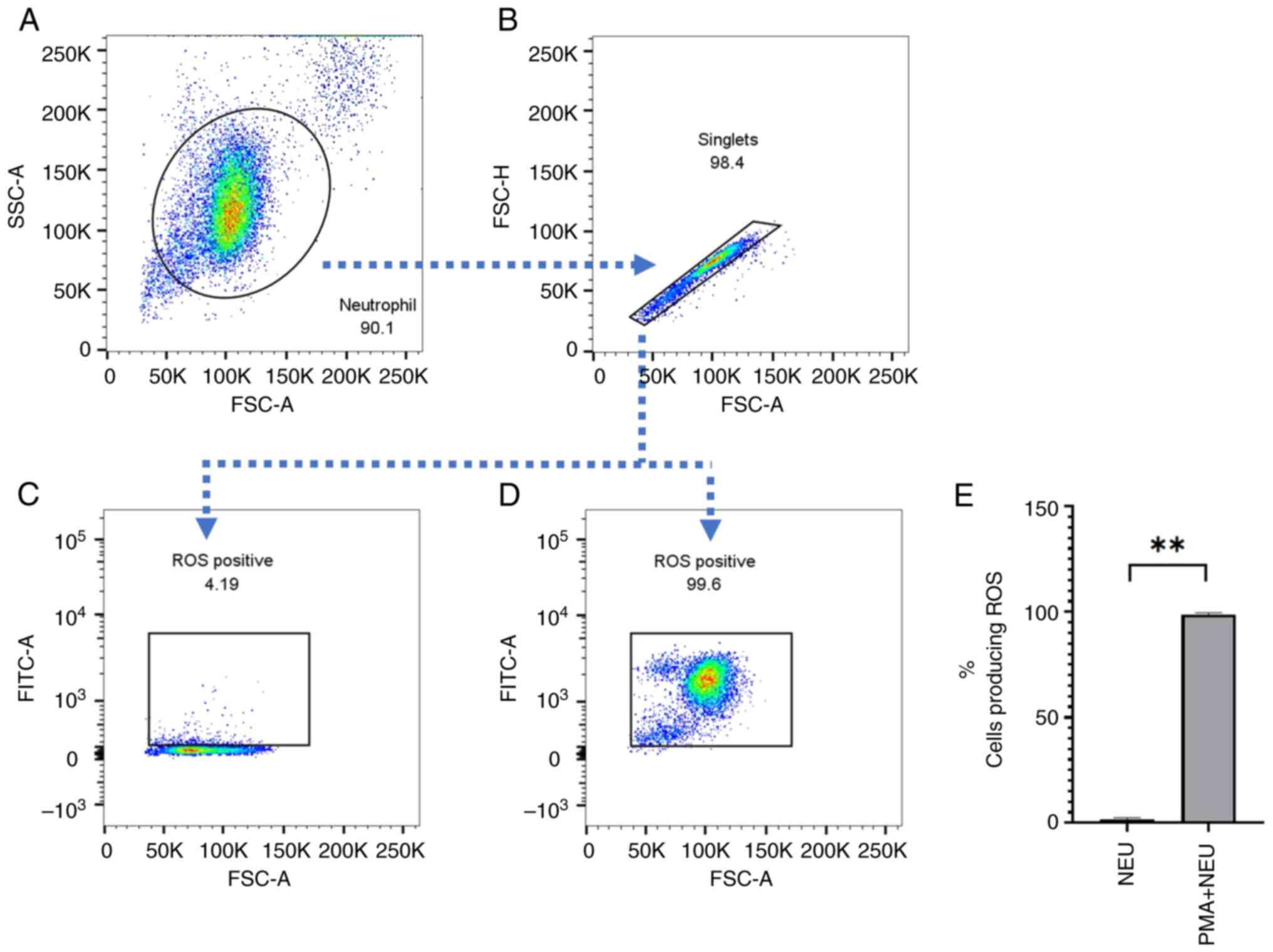
Gating strategy
The gating strategy for ROS detection is shown in
Fig. 2. Neutrophils were gated by
forward and side scatter (Fig. 2A)
and then single neutrophils were analyzed by forward scatter area
and forward scatter high (Fig. 2B).
The cut-off value for a positive signal was identified based on
comparisons of negative control values (Fig. 2C) vs. positive control values with
PMA stimulation (Fig. 2D).
Statistical analysis
All data are presented as the mean ± SEM.
Statistical comparisons were performed using a Mann-Whitney U test
between groups. P<0.05 was considered to indicate a
statistically significant difference. All data were analyzed using
GraphPad Prism version 8.0 (GraphPad Software, Inc.).
Results
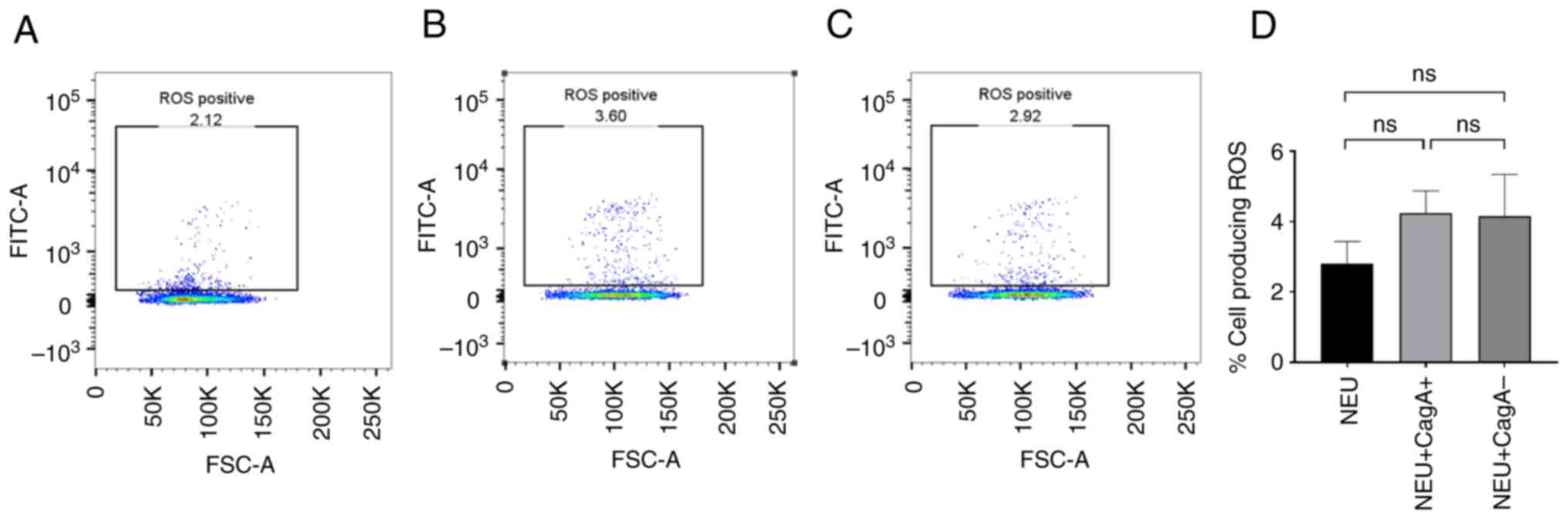
cagA+ and cagA- H. pylori extracts do
not directly trigger ROS production in purified neutrophils
To identify whether H. pylori extracts can
trigger ROS production directly, purified neutrophils were
co-cultured with cagA+ and cagA- H. pylori
extracts. Very few neutrophils produced ROS in the untreated
control samples (<5%, Fig. 3A),
whereas PMA effectively stimulated the majority of the neutrophils
to release ROS. Therefore, the control system was reliable.
Neither cagA+ (P=0.200) nor cagA-
(P=0.3429) H. pylori extract increased the number of
ROS-producing cells (Fig. 3B and
C, respectively) when compared to
the untreated control (Fig. 3D).
Thus, it was concluded that these extracts could not induce ROS
production directly, or otherwise, neutrophils require other
factors to prime them, such as cytokines from PBMCs before
encountering the antigens.
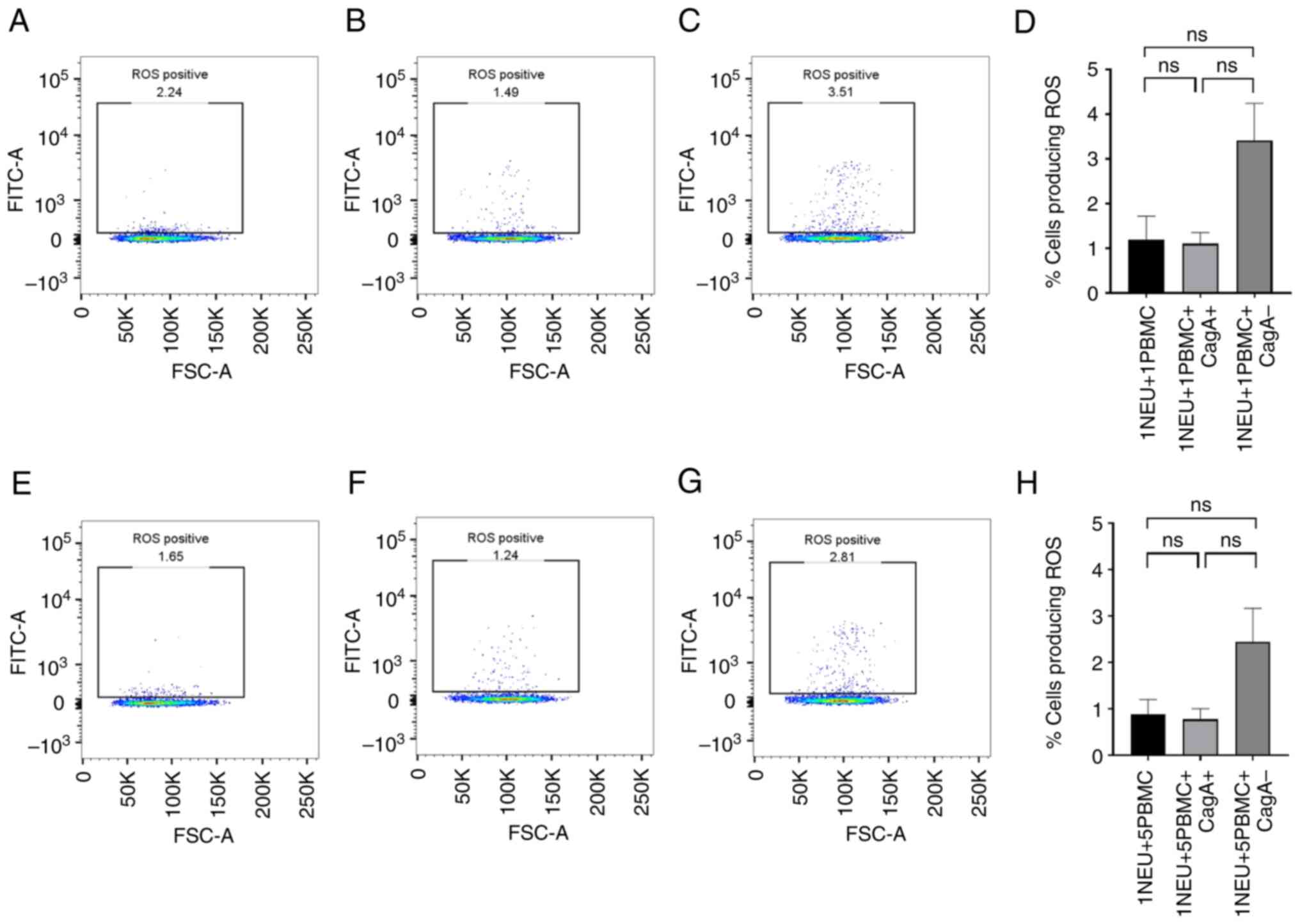
cagA+ and cagA- H. pylori extracts do
not trigger the production of ROS by neutrophils in the presence of
PBMCs
To determine whether cell-to-cell contact with PBMCs
induced neutrophil ROS production, purified PBMCs were added to the
neutrophils before incubation with cagA+ and cagA-
H. pylori extracts, and the ROS levels were measured. There
were no notable levels of ROS detected in the control group (only
neutrophils and PBMCs) (Fig. 4A)
nor in the cagA+ (Fig. 4B)
or in the cagA- H. pylori-treated neutrophils
(Fig. 4C). we increased the ratio
of PBMCs to neutrophil was increased to 5:1, there was still no
measurable ROS production detected (Fig. 4E-G).
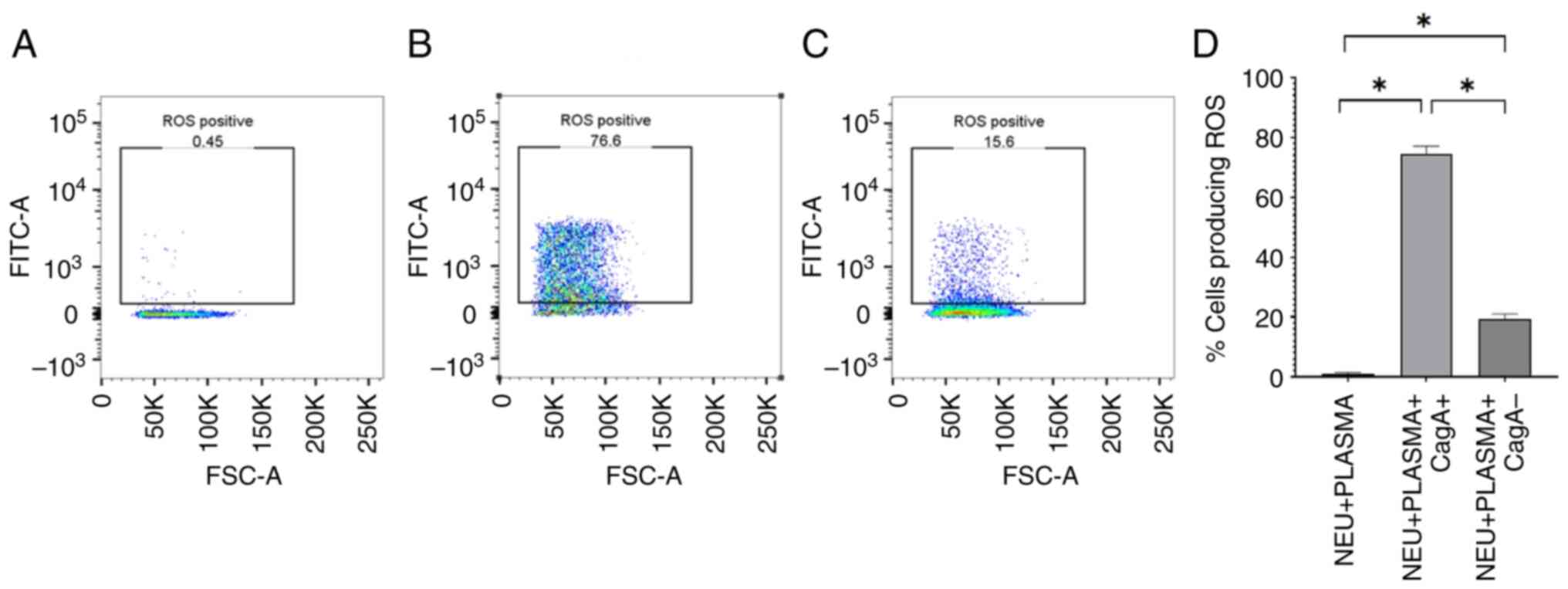
cagA+ and cagA- H. pylori extracts
induce ROS production by neutrophils in the presence of human
plasma
To investigate whether human plasma affects ROS
production by neutrophils in response to cagA+ and
cagA- H. pylori extracts, autologous plasma was used
to pre-treat neutrophils, with or without co-culture with
cagA+ and cagA- H. pylori extracts, and
measured ROS production after 1 h. There was no ROS production in
the negative controls (plasma-treated neutrophils without H.
pylori extracts; Fig. 5A).
However, in the presence of plasma, both cagA+ (Fig. 5B; P=0.0286) and cagA- H.
pylori extracts stimulated neutrophils to produce significantly
more ROS than the negative controls (Fig. 5C; P=0.0286). Additionally,
cagA+ H. pylori extract induced the production
of significantly more ROS by neutrophils than cagA- H.
pylori extract in the presence of plasma (Fig. 5D).
Discussion
This study shows, for the first time, that complex
host-pathogen inflammatory processes regulate the activation of
human neutrophils using H. pylori extracts. It was found
that these extracts could only activate ROS production by human
neutrophils in the presence of plasma, and that this activation
could not be replicated by the addition of PBMCs. It was also shown
that H. pylori extracts containing the cagA protein
generated significantly higher levels of ROS than extracts devoid
of this protein. This is an important observation in view of the
fact that infection with cagA+ H. pylori strains usually
results in more adverse pathological outcomes, such as an increased
risk of gastric cancer, than those that do not express this
pathogenicity factor (15). The
results of the present study indicated that serum factors are
necessary to prime neutrophils to generate ROS after incubation
with these extracts, but also showed that extracellular CagA
activated neutrophils in the presence of these plasma factors.
Several components in plasma may be involved in the
process of ROS activation of neutrophils. Firstly, following
interaction with immune cells, activated platelets can release
chemokines (to attract neutrophils) and proinflammatory cytokines
such as CD40L and IL-1β (16),
which may prime neutrophil functions. Second, immunoglobulins in
plasma may elicit neutrophil ROS production, either alone or via
the formation of immune complexes after interactions with their
cognate antigen. It has been shown that immunoglobulins for
intravenous use, named Gamimune N, Sandoglobuin, and Intraglobin F
can enhance neutrophil respiratory-burst activity in-vitro
(17). Moreover, these
immunoglobulins also promote killing activity towards
multi-drug-resistant bacteria and autophagy of neutrophils in
immunocompromised patients (18).
However, whether the plasma of the volunteers contained
anti-Helicobacter antibodies was not determined, although this is
now being explored in the follow-up studies. Third, complement
proteins such as C3a, C5a, and the surface-bound opsonins, C3b and
C4b, can enhance the ability of neutrophils to phagocytose
opsonized particles, release proinflammatory cytokines, generate
ROS, and form neutrophil extracellular traps (NETs) (19). It is postulated that these
components in plasma can prime neutrophils, and once these primed
neutrophils encounter specific antigens in H. pylori
extracts, they become activated. It is also possible that
components in plasma interact with H. pylori proteins,
subsequently activating neutrophils. Nevertheless, these results
are the first to demonstrate that neutrophil ROS production can be
stimulated by H. pylori extracts even without phagocytosis
of intact live bacteria; however, factors present within plasma are
required for this ROS production to occur.
It is hypothesized that cell-to-cell contact with
PBMCs may facilitate neutrophil ROS production in the presence of
H. pylori extracts. However, this was shown not to be the
case. The results of the present study suggest that cell-to-cell
contact with, or cytokines from, PBMCs do not contribute to
neutrophil ROS production at least after incubation with
cagA+ and cagA- H. pylori extract under the
experimental conditions employed in the present study.
H. pylori has been studied largely given its
role in gastric cancer development and several in vitro
experiments support this property. For example, co-culture of H.
pylori extract with gastric epithelial cell lines leads to
elevated cell proliferation as well as inhibition of cell apoptosis
and autophagy (20). In addition,
H. pylori extract induces mRNA expression of gastric cancer
biomarkers such as chloride channel-3 and slingshot protein
phosphatase 1, which suggests that H. pylori extract may
contribute to gastric cancer progression (20). H. pylori extract also causes
extra-gastric disorders. For example, an animal-model study
indicated that this extract promoted the expression of chemokines
and elevated the levels of TGF-β1/NF-κB-mediated inflammation in a
rat hepatic stellate cell line (21). For these reasons, H. pylori
extract has been used for vaccine development. Flagella are
important for H. pylori motility and colonization, and
flagella-sheath proteins or total protein lysate have been used to
vaccinate mice (22). These
immunized mice were then infected with H. pylori orally and
both forms of vaccination led to an equally significant decrease in
H. pylori burden relative to non-vaccinated controls
(22).
A major finding of the present study was that
cagA+ H. pylori extract induced significantly higher
ROS levels than cagA-extracts, which suggests a novel
immunopathogenic pathway induced by cagA+ H. pylori.
As the two strains that were used in the present study were
isogenic (that is, the cagA-strain was identical to the
wild-type strain except that it was specifically depleted of
cagA), the only difference in protein composition of the two
extracts used was the absence or presence of CagA. Thus, this
pathogenicity factor induced high levels of ROS production in
plasma-treated neutrophils, and it is hypothesized that this
mechanism plays a role in tissue damage associated with this
organism in human diseases.
cagA is normally inserted into host cells via
the type-4 secretory system and once within the cytoplasm, it
becomes phosphorylated and then interacts with and activates
intracellular signaling pathways leading to altered gene expression
and oncogenesis (3). Intact H.
pylori are phagocytosed by human neutrophils and can survive
intracellularly and delay neutrophil apoptosis (23). It is noteworthy that in the present
study, extracellular cagA activated human neutrophils (in
the presence of serum) to generate ROS. Previously, it has been
reported that in addition to their role in gastric diseases,
Helicobacter spp are responsible for a number of
hepatobiliary pathologies including several types of liver cancer
(24-27).
In addition, cagA+ H. pylori is detected at considerably
higher levels in individuals infected with the liver fluke,
Opisthorchis viverrini (compared to uninfected controls) and
at even higher levels in those with advanced periductal fibrosis,
which is an outcome of liver fluke infection (28). O. viverrini is a reservoir
for H. pylori and hence carries this bacterial pathogen to
the bile ducts during liver fluke infection (29). At a 2-year follow-up, >40% of
those initially diagnosed with liver fibrosis were now parasite
free but had persistent or worsening fibrosis (30) and these individuals had
significantly higher levels of cagA + H. pylori
(31).
The present study highlights the importance of
cagA as an important pathogenicity factor that can activate
neutrophils to generate molecules that may damage host tissues, as
ROS may induce oxidative stress, resulting in DNA damage.
Identification of the molecular mechanisms responsible for the
receptor/intracellular signaling processes mediated by cagA
is important in order, not only to define this mechanism, but to
identify ways by which this pathway can be experimentally blocked.
Further work will also include identifying the factor(s) within
plasma that can regulate this process and establish the full range
of neutrophil functions (including secreted proteases and
cytokines/chemokines) that are activated by this novel
mechanism.
In conclusion, total extracts from cagA+ and
cagA- H. pylori had little effect on neutrophil ROS
production in the absence of plasma. However, the addition of
plasma significantly primed human neutrophils to generate ROS in
response to the extracts. cagA+ H. pylori
extract-treated neutrophils produced significantly more ROS than
did neutrophils treated with cagA- H. pylori
extracts. These data show that H. pylori proteins, perhaps
actively secreted by the bacteria or after release from dead
bacteria, can, in the presence of plasma factors, activate ROS by
neutrophils. CagA+ H. pylori extract significantly
induced higher ROS than cagA-extract, which suggests a novel
immunopathogenic pathway of cagA+ H. pylori.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
Faculty of Medicine, Khon Kaen University, Thailand (grant no.
IN66011) and partly supported by a Royal Society International
Collaboration Award (grant no. ICA\R1\201299).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TDT, SWE and KS conceived and designed the study.
TDT, CC, DW, and KS performed the experiments. TDT, KF, BS, SWE and
KS analysed and interpreted the data. TDT and KS wrote the first
draft of the manuscript. TDT, KF, BS, SE and KS edited and
finalized the manuscript. All authors have read and approved the
final manuscript. TDT, SWE and KS confirm the authenticity of all
the raw data.
Ethics approval and consent to
participant
The present study was approved by the Ethics
Committee of Khon Kaen University, Faculty of Medicine (Khon Kaen,
Thailand; approval no. HE651442) and written informed consent was
obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baj J, Forma A, Sitarz M, Portincasa P,
Garruti G, Krasowska D and Maciejewski R: Helicobacter pylori
virulence factors-mechanisms of bacterial pathogenicity in the
gastric microenvironment. Cells. 10(27)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Noto JM and Peek RM Jr: The helicobacter
pylori cag Pathogenicity Island. Methods Mol Biol. 921:41–50.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hatakeyama M: Structure and function of
Helicobacter pylori CagA, the first-identified bacterial protein
involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci.
93:196–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamaoka Y, Kita M, Kodama T, Sawai N and
Imanishi J: Helicobacter pylori cagA gene and expression of
cytokine messenger RNA in gastric mucosa. Gastroenterology.
110:1744–1752. 1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crabtree JE, Farmery SM, Lindley IJ,
Figura N, Peichl P and Tompkins DS: CagA/cytotoxic strains of
Helicobacter pylori and interleukin-8 in gastric epithelial cell
lines. J Clin Pathol. 47:945–950. 1994.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tohidpour A: CagA-mediated pathogenesis of
Helicobacter pylori. Microb Pathog. 93:44–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wright HL, Moots RJ and Edwards SW: The
multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev
Rheumatol. 10:593–601. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salao K, Spofford EM, Price C, Mairiang E,
Suttiprapa S, Wright HL, Sripa B and Edwards SW: Enhanced
neutrophil functions during Opisthorchis viverrini infections and
correlation with advanced periductal fibrosis. Int J Parasitol.
50:145–152. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Han L, Shu X and Wang J: Helicobacter
pylori-mediated oxidative stress and gastric diseases: A Review.
Front Microbiol. 13(811258)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Psychogios N, Hau DD, Peng J, Guo AC,
Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R,
Gautam B, et al: The human serum metabolome. PLoS One.
6(e16957)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Perez-Figueroa E, Torres J, Sanchez-Zauco
N, Contreras-Ramos A, Alvarez-Arellano L and Maldonado-Bernal C:
Activation of NLRP3 inflammasome in human neutrophils by
Helicobacter pylori infection. Innate Immun. 22:103–112.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Faass L, Hauke M, Stein SC and Josenhans
C: Innate immune activation and modulatory factors of Helicobacter
pylori towards phagocytic and nonphagocytic cells. Curr Opin
Immunol. 82(102301)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pham KT, Weiss E, Jimenez Soto LF,
Breithaupt U, Haas R and Fischer W: CagI is an essential component
of the Helicobacter pylori Cag type IV secretion system and forms a
complex with CagL. PLoS One. 7(e35341)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Almaraz-Arreortua A, Sosa-Luis SA,
Rios-Rios WJ, Romero-Tlalolini MLÁ, Aguilar-Ruiz SR,
Baltiérrez-Hoyos R and Torres Aguilar H: Morphological and
compositional analysis of neutrophil extracellular traps induced by
microbial and chemical stimuli. J Vis Exp. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Parsonnet J, Friedman GD, Orentreich N and
Vogelman H: Risk for gastric cancer in people with CagA positive or
CagA negative Helicobacter pylori infection. Gut. 40:297–301.
1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Seyoum M, Enawgaw B and Melku M: Human
blood platelets and viruses: Defense mechanism and role in the
removal of viral pathogens. Thromb J. 16(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lawton JW, Robinson JP and Till GO: The
effect of intravenous immunoglobulin on the in vitro function of
human neutrophils. Immunopharmacology. 18:97–105. 1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsuo H, Itoh H, Kitamura N, Kamikubo Y,
Higuchi T, Shiga S, Ichiyama S, Kondo T, Takaori-Kondo A and Adachi
S: Intravenous immunoglobulin enhances the killing activity and
autophagy of neutrophils isolated from immunocompromised patients
against multidrug-resistant bacteria. Biochem Biophys Res Commun.
464:94–99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Halbgebauer R, Schmidt CQ, Karsten CM,
Ignatius A and Huber-Lang M: Janus face of complement-driven
neutrophil activation during sepsis. Semin Immunol. 37:12–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He Y, Wang C, Zhang X, Lu X, Xing J, Lv J,
Guo M, Huo X, Liu X, Lu J, et al: Sustained exposure to
helicobacter pylori lysate inhibits apoptosis and autophagy of
gastric epithelial cells. Front Oncol. 10(581364)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ki MR, Goo MJ, Park JK, Hong IH, Ji AR,
Han SY, You SY, Lee EM, Kim AY, Park SJ, et al: Helicobacter pylori
accelerates hepatic fibrosis by sensitizing transforming growth
factor-β1-induced inflammatory signaling. Lab Invest. 90:1507–1516.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Skene C, Young A, Every A and Sutton P:
Helicobacter pylori flagella: Antigenic profile and protective
immunity. FEMS Immunol Med Microbiol. 50:249–256. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Whitmore LC, Weems MN and Allen LH:
Cutting Edge: Helicobacter pylori Induces nuclear hypersegmentation
and subtype differentiation of human neutrophils in vitro. J
Immunol. 198:1793–1797. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gros B, Gomez Perez A, Pleguezuelo M,
Serrano Ruiz FJ, de la Mata M and Rodriguez-Peralvarez M:
Helicobacter species and hepato-biliary tract malignancies: A
systematic review and meta-analysis. Cancers (Basel).
15(595)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Osaki T, Lin Y, Sasahira N, Ueno M,
Yonezawa H, Hojo F, Okuda M, Matsuyama M, Sasaki T, Kobayashi S, et
al: Prevalence estimates of Helicobacter species infection in
pancreatic and biliary tract cancers. Helicobacter.
27(e12866)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou D, Wang JD, Weng MZ, Zhang Y, Wang
XF, Gong W and Quan ZW: Infections of Helicobacter spp. in the
biliary system are associated with biliary tract cancer: A
meta-analysis. Eur J Gastroenterol Hepatol. 25:447–454.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aviles-Jimenez F, Guitron A, Segura-Lopez
F, Méndez-Tenorio A, Iwai S, Hernández-Guerrero A and Torres J:
Microbiota studies in the bile duct strongly suggest a role for
Helicobacter pylori in extrahepatic cholangiocarcinoma. Clin
Microbiol Infect. 22:178 e11–178 e22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deenonpoe R, Mairiang E, Mairiang P,
Pairojkul C, Chamgramol Y, Rinaldi G, Loukas A, Brindley PJ and
Sripa B: Elevated prevalence of Helicobacter species and virulence
factors in opisthorchiasis and associated hepatobiliary disease.
Sci Rep. 7(42744)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Deenonpoe R, Chomvarin C, Pairojkul C,
Chamgramol Y, Loukas A, Brindley PJ and Sripa B: The carcinogenic
liver fluke Opisthorchis viverrini is a reservoir for species of
Helicobacter. Asian Pac J Cancer Prev. 16:1751–1758.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mairiang E, Laha T, Kaewkes S, Loukas A,
Bethony J, Brindley PJ and Sripa B: Hepatobiliary morbidities
detected by ultrasonography in Opisthorchis viverrini-infected
patients before and after praziquantel treatment: A five-year
follow up study. Acta Trop. 217(105853)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Phung HTT, Deenonpoe R, Suttiprapa S,
Mairiang E, Edwards SW and Sripa B: Persistent advanced periductal
fibrosis is associated with cagA-positive Helicobacter pylori
infection in post-praziquantel treatment of opisthorchiasis.
Helicobacter. 27(e12897)2022.PubMed/NCBI View Article : Google Scholar
|