Introduction
Gastric cancer (GC) has the 5th highest incidence of
all cancers worldwide, and it is the 3rd leading cause of
cancer-related death (1,2). The clarity mechanism leading to the
tumorigenesis and progress of GC is still unknown. There are
certain factors which play an important role in the development of
GC, with Helicobacter pylori (HP) being the main one (3). Contrary to Western countries, numerous
countries in Asia, such as Japan, are more affected by HP (4).
Type 2 diabetes mellitus (T2DM) is also one of the
leading causes of death in the world (5), and the incidence of this disease has
gradually increased over the years. By 2045, the prevalence of
diabetes is expected to be 21.1% in middle-income countries
(6). T2DM can increase the risk of
cardiovascular disease (7,8), and it is positively associated with
the incidence of GC (9,10). A previous prospective study showed
that hyperglycemia was an independent risk factor of GC (11). Although the mechanism remains
unclear, hyperglycemia is more common in patients with T2DM, which
could increase the risk of GC. Therefore, it is apparent that there
is a close relationship between T2DM and GC.
The impact of T2DM on the outcomes of patients with
GC following gastrectomy remains controversial (12,13),
and there is a need to analyze the long-term outcomes of patients
with GC between the T2DM group and the non-T2DM group. Previous
studies which analyzed the relationship between GC and T2DM could
have been affected by selection biases (9,10). The
propensity score matching (PSM) method could reduce selection bias
in terms of covariates, such as sex and age (14,15).
Therefore, the aim of the current study was to
analyze the outcomes of patients with GC in the T2DM group and the
non-T2DM group.
Materials and methods
Search strategy
The PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (https://www.embase.com/landing?status=grey) and
Cochrane Library (https://www.cochranelibrary.com) databases were
searched from inception to March 8, 2022, to identify eligible
studies for inclusion in the present study. The search strategy for
GC was as follows: ‘Stomach tumor’ OR ‘stomach neoplasm’ OR
‘stomach cancer’ OR ‘cancer of the stomach’ OR ‘gastric neoplasm’
OR ‘gastric cancer’. The search strategy for T2DM was as follows:
‘Diabetes’ OR ‘type 2 diabetes’ OR ‘diabetes mellitus’.
The search strategy used complied with the
guidelines of the Preferred Reporting Items for Systematic Reviews
and Meta-Analyses published in 2009(16). The PICO criteria used in the present
study were: i) Patients (P), patients with GC who underwent
gastrectomy; ii) intervention (I), patients with GC and T2DM; iii)
comparison (C), patients with GC but without T2DM; and iv) outcome
(O), outcomes of patients with GC with or without T2DM, such as
complications, OS and DFS.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Studies
that used PSM analysis; ii) studies that provided data suitable for
the evaluation of outcomes, such as total complications, overall
survival (OS), disease-free survival (DFS) and cancer-specific
survival (CSS); iii) studies that provided data allowing
calculation of either the hazard ratio (HR) or the odds ratio (OR)
with 95% confidence interval (CI) (4); and iv) studies that provided the
sample size and other appropriate data, such as sex, age,
complications and surgery information.
The exclusion criteria were: i) Non-reporting of
predefined outcomes for patients with GC in the T2DM group and the
non-T2DM group, or inability to extract the number of outcome
events from the published results; ii) articles that were
considered letters, commentaries, correspondences, editorials and
reviews, including meta-analyses; and iii) retrospective studies
that had no matched control which used PSM to eliminate the effect
of covariates.
Study selection and data
extraction
The databases were independently searched by two
reviewers, and duplicate records were removed. After titles and
abstracts were screened, full texts were evaluated based on the
aforementioned inclusion, exclusion and PICO criteria. Two
reviewers conducted the study selection, and any disagreements were
resolved by group discussion and consensus.
The data were extracted and cross-checked by two
reviewers who extracted the following information from every
eligible study: First author, country, year, time quantum, center,
study design, type of analysis, sample size before and after
matching, baseline information, surgical information, postoperative
total complications and survival information. If the required data
could not be extracted from a study, the original authors of that
study were conducted directly, whenever possible.
Quality assessment
The Newcastle-Ottawa Scale (NOS) scoring system was
used to independently assess the quality of the four studies
selected by the two reviewers (17). The maximum scale was 9 points.
Studies of high quality scored 9 points, studies of medium quality
scored 7-8 points, and studies of low quality scored <7 points
(18).
Data synthesis and statistical
analysis
Review Manager (version 5.4; The Cochrane
Collaboration) was used in the present study. Statistical
heterogeneity was assessed by the I2 value and the
result of the χ2 test. When I2>50%, a
fixed-effects model was used. By contrast, when I2≤50%,
a random-effects model was used and the tau2 value was
calculated.
For dichotomous and continuous variables, OR, mean
difference (MD) and 95% CI were calculated. The pooled HR and the
95% CI of every study were calculated to estimate the survival
outcomes.
Statistical heterogeneity was calculated using the
I2 statistic, which determined the proportion of total
variation across studies that was due to heterogeneity rather than
chance. Forest plots were shown in order by weight for every study.
P<0.05 was considered to indicate a statistically significant
significance. Meanwhile, the Z-score was calculated to measure the
relative position of the sample data of the present study in the
population; therefore, it was determined whether data were
considered an outlier.
Results
Study identification and
eligibility
A total of 1,632 studies were identified after
electronic search, of which 533, 229 and 870 studies were
identified in PubMed, Cochrane and Embase databases, respectively.
A total of 454 studies were regarded as duplicates based on title
search. Based on the selection and PICO criteria, there were 22
studies remaining, which were accessed by full-text scanning, with
four studies being eligible for inclusion in the present study
(13,19-21).
After PSM, a total of 834 patients were included. A flow diagram of
the analysis protocol is shown in Fig.
1.
Characteristics of the included
studies
Three studies originated from China, and one study
originated from Japan. The publication year of the four included
studies ranged from 2020 to 2022. The time quantum was from April
2008 to December 2019. The characteristics and detailed information
of the sample size of the four included studies are shown in
Table I. Although the four studies
were retrospective, single-center studies, PSM analysis was
used.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | Simple size before
matching, n | Simple size after
matching, n | |
|---|
| First author,
year | Country | Time quantum,
year.month | Center | Design | Analysis | DM | Non- DM | DM | Non- DM | NOS score | (Refs.) |
|---|
| Matsui et
al, 2022 | Japan | 2008.4-2018.6 | Single | Retrospective | PSM | 92 | 420 | 72 | 216 | 7 | (19) |
| Sheng et al,
2020 | China |
2008.11-2015.12 | Single | Retrospective | PSM | 84 | 215 | 84 | 84 | 8 | (20) |
| Chen et al,
2020 | China | 2004.4-2015.12 | Single | Retrospective | PSM | 71 | 1621 | 71 | 139 | 7 | (13) |
| Cheng et al,
2022 | China | 2014.1-2019.12 | Single | Retrospective | PSM | 84 | 619 | 84 | 84 | 8 | (21) |
Data adjustment in the included
studies
The summary of the information in the T2DM group and
the non-T2DM group is shown in Table
II. There were no significant differences between the T2DM
group and the non-T2DM group in either of the four studies or the
current study.
 | Table IISummary of information between the DM
group and the non-DM group. |
Table II
Summary of information between the DM
group and the non-DM group.
| | Mean
difference | Heterogeneity |
|---|
|
Characteristics | Studies, n | Patients with
DM/non-DM, n | OR (95% CI) | P-value | I2
(%) | P-value |
|---|
| Sex | | | | | | |
|
Male | 4 | 213/367 | 0.95
(0.70-1.30) | 0.76 | 0 | 0.89 |
|
Female | 4 | 98/156 | 1.05
(0.77-1.44) | 0.76 | 0 | 0.89 |
| Age, years | 3 | 240/384 | 0.47
(-1.12-2.05) | 0.56 | 0 | 0.73 |
| BMI,
kg/m2 | 2 | 156/230 | 0.15
(-0.53-0.83) | 0.66 | 0 | 0.68 |
| Pathological
stage | | | | | | |
|
I-II | 3 | 106/187 | 0.99
(0.70-1.38) | 0.93 | 0 | 0.74 |
|
III-IV | 3 | 134/197 | 1.02
(0.72-1.43) | 0.93 | 0 | 0.74 |
| Surgical
procedure | | | | | | |
|
Subtotal
gastrectomy | 3 | 146/282 | 1.00
(0.71-1.41) | 0.98 | 0 | 0.42 |
|
Total
gastrectomy | 3 | 81/157 | 1.00
(0.71-1.40) | 0.98 | 0 | 0.42 |
|
Chemotherapy | 2 | 76/116 | 0.58
(0.20-1.71) | 0.33 | 0 | 0.06 |
| Surgical
approach | | | | | | |
|
Laparoscopic
surgery | 3 | 159/286 | 0.87
(0.59-1.27) | 0.46 | 0 | 0.84 |
|
Open
surgery | 3 | 68/153 | 1.15
(0.79-1.69) | 0.46 | 0 | 0.84 |
| Reconstruction
methods | | | | | | |
|
Roux Y | 3 | 112/239 | 1.01
(0.71-1.43) | 0.95 | 0 | 0.68 |
|
Others | 3 | 115/200 | 0.99
(0.70-1.40) | 0.95 | 0 | 0.68 |
| Total
complications | 2 | 42/74 | 1.01
(0.64-1.60) | 0.95 | 0 | 0.45 |
Quality of included studies
All four included studies were assessed using the
NOS scoring system, and the results are shown in Table I.
Association between T2DM and
outcomes
Data on total complications were available for two
studies (19,21). After pooling up the data, there were
no significant differences between the T2DM group and the non-T2DM
group (OR, 1.01; 95% CI, 0.64-1.60; P=0.95; Fig. 2A).
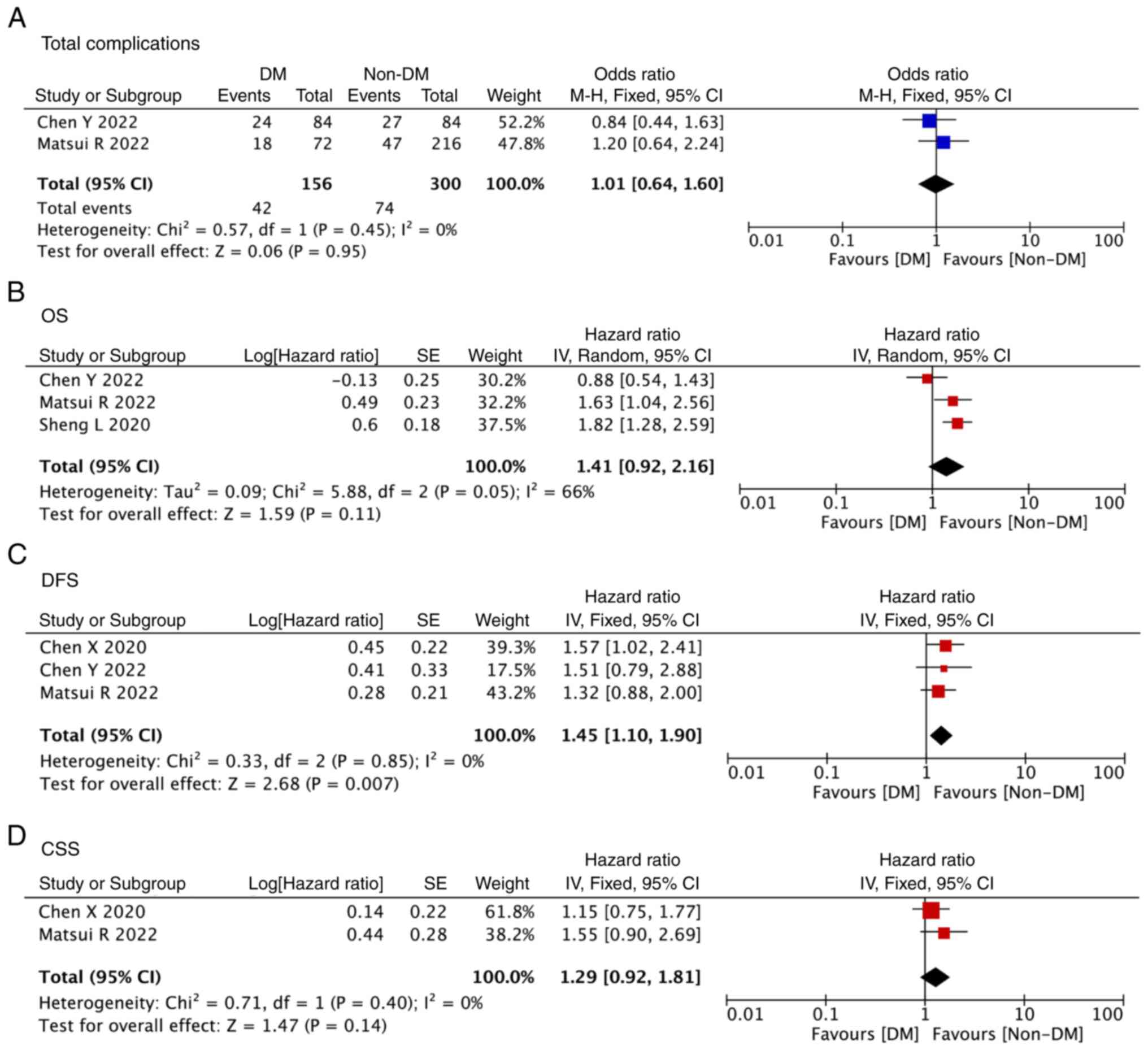 | Figure 2Forest plot showing the outcomes of
patients with GC between the T2DM group and the non-T2DM group. (A)
Total complications, (B) OS, (C) DFS and (D) CSS of patients with
GC between the T2DM group and the non-T2DM group. T2DM, type 2
diabetes mellitus; GC, gastric cancer; OS, overall survival; DFS,
disease-free survival; CSS, cancer-specific survival; CI,
confidence interval; DM, diabetes mellitus; df, degrees of
freedom. |
A total of three studies included data on OS
(19-21),
three studies included data on DFS (13,19,21),
and two studies included data on CSS (13,19).
The DFS in the T2DM group was significantly worse than that in the
non-T2DM group (HR, 1.45; 95% CI, 1.10-1.90; P=0.007; Fig. 2C). However, after pooling up the
data, there were no significant differences between the T2DM group
and the non-T2DM group in terms of OS (HR, 1.41; 95% CI, 0.92-2.16;
P=0.11; Fig. 2B) and CSS (HR, 1.29;
95% CI, 0.92-1.81; P=0.14; Fig.
2D).
Discussion
Research in GC has increased due to the high
incidence and mortality of the disease (1,2). HP
infection has been recognized as an important factor of GC
(3). Only a fraction of individuals
with HP infection developed GC, which showed that HP infection was
not an independent etiologic factor of GC (22). Ikeda et al (11) showed that hyperglycemia might
enhance the ability of HP to cause GC. This finding indicates the
close relationship between T2DM and GC.
Numerous studies investigated the association of
T2DM with GC regarding patient outcomes, and a meta-analysis
discussed the controversial results of those studies (9,12,22).
However, in terms of survival, the meta-analytic study only
discussed the risk of the incidence and mortality of patients with
GC and DM; the association of OS, DFS and CSS was not involved.
Additionally, the included studies of the present meta-analysis did
not uniform the baseline information by PSM (22). Therefore, the current meta-analysis
that included four studies using PSM (13,19-21)
could assist in reducing selection biases (17,18).
Furthermore, more detailed data, including complications, OS, DFS
and CSS were analyzed. A total of 834 patients were included in the
current meta-analysis. There were 311 and 523 patients in the T2DM
group and the non-T2DM group, respectively. The baseline
characteristics of the two groups were adjusted using PSM in all
the four included studies. After pooling up the data, the DFS in
the T2DM group was significantly worse than that in the non-T2DM
group. However, no significant difference was found between the
T2DM group and the non-T2DM group in terms of OS, CSS and total
complications.
The exact link between GC and T2DM is still debated.
Some studies hypothesized that T2DM might influence the prognosis
of patients with GC in terms of OS, DFS and CSS (15,20).
However, other studies reported no significant relationship between
T2DM and GC in terms of prognosis (23,24).
Therefore, the impact of T2DM on the outcomes of patients with GC
after gastrectomy remains controversial (14,17).
It is essential to compare the long-term outcomes of patients with
GC in the T2DM group and the non-T2DM group. Moreover, there were a
few studies discussing the outcomes of patients with GC after
gastrectomy between the T2DM group and the non-T2DM group through
PSM (13,19-21).
The current meta-analysis included four of those studies using PSM
to explore the postoperative outcomes of patients with GC between
the T2DM group and the non-T2DM group (13,19-21).
Zylla et al (25) reported that T2DM was associated with
higher infection and readmission rates. Other studies also showed
that T2DM was associated with increased postoperative
complications, for example, anastomotic leakage and hemorrhage
(12,25,26).
The possible mechanism was as follows: i) Glycolysis is highly
dependent on glucose, and a number of tumour cells rely on
glycolysis for energy supply while in the state of hyperglycemia,
making uncontrolled hyperglycemia beneficial to cancer cell
proliferation (27); and ii)
chronic inflammatory disease, such as T2DM, may result in malignant
tumors (28). However, in the
present study, after pooling up the data, it was shown that there
was no significant difference in complications between the T2DM
group and the non-T2DM group.
Chen et al (13), as well as other studies with
(20) and without PSM (12), reported that T2DM could lead to
worse OS. While more studies with PSM showed that there were no
significant differences in OS (19,21),
in the current study, T2DM was associated with worse OS, but the
difference was not statistically significant. The meta-analysis by
Tian et al (22) showed that
DM lead to higher mortality in patients with GC, which is different
from what was found in the present study. A possible explanation
for the discrepancy could be that the study by Tian et al
(22) did not distinguish T1DM from
T2DM. Evidence suggested that individuals with T1DM had higher risk
of GC than T2DM (29). Chen et
al (13) hypothesized that T2DM
was associated with worse DFS, which was different to the
hypothesis in two other studies (20,21).
The current study showed that T2DM was related to significantly
worse DFS. It seemed there was a contradictory result between OS
and DFS. T2DM has a significant relationship with worse DFS, but OS
was similar in the T2DM group and the non-T2DM group. Therefore, a
larger-data prospective study would enable the investigation of the
exact impact of T2DM on the prognosis of patients with GC.
Regarding CSS, all the studies included in the present study showed
that there was no significant difference between the T2DM group and
the non-T2DM group.
All the four studies included in the current study
originated from Asian countries; three originated from China and
one from Japan. There is probably a connection with the high
incidence of GC in Asian countries, including China, Japan and
Korea (30). Three out of the four
of studies included in the present study showed a significant
correlation between T2DM and prognosis in GC, which was consistent
with previous studies (21,31,32).
By contrast, the incidence of GC in Western countries and the
association between GC and T2DM are poorer. The possible
explanation may be related to the difference in ethnic backgrounds
and dietary habits.
However, some baseline information could not be
analyzed. Because the baseline characteristics of the four studies
were inconsistent. For example, coronary heart disease, chronic
obstructive pulmonary disease, lymph node dissection and
other-cause survival could not be analyzed.
To the best of our knowledge, the current study is
the first meta-analysis that included only PSM studies. The PSM
method can reduce the selection biases such as age and sex, and the
inconformity of baseline information (14,15),
which could increase the credibility of the present
meta-analysis.
The present meta-analysis also has several
limitations. Firstly, although all connected PSM studies had been
selected, the number of studies selected for inclusion was
relatively small. Secondly, all four included studies were
single-center, retrospective studies. Thirdly, none of the studies
described all the aforementioned measures of postoperative
survival. Fourthly, the data on total complications were identified
in only two studies. Fifthly, all the four studies originated from
Asian countries, therefore, results could be ethic origin-specific.
Additional multi-center, prospective, worldwide studies are
needed.
To sum up, patients with GC and T2DM show poor DFS.
However, there were no significant differences between the T2DM
group and the non-T2DM group in terms of OS, CSS and total
complications.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by the Chongqing
Medical Scientific Research Project (Joint Project of Chongqing
Health Commission and Science and Technology Bureau; grant no.
2023QNXM020).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YQ carried out literature search, prepared the
figures, completed the study design, performed data collection,
analysis and interpretation, and wrote the manuscript. DP carried
out literature search, and data collection, analysis and
interpretation. KT completed the study design, carried out data
interpretation and wrote the manuscript. YX wrote and revised the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tao W, Cheng YX, Zou YY, Peng D and Zhang
W: Aorta calcifification increases the risk of anastomotic leakage
after gastrectomy in gastric cancer patients. Cancer Manag Res.
13:3857–3865. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shirani M, Pakzad R, Haddadi MH, Akrami S,
Asadi A, Kazemian H, Moradi M, Kaviar VH, Zomorodi AR, Khoshnood S,
et al: The global prevalence of gastric cancer in Helicobacter
pylori-infected individuals: A systematic review and meta-analysis.
BMC Infect Dis. 23(543)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
An international association between
Helicobacter pylori infection and gastric cancer. The EUROGAST
Study Group. Lancet. 341:1359–1362. 1993.PubMed/NCBI
|
|
5
|
Saeedi P, Salpea P, Karuranga S, Petersohn
I, Malanda B, Gregg EW, Unwin N, Wild SH and Williams R: Mortality
attributable to diabetes in 20-79 years old adults, 2019 estimates:
Results from the International Diabetes Federation Diabetes Atlas,
9th edition. Diabetes Res Clin Pract. 162(108086)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garza-Campos A, Prieto-Correa JR,
Domínguez-Rosales JA and Hernández-Nazará ZH: Implications of
receptor for advanced glycation end products for progression from
obesity to diabetes and from diabetes to cancer. World J Diabetes.
14:977–994. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mazzone T: Intensive glucose lowering and
cardiovascular disease prevention in diabetes: Reconciling the
recent clinical trial data. Circulation. 122:2201–2211.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Emerging Risk Factors Collaboration.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio
E, Ingelsson E, Lawlor DA, Selvin E, et al: Diabetes mellitus,
fasting blood glucose concentration, and risk of vascular disease:
A collaborative metaanalysis of 102 prospective studies. Lancet.
375:2215–2222. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang HJ, Kang D, Chang Y, Ahn J, Ryu S,
Cho J, Guallar E and Sohn CI: Diabetes mellitus is associated with
an increased risk of gastric cancer: A cohort study. Gastric
Cancer. 23:382–390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miao ZF, Xu H, Xu YY, Wang ZN, Zhao TT,
Song YX and Xu HM: Diabetes mellitus and the risk of gastric
cancer: A meta-analysis of cohort studies. Oncotarget.
8:44881–44892. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ikeda F, Doi Y, Yonemoto K, Ninomiya T,
Kubo M, Shikata K, Hata J, Tanizaki Y, Matsumoto T, Iida M and
Kiyohara Y: Hyperglycemia increases risk of gastric cancer posed by
Helicobacter pylori infection: A population-based cohort study.
Gastroenterology. 136:1234–1241. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wei ZW, Li JL, Wu Y, Xia GK, Schwarz RE,
He YL and Zhang CH: Impact of pre-existing type-2 diabetes on
patient outcomes after radical resection for gastric cancer: A
retrospective cohort study. Dig Dis Sci. 59:1017–1024.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen X, Chen Y, Li T, Jun L, Lin T, Hu Y,
Huang H, Chen H, Liu H, Li T, et al: Impact of diabetes on
prognosis of gastric cancer patients performed with gastrectomy.
Chin J Cancer Res. 32:631–644. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rubin DB and Thomas N: Matching using
estimated propensity scores: Relating theory to practice.
Biometrics. 52:249–264. 1996.PubMed/NCBI
|
|
15
|
Rubin DB: The design versus the analysis
of observational studies for causal effects: Parallels with the
design of randomized trials. Stat Med. 26:20–36. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred Reporting Items for Systematic Reviews
and Meta-Analyses: The PRISMA Statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wells GA, O'Connell D and Peterson J: The
Newcastle-Ottawa scale (NOS) for assessing the quality of
nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Accessed Nov 1, 2011.
|
|
18
|
Admiraal WM, Celik F, Gerdes VE, Dallal
RM, Hoekstra JB and Holleman F: Ethnic differences in weight loss
and diabetes remission after bariatric surgery: A meta-analysis.
Diabetes Care. 35:1951–1958. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsui R and Inaki Nand Tsuji T: Impact of
diabetes mellitus on long-term prognosis after gastrectomy for
advanced gastric cancer: A propensity score matching analysis. Surg
Today. 28(1382)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sheng L, Peng H, Pan Y, Wang C and Zhu Y:
Evaluating the effect of diabetes on the prognosis of gastric
cancer using a propensity score matching method. J Gastrointest
Oncol. 11:999–1008. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cheng YX, Tao W, Kang B, Liu XY, Yuan C,
Zhang B and Peng D: Impact of preoperative type 2 diabetes mellitus
on the outcomes of gastric cancer patients following gastrectomy: A
propensity score matching analysis. Front Surg.
9(850265)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tian T, Zhang LQ, Ma XH, Zhou JN and Shen
J: Diabetes mellitus and incidence and mortality of gastric cancer:
A meta-analysis. Exp Clin Endocrinol Diabetes. 120:217–223.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Coughlin SS, Calle EE, Teras LR, Petrelli
J and Thun MJ: Diabetes mellitus as a predictor of cancer mortality
in a large cohort of US adults. Am J Epidemiol. 159:1160–1167.
2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marimuthu SP, Vijayaragavan P, Moysich KB
and Jayaprakash V: Diabetes mellitus and gastric carcinoma: Is
there an association? J Carcinog. 10(30)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zylla D, Gilmore G, Eklund J, Richter S
and Carlson A: Impact of diabetes and hyperglycemia on health care
utilization, infection risk, and survival in patients with cancer
receiving glucocorticoids with chemotherapy. J Diabetes
Complications. 33:335–339. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee KC, Chung KC, Chen HH, Cheng KC, Wu
KL, Song LC and Hu WH: The impact of comorbid diabetes on
short-term postoperative outcomes in stage I/II colon cancer
patients undergoing open colectomy. Biomed Res Int.
2020(2716395)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lega IC and Lipscombe LL: Review:
Diabetes, obesity and cancer-pathophysiology and clinical
implications. Endocr Rev. 41(bnz014)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ohara N, Kobayashi M, Ikeda Y, Hoshi T,
Morita S, Kanefuji T, Yagi K, Suda T, Takada T, Hasegawa G, et al:
Non-insulin-dependent diabetes mellitus induced by immune
checkpoint inhibitor therapy in an insulinoma-associated antigen-2
autoantibody-positive patient with advanced gastric cancer. Intern
Med. 59:551–556. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guo J, Liu C, Pan J and Yang J:
Relationship between diabetes and risk of gastric cancer: A
systematic review and meta-analysis of cohort studies. Diabetes Res
Clin Pract. 187(109866)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh
KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al: Screening
for gastric cancer in Asia: Current evidence and practice. Lancet
Oncol. 9:279–287. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M and
Samet JM: Fasting serum glucose level and cancer risk in Korean men
and women. JAMA. 293:194–202. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koskinen SV, Reunanen AR, Martelin TP and
Valkonen T: Mortality in a large population-based cohort of
patients with drug-treated diabetes mellitus. Am J Public Health.
88:765–770. 1998.PubMed/NCBI View Article : Google Scholar
|
















