Introduction
Anti-N-methyl-D-aspartate receptor (anti-NMDAR)
encephalitis, the most common type of autoimmune encephalitis, was
first described by Vitaliani et al (1) in 2005, and related autoantigens were
first reported in 2007(2).
Anti-NMDAR encephalitis is attributed to a disrupted autoimmune
mechanism and the relevant autoantibodies mainly target the NR1
subunit of NMDARs on the neuronal surface or synaptic protein
(2). Patients with anti-NMDAR
encephalitis often present with psychiatric/behavioral
abnormalities, cognitive impairment, seizures and movement
disorders, and may have a favorable prognosis with early and
comprehensive treatment, especially immunotherapy (3,4). A
previous study has demonstrated that the cognitive recovery process
in patients with anti-NMDAR encephalitis is time-dependent and
improves gradually after initial treatment (5). Immunotherapy is the most important
treatment for anti-NMDAR encephalitis, and various clinical
symptoms are examined to evaluate the degree of cognitive
impairment, including alertness, which is expected to improve
gradually after treatment (6-8).
However, the majority of patients still suffer from long-term
deficits, particularly cognitive abnormalities such as memory
deficit (9). To better understand
the potential pathogenetic mechanism of anti-NMDAR encephalitis,
multimodal imaging investigations have been increasingly conducted
to explore the influence of functional and structural abnormalities
on neuropsychological disorders (10).
Reversible damage to neurons can be attributed to
the elimination of anti-NMDAR antibodies, which can be observed on
a small scale. However, cerebral functional and structural
abnormalities involving neural functional activity, cortical and
subcortical volumes, white matter and cerebral blood perfusion have
also been reported on a larger scale in previous studies. For
instance, Peer et al (11)
found that in patients with anti-NMDAR encephalitis, functional
connectivity (FC) was disrupted between and within subnetworks,
including the default mode network (DMN), medial-temporal lobe
network, sensorimotor network and visual network. Furthermore,
connections between brain regions were also disrupted and mostly
located in the frontal lobe, medial temporal lobe and inferior
parietal lobe on the large-scale network level. Using surface-based
morphometric analyses, decreased cortical and subcortical volumes
have been found in the DMN, language network and left cornu ammonis
1 region of the hippocampus, and these changes were suggested to
contribute to different aspects of cognitive impairment (12). Analysis of white matter has revealed
a widespread reduction in fractional anisotropy across the entire
white matter skeleton, which was prominently located in the
bilateral cingulum, right middle temporal gyrus and left middle
cerebellar peduncle (13,14). Widespread superficial white matter
impairments have been observed in patients with anti-NMDAR
encephalitis (15). An abnormal
glucose metabolic pattern has also been identified in patients with
anti-NMDAR encephalitis, which dynamically changes between
hypermetabolism and hypometabolism, based on the recovery mode,
disease course and clinical features (16-18).
The studies mentioned above may contribute to elucidating the
pathophysiology of anti-NMDAR encephalitis and providing promising
diagnostic methods for treating this disease from different
perspectives, including functional activity, brain structure,
neuropsychological deficits and disease course. Although a certain
understanding of the cerebral damage in patients with anti-NMDAR
encephalitis from the perspective of multimodal imaging exists,
relevant reports are scarce and are insufficient for evaluating
other neurological comorbidity, such as seizure, depression and
psychiatric symptoms. Furthermore, little attention has been given
to the functional hubs and related networks contributing to
cognitive impairment in patients with anti-NMDAR encephalitis based
on the previous studies (12-14).
The brain is a complex system in which certain
regions perform primary functions, and other adjacent or even
distant brain regions need to closely cooperate to form neural
networks and complete different tasks together. In a previous study
by our group (19), voxel-mirrored
homotopic connectivity was used to estimate the resting-state FC
between a voxel within one hemisphere and its mirrored counterpart
within the opposing hemisphere, which was found to serve an
important role in the diagnosis of encephalitis. To date, a graph
theory-based approach has been used to explore the changes in the
functional and structural neural networks in patients with
anti-NMDAR encephalitis. By combining multimodal MRI data and
graph-based network approaches, various network parameters,
including global network metrics, nodal metrics and connections
between different brain regions, have been shown to be altered in
patients with anti-NMDAR encephalitis (13-15).
Furthermore, in graph theory-based analysis, degree centrality (DC)
is the most reliable property for investigating abnormalities in
the FC matrix at the large-scale level, when the regions of
interest (ROIs) are not initially defined (20,21).
DC can be used to evaluate the importance of each node in the brain
network by determining its direct association with the remaining
nodes in the whole brain at the global network level. Therefore, DC
is a measure of the hub distribution that reflects information
processing and communication abilities throughout functional brain
networks (22,23). By defining hubs as ROIs in
subsequent analyses using a traditional FC approach, disrupted
networks between the hub and other brain regions can be further
displayed at the global level to reveal pathogenetic mechanisms
from different perspectives (24).
However, to the best of our knowledge, this combination analysis
has not been applied to patients with anti-NMDAR encephalitis. The
present study aimed to combine DC and seed-based FC to explore
abnormal cerebral functional activity in patients with anti-NMDAR
encephalitis in a comprehensive manner.
In the present study, abnormal functional activity
at the local and global cerebral levels in patients with anti-NMDAR
encephalitis was explored, and its effects on neuropsychological
impairments were examined. The DC method was first used to compare
the distribution of abnormal functional hubs at the local level
between 26 healthy controls (HCs) and 29 patients with anti-NMDAR
encephalitis based on resting-state functional MRI (rs-fMRI).
Subsequently, brain regions with significant DC differences between
groups were defined as ROIs for subsequent FC analysis to
investigate the potential disrupted hub network in the entire
brain. Furthermore, correlation analysis was performed to reveal
the influence of the aberrant functional activity and related
networks of cerebral hubs on cognitive impairment in patients with
anti-NMDAR encephalitis. Finally, multivariate pattern analysis
(MVPA) was performed to explore neuroimaging characteristics based
on the rs-fMRI data of the patients. The present study could be
conducive to revealing the functional hub distributions and
abnormalities in patients with anti-NMDAR encephalitis, and to
further elucidate the pathological mechanisms of clinical deficits
in these patients.
Subjects and methods
Subjects
The present study was approved by the Medical
Research Ethics Committee of The First Affiliated Hospital of
Guangxi Medical University (approval no. 2015-KY-National Fund-064;
Nanning, China). All participants provided detailed written
informed consent for the entire study. A total of 29 patients (age
range, 18-65 years) with anti-NMDAR encephalitis after 3 months
after their first diagnosis were recruited at the Department of
Neurology, The First Affiliated Hospital of Guangxi Medical
University (Nanning, China), between January 2019 and December
2020. All patients met the diagnostic standard for anti-NMDAR
encephalitis as follows: i) Typical clinical features, such as
psychological and behavioral abnormalities, cognitive deficits,
seizures, disturbance of consciousness, and autonomic dysfunction,
were observed rapidly <3 months from onset; and ii) anti-NMDAR
IgG antibodies were detected to be present in the cerebrospinal
fluid of the patients (25).
Patients who suffered from other central nervous system disorders,
such as intracranial infection, metabolic diseases or brain tumors,
were excluded. A total of 26 HCs matched for age (age range, 18-65
years), sex and education level were enrolled. The HCs had no
neuropsychological disease. All participants were right-handed and
completed the entire experimental process. The Montreal Cognitive
Assessment (MoCA) test was used to evaluate cognitive function
(26), and the Hamilton Anxiety
Scale (HAMA) and Hamilton Depression Scale (HAMD24) were used to
assess anxiety and depression, respectively (27). Furthermore, the participants
underwent attention network tests to assess alertness (28).
Rs-fMRI data acquisition
Rs-fMRI scans were performed at The First Affiliated
Hospital of Guangxi Medical University. An Achieva 3T MRI scanner
(Philips Medical Systems Nederland B.V.) was used for the
acquisition of MRI data. The parameters of the gradient-echo planar
image sequence were as follows: Repetition time, 2,000 msec; echo
time; 30 msec, flip angle, 90˚; field of view, 220x220 mm; voxel
size, 3.44x3.44x3.50 mm; matrix size, 64x64; slice number, 41;
slice gap, 0.5 mm; and volume number, 225 slices. During the
scanning process, all participants were kept in a quiet and relaxed
state, without any particular thoughts, keeping their eyes closed
and staying awake. Spongy pads provided stability for the head, and
wearing headphones helped to minimize the impact of noise. The
entire scanning process lasted ~8 min.
Rs-fMRI data preprocessing
Rs-fMRI data were preprocessed using Data Processing
& Analysis of Brain Imaging version 5.0 (DPABI 5.0, http://rfmri.org/dpabi) and Statistical Parametric
Mapping 12 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12).
The detailed procedure was as follows: Conversion to the
Neuroimaging Informatics Technology Initiative format; removal of
the first 10 volumes; slice timing correction; head movement
correction (displacement <2 mm or angular rotation <2 in all
directions); normalization to the Montreal Neurological Institute
template (29); resampling to 3x3x3
mm3 resolution; temporal bandpass filtering between 0.01
and 0.08 Hz; spatial smoothing with a 6-mm full-width at
half-maximum Gaussian kernel and nuisance signal regression,
including 24 head motion parameters; and mean white matter signals
and cerebrospinal fluid signals.
Functional activity analysis
After preprocessing, the rs-fMRI data were subjected
to DC and FC analyses using DPABI software. For the DC analysis,
the voxel-based functional Pearson correlation coefficients of all
pairs of brain voxels were first calculated to obtain the ‘n x n’
matrix depicting the FC pattern across the entire brain.
Subsequently, functional correlation coefficients were subjected to
Fisher's z-score transformation. An undirected adjacency matrix was
obtained by setting the suprathreshold correlation value at 0.25 to
eliminate possible spurious connectivity at the individual level.
Furthermore, DC was subsequently calculated by counting the number
of remaining functional correlations at the individual level. The
resulting data were spatially smoothed with a Gaussian kernel of
6x6x6 mm3 full width at half-maximum. Finally, DC z-maps
from each group were compared using a two independent-samples
t-test (Gaussian random field correction; voxel-level P<0.001;
cluster-level P<0.05).
FC analysis was performed based on the ROIs. The
brain regions with significant differences in DC between groups
were selected as ROIs for subsequent FC analyses. The average time
series from each ROI was extracted, and correlation analyses were
subsequently performed with the remaining voxels in the entire
brain. Subsequently, Fisher's-to-z transformation was conducted for
a z-score FC map for each participant. The z-score FC maps in each
group were subjected to a two independent-samples t-test (false
discovery rate correction, P<0.01) for between-group
comparisons. Age, sex and education level served as nuisance
covariates in the group comparisons. T-values were used to
represent the strength of functional activity of the cerebral
regions with significant differences between groups.
MVPA
MVPA has gained increasing attention for analysis of
specific characteristics of brain signals in MRI data (30,31).
MVPA was applied to the DC signals of each participant in the two
groups using PRONTO software 2.0 version on the MATLAB2018 platform
(https://ww2.mathworks.cn) (32). A binary support vector machine was
employed to construct the anti-NMDAR encephalitis and HC group
classification model. A permutation test was performed 1,000 times
to assess the statistical significance of the differences in brain
region voxels. Ultimately, the classification plot, receiver
operating characteristic curve and weight map for the different
brain regions were used to present the diagnostic value of the DC
maps in differentiating the patient and HC groups.
Statistical analysis
SPSS 16.0 (SPSS, Inc.) software was used for
statistical analyses. The data are presented as the mean ± standard
deviation or numbers. Two independent-samples t-tests were used for
group comparisons of age, education level, alerting effect, MoCA,
HAMA and HAMD24 scores. The χ2 test was used for sex
comparisons. The functional activity strengths of the significant
brain regions in the FC and DC analyses were extracted to analyze
their Pearson correlation with clinical features. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic information and clinical
characteristics
A total of 29 patients with anti-NMDAR encephalitis
(13 men and 16 women) and 26 sex-, age- and education-matched HCs
(11 men and 15 women) were recruited. The patients participated in
the study after 3 months after their first diagnosis. There was no
difference in alertness between the patients with anti-NMDAR
encephalitis and HCs. However, although the scores were within the
normal range, the HAMA and HMAD24 scores of anti-NMDAR patients
were significantly higher than those of the normal control group
(4.10±3.27 vs. 0.11±0.43, P<0.001; 5.69±5.66 vs. 0.69±1.05,
P<0.001). As expected, MOCA scores were lower in NMDAR-resistant
patients than in normal controls (23.93±4.14 vs. 28.54±1.70,
P<0.001). The patient group (11/29) showed abnormal T2 or
fluid-attenuated inversion recovery signals on conventional brain
MRI. These signals were mainly located unilaterally or bilaterally
in the frontal lobe, temporal lobe and limbic system, and even in
the deep brain nuclei and meninges. Typical clinical manifestations
in the patient group included at least one of the following
syndromes: Acute neuropsychiatric symptoms, cognitive deficits,
memory impairments and seizures. The detailed clinical
characteristics of all participants are presented in Table I.
 | Table IClinical information of all
participants. |
Table I
Clinical information of all
participants.
| Characteristic | Patients with
anti-NMDAR encephalitis | HCs | P-value |
|---|
| Age, years | 26.90±8.60 | 27.50±5.36 | 0.759 |
| Sex, n
(male/female) | 13/16 | 11/15 | 0.851 |
| Education,
years | 12.55±3.05 | 13.58±2.87 | 0.207 |
| Disease duration,
years | 1.33±1.14 | - | - |
| MoCA, score | 23.93±4.14 | 28.54±1.70 | <0.001 |
| HAMD24, score | 5.69±5.66 | 0.69±1.05 | <0.001 |
| HAMA, score | 4.10±3.27 | 0.11±0.43 | <0.001 |
| Alerting effect RT,
msec | 52.47±14.35 | 54.10±14.97 | 0.683 |
| Abnormalities in
conventional MRI, n | 11 | 0 | <0.001 |
| Neuropsychiatric
symptoms, n | 23 | 0 | <0.001 |
| Cognitive deficit,
n | 9 | 0 | <0.001 |
| Memory disorder,
n | 6 | 0 | <0.001 |
| Seizure, n | 23 | 0 | <0.001 |
DC analysis
The differences between the patient group and the HC
group according to the results of the DC analysis are shown in
Fig. 1 and Table II presents the value of the
different brain regions in DC. As indicated in Fig. 1 and Table II, compared with the HCs, the
patients with anti-NMDAR encephalitis exhibited increased DC
strength in the anterior lobe of the cerebellum. Furthermore, the
patient group also exhibited decreased DC strength in the left
rectus gyrus (LRG), left caudate nucleus (LCN) and bilateral
superior medial frontal gyrus (BSMFG).
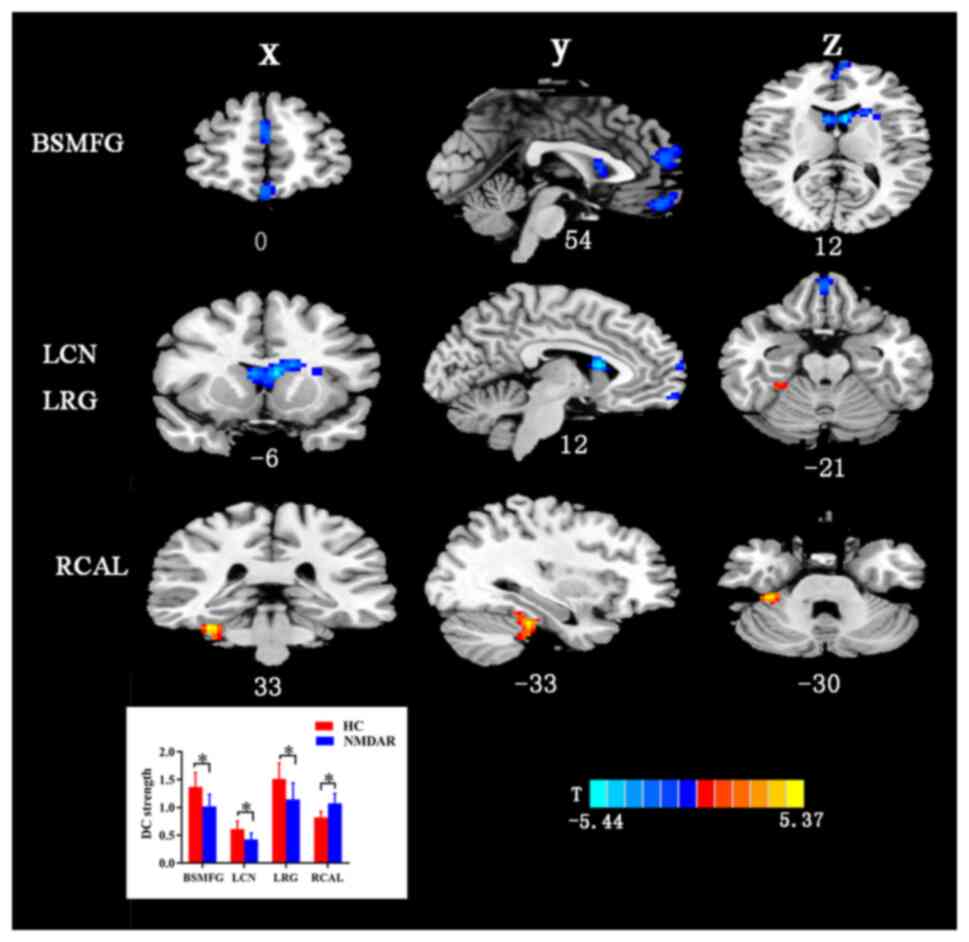 | Figure 1Alterations in DC strength in
patients with anti-NMDAR encephalitis. The altered brain regions
were located in the BSMFG, LCN, LRG and RCAL. Gaussian random field
correction was set at voxel-level P<0.001 and cluster-level
P<0.05. The color bar with T-values indicates the strength of
functional activity (a warm color represents increased functional
strength, while a cool color represents decreased functional
strength). *P<0.05. X, coronal plane; Y, sagittal
plane; Z, transverse plane; numbers, coordinates. BSMFG, bilateral
superior medial frontal gyrus; LCN, left caudate nucleus; LRG, left
rectus gyrus; RCAL, right cerebellum anterior lobe; DC, degree
centrality; NMDAR, N-methyl-D-aspartate receptor; HC, healthy
control. |
 | Table IIBrain regions with significant
differences between patients with anti-N-methyl-D-aspartate
receptor encephalitis and HCs according to degree centrality
analysis. |
Table II
Brain regions with significant
differences between patients with anti-N-methyl-D-aspartate
receptor encephalitis and HCs according to degree centrality
analysis.
| Brain region | FS in HCs | FS in patients | Cluster size
(voxel) | MNI coordination
(x, y, z) | T-value |
|---|
| Cerebellar anterior
lobe_R | 0.8204±0.1063 | 1.0768±0.1729 | 75 | 33, -33, -30 | 5.7453 |
| Rectal gyrus_L | 1.5119±0.2853 | 1.1465±0.2899 | 55 | 0, 51, -21 | -4.5669 |
| Caudate
nucleus_L | 0.6117±0.1438 | 0.4246±0.1145 | 171 | -6, 12, 12 | -5.8239 |
| Bilateral superior
medial frontal gyrus | 1.3678±0.2600 | 1.0220±0.2121 | 104 | 0, 54, 12 | -4.5116 |
FC analysis
The AAL template has a total of 116 regions, but
only 90 belong to the brain, and the remaining 26 belong to the
cerebellar structure, which is less studied. In the present study,
116 brain regions were compared between the two groups. Brain
regions exhibiting statistically significant differences in DC
density were selected as ROIs for subsequent seed-based FC
analyses. Compared with the HCs, the patients with anti-NMDAR
encephalitis showed decreased FC strength between the LCN and the
left precuneus and bilateral middle frontal gyrus (Fig. 2 and Table III). Table III presents the value of the
different brain regions in FC strength corresponding to Fig. 2. The other brain ROIs of Anatomical
Automatic Labeling exhibited no differences in functional maps at
the whole-brain level between the patient and HC groups.
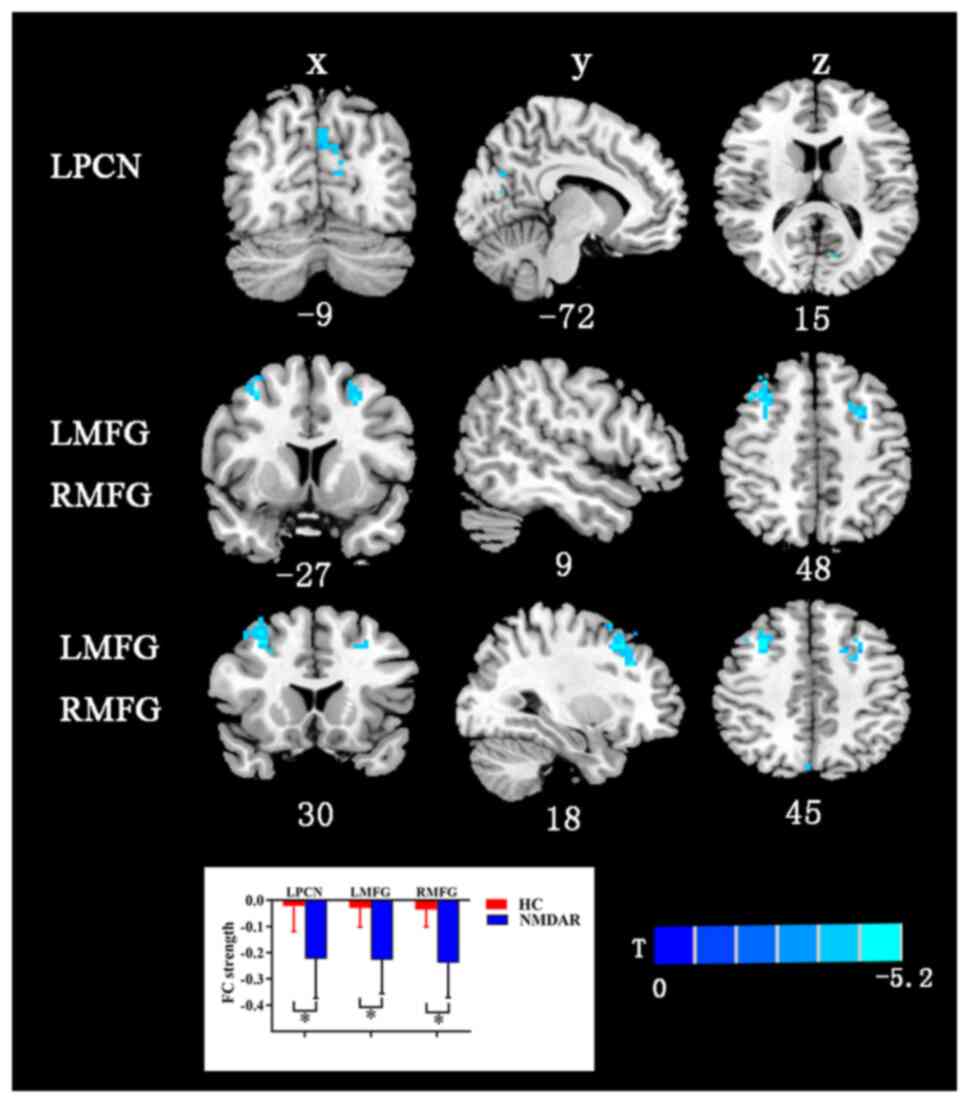 | Figure 2Differences in FC between patients
with anti-NMDAR encephalitis and HCs. The regions with significant
degree centrality values were selected as seeds for FC analysis.
The FC between left caudate nucleus with LMFG, RMFG and RPCN was
decreased. False discovery rate correction was performed at
P<0.01. The color bar with T-values indicates the functional
strength (a warm color indicates increased functional strength,
while a cool color indicates decreased functional strength).
*P<0.05. X, coronal plane; Y, sagittal plane; Z,
transverse plane; numbers, coordinates. LPCN, left precuneus; LMFG,
left middle frontal gyrus; RMFG, right middle frontal gyrus; FC,
functional connectivity; NMDAR, N-methyl-D-aspartate receptor; HC,
healthy control. |
 | Table IIIBrain regions showing functional
connectivity alterations in patients with anti-N-methyl-D-aspartate
receptor encephalitis compared with HCs. |
Table III
Brain regions showing functional
connectivity alterations in patients with anti-N-methyl-D-aspartate
receptor encephalitis compared with HCs.
| Brain region | FS in HCs | FS in patients | MNI coordination
(x, y, z) | Cluster size
(voxel) | T-value |
|---|
| Left precuneus | -0.0218±0.0973 | -0.2229±0.1506 | -9, -72, 15 | 55 | -5.1393 |
| Right middle
frontal gyrus | -0.0356±0.0654 | -0.2389±0.1318 | 30, 18, 45 | 123 | -6.3482 |
| Left middle frontal
gyrus | -0.0290±0.0726 | -0.2272±0.1287 | -27, 9, 48 | 45 | -5.774 |
Correlations between clinical features
and functional activity
Correlations between clinical features (disease
duration, alerting effect, MoCA, HAMA and HAMD24 scores) and
functional activity in the brain regions exhibiting significant
differences in the DC and FC analyses were further analyzed
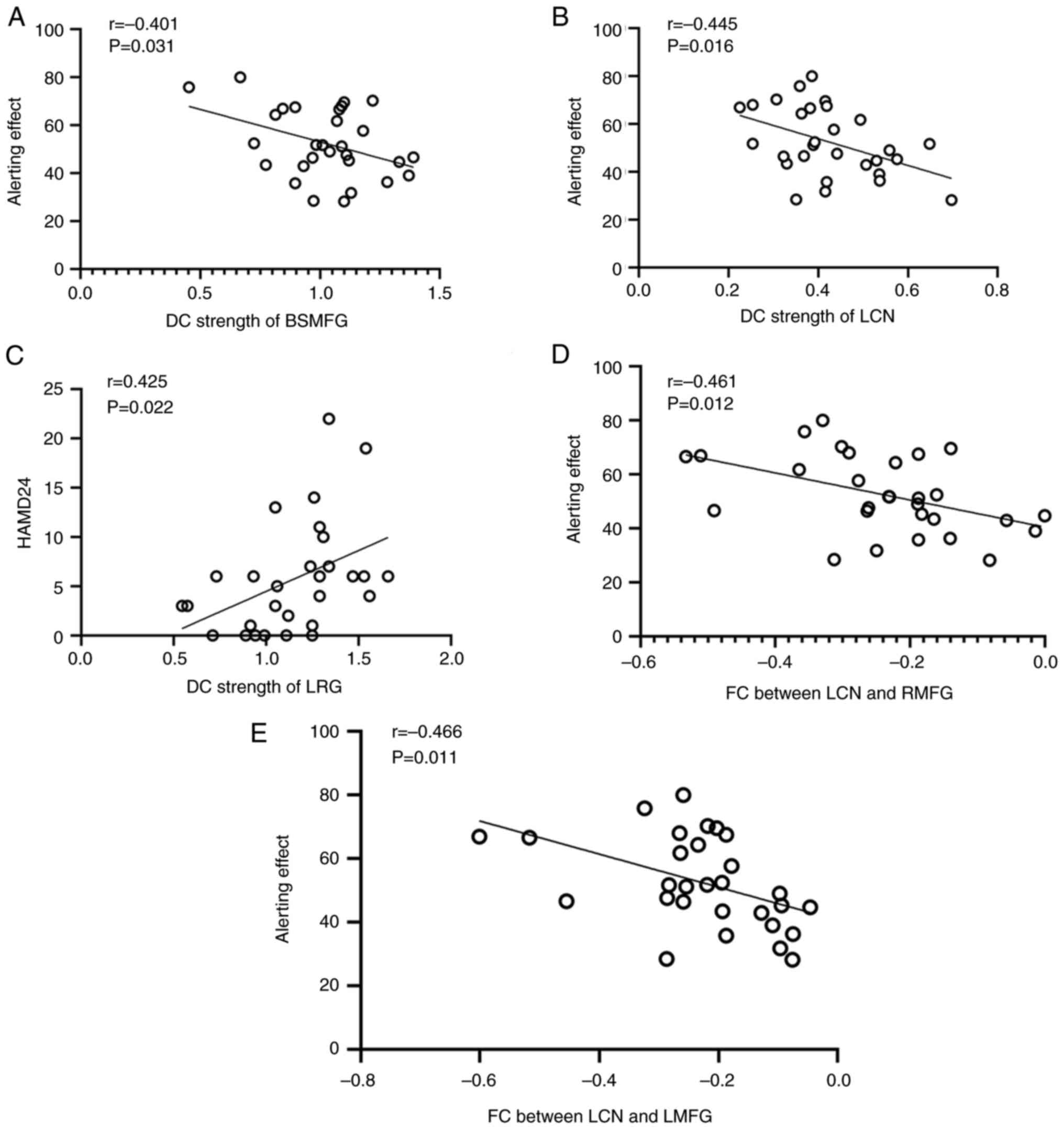
(Fig. 3). The DC strength in the
LRG was positively correlated with the HAMD24 score (r=0.425;
P=0.022; Fig. 3C), while negative
associations were observed between the alerting effect and the DC
strength in the LCN (r=-0.445; P=0.016; Fig. 3B) and BSMFG (r=-0.401; P=0.031;
Fig. 3A) in the patient group.
According to the FC analysis, the FC strengths between the LCN and
the right and left middle frontal gyri were both negatively
correlated with the alerting effect (r=-0.461; P=0.012; and
r=-0.466; P=0.011; Fig. 3D and
E, respectively).
MVPA
In the present study, MVPA was used to analyze
neural signals based on DC maps to identify specific spatial
functional activities in patients with anti-NMDAR encephalitis
compared with HCs. The specific brain regions were mainly located
in the cerebellum, left middle orbital frontal gyrus, left superior
orbital frontal gyrus, right precuneus, right postcentral gyrus,
right superior medial frontal gyrus, right inferior parietal gyrus,
right supplementary motor area, left medial orbital frontal gyrus,
right superior parietal gyrus, left angular gyrus and LCN (Table IV). These regions together yielded
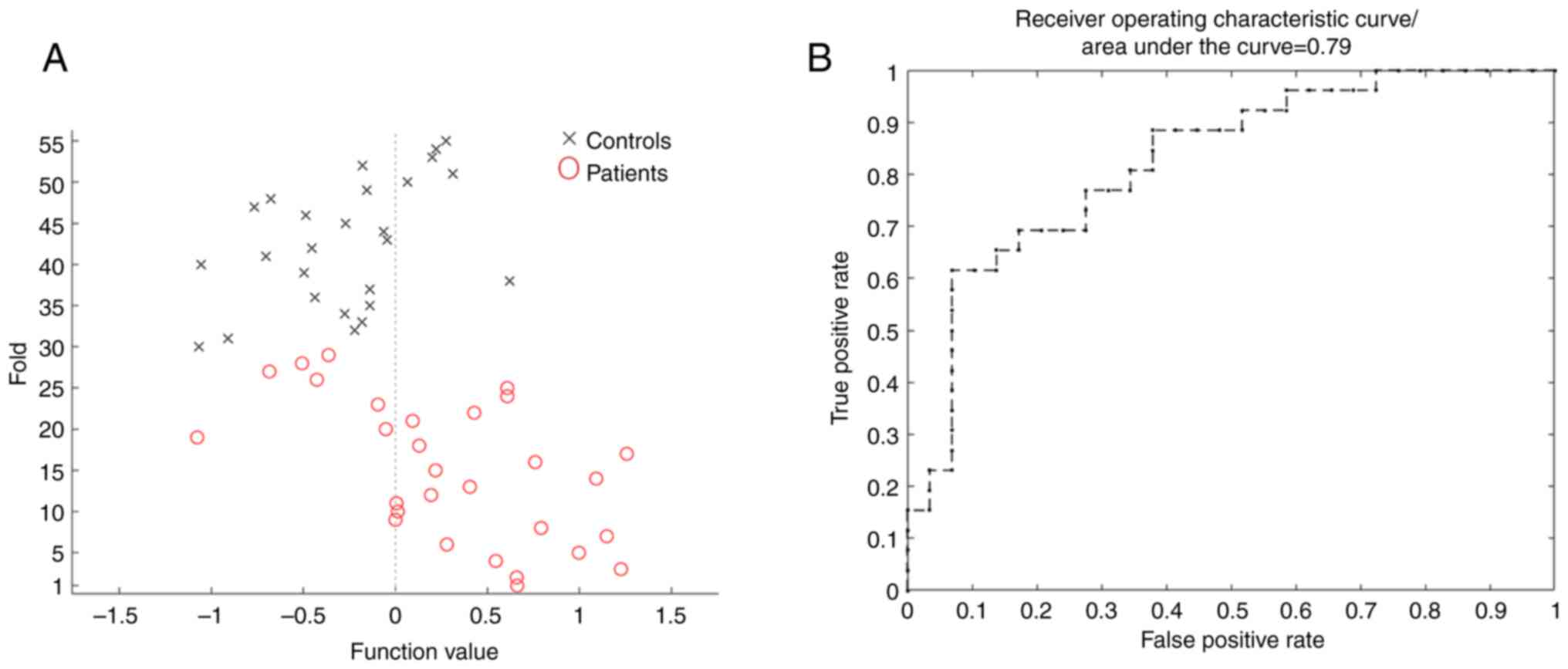
an area under the curve of 0.79 (Fig.
4B), with an overall classifier accuracy, sensitivity and
specificity of 76.36, 75.86 and 76.92%, respectively (Fig. 4A). Based on this analysis, the MVPA
classifier can be used to distinguish patients with anti-NMDAR
encephalitis from HCs.
 | Table IVBrain regions with the largest ROI
weight between patients with anti-N-methyl-D-aspartate receptor
encephalitis and healthy controls using multivariate pattern
analysis classification. |
Table IV
Brain regions with the largest ROI
weight between patients with anti-N-methyl-D-aspartate receptor
encephalitis and healthy controls using multivariate pattern
analysis classification.
| Brain region | ROI size,
voxels | ROI weight, % |
|---|
| Cerebellum_9_L | 599 | 1.8768 |
| Vermis_9 | 75 | 1.7412 |
| Cerebellum_8_R | 517 | 1.6727 |
| Vermis_1_2 | 41 | 1.5827 |
|
Cerebellum_7b_R | 129 | 1.5812 |
| Parietal_sup_R | 558 | 1.4246 |
| Cerebellum_3_L | 550 | 1.4048 |
|
Cerebellum_7b_L | 507 | 1.3974 |
|
Frontal_med_orb_L | 614 | 1.3081 |
|
Frontal_sup_medial_R | 785 | 1.3008 |
|
Cerebellum_crus2_L | 726 | 1.2649 |
| Parietal_inf_R | 662 | 1.2065 |
|
Cerebellum_crus2_R | 556 | 1.2021 |
| Postcentral_R | 993 | 1.2012 |
| Angular_L | 547 | 1.1960 |
|
Supp_motor_area_R | 627 | 1.1869 |
|
Frontal_sup_orb_L | 1,089 | 1.1839 |
| Cerebellum_8_L | 124 | 1.1745 |
| Caudate_L | 200 | 1.1702 |
|
Frontal_mid_orb_L | 1,431 | 1.1599 |
| Precuneus_R | 1,041 | 1.1184 |
Discussion
The present study investigated alterations in the
hub distribution and functional activities at the local and global
cerebral levels based on DC and FC analyses in patients with
anti-NMDAR encephalitis. The patient group exhibited increased DC
in the cerebellum anterior lobe and decreased DC in the LRG, LCN
and BSMFG, compared with the HC group. In subsequent FC analyses
based on the ROIs of the aforementioned brain regions, the LCN
showed decreased FC with the left precuneus and bilateral middle
frontal gyrus in the patient group compared with HCs. Furthermore,
these abnormal functional activities were associated with cognitive
and psychological impairments. By performing MVPA, disrupted DC
maps could distinguish patients with anti-NMDAR encephalitis from
HCs with high classification accuracy and sensitivity. In summary,
the present study effectively revealed the features of disrupted
functional hubs and related networks in patients with anti-NMDAR
encephalitis, which provides a more comprehensive understanding of
the mechanism underlying pathological damage in this disease.
Brain regions are closely connected and coordinated
with each other while engaging in task processes, and different
regions may display predominant functions in diverse neurological
states (33). DC analyses can
indicate the ability of each voxel to process information by
evaluating its number of direct connections with other voxels
(34). Disrupted functional
activities of hub regions are considered to participate in
processes underlying abnormal neuropsychological function. In the
present study, it was demonstrated that brain regions with abnormal
strength were mainly located in the cerebellar anterior lobe, LRG,
LCN, BSMFG, left precuneus and bilateral middle frontal gyrus.
These findings indicated that disrupted functional activities were
not limited to the limbic system, but were also widely distributed
from the frontal lobe to the subcortical region and even the
distant cerebellum in patients with anti-NMDAR encephalitis. These
results provide evidence that anti-NMDAR encephalitis is a brain
network disease.
The prefrontal lobe is the association cortex of the
frontal lobe and constitutes nearly one-third of the neocortex
(35). The superior medial frontal
gyrus, middle frontal gyrus and rectus gyrus are important parts of
the prefrontal lobe. A total of five separate prefrontal functions
exist, namely energization, task setting, monitoring,
meta-cognition and behavioral/emotional regulation (36). It has been widely recognized that
the prefrontal lobe is the predominant brain region that transmits
signals to and receives signals from other cortical regions,
subcortical structures and even the remote cerebellum in
high-cognitive processes (36-39).
The prefrontal cortex (PFC) is the key node in the executive
control network (ECN), and disrupted functional and structural
parameters of the PFC contribute to neuropsychological dysfunction,
including executive disorders and anxiety (38). It has been demonstrated that older
subjects with amnestic mild cognitive impairment exhibited reduced
regional cerebral blood flow in the PFC in a retrieval task,
causing memory deficit (40). Using
the Delis-Kaplan Executive Function Scale and a classic
neuropsychological test, a previous study indicated that a larger
volume of the lateral PFC was related to greater executive function
(41). The correlation analysis in
the present study also indicated that disrupted PFC activity was
negatively correlated with alerting function. Thus, disruptions of
the PFC in the ECN may contribute to complex and diverse clinical
disorders of neuropsychological function in patients with
anti-NMDAR encephalitis.
The deep nuclei act as information transfer stations
in information processing (42).
The caudate nucleus is one of the brain regions in the
extrapyramidal system involved in motor regulation, and
abnormalities in the caudate nucleus cause deficits in motor
performance (43). However,
converging evidence has indicated that the caudate nucleus receives
outputs from the PFC and contributes to different cognitive
processes (42). This has been
demonstrated in various cognitive tasks such as reading and
language showing increased activity both in the caudate nucleus and
prefrontal and temporal-parietal lobes (41). Furthermore, the caudate nucleus is
also a subcortical component of the DMN and is implicated in
numerous clinical disorders such as temporal lobe epilepsy and
attention-deficit hyperactivity disorder, including memory,
emotion, cognitive function and the processing of emotionally
salient stimuli (44,45). In older patients with depression,
psychomotor retardation is an important cognitive symptom, and this
phenomenon can be predicted through the observation of a reduced
volume in the caudate nucleus (40). The present study demonstrated that
the LCN exhibited abnormal FC with the left precuneus and bilateral
middle frontal gyrus in patients with anti-NMDAR encephalitis. The
precuneus is also the core node in the DMN and has been
demonstrated to participate in a wide spectrum of higher-order
cognition, consciousness and attention regulation (46). There are four specific major types
of anatomic connections of the precuneus, namely connections with
the superior parietal lobule, occipital cortex, frontal lobe and
temporal lobe, which provide an additional illustration of the
integration of higher-order information throughout the entire brain
network (47). Hebscher et
al (48) reported that the
precuneus serves a causal role in the retrieval of autobiographical
memories and that precuneus stimulation leads to disrupted dynamics
in the retrieval process. Combined with the findings of previous
studies (47,48), our findings suggest that abnormal FC
between the caudate nucleus and the ECN and DMN may contribute to
higher-order and motor deficits in patients with anti-NMDAR
encephalitis.
In the DC analysis, disrupted activities in the
cerebellum were also observed. The cerebellum has been
traditionally recognized as the dominant region of motor
regulation. However, with the increasing knowledge of the
cerebellar function, its role in non-motor tasks, such as emotion,
social behavior, executive function and working memory language
function, has been widely recognized (49,50).
In functional and structural explorations, widespread connections
from/to the cerebellum in the cerebrum were identified, involving
the frontal lobe, parietal lobe, temporal lobe, occipital lobe and
subcortical brain regions, which are the basis of motor and
non-motor functions (49,51,52).
Lesions in the cerebellum are considered to contribute to
cerebellar cognitive affective syndrome, which is associated with
more pronounced cognitive deficits (53). In previous studies, abnormal
functional activity and fiber damage in the cerebellum have been
observed in patients with anti-NMDAR encephalitis (14,54).
Recently, compensatory effects in the cerebellum in
neurodegenerative disease such as Parkinson's disease have been
proposed (55), and increased
functional activity, increased volume and metabolic enhancement are
considered to alleviate clinical symptoms and delay the course of
the disease. Thus, the increased DC strength in the cerebellum
observed in the patient group in the present study indicates a more
intensive information processing ability that could enhance motor
and cognitive function in patients with anti-NMDAR
encephalitis.
In the present study, correlation analyses were
performed between clinical parameters and the brain regions with
significant differences in DC and FC analyses. It was observed that
the DC strength of the BSMFG and LCN was negatively correlated with
the alerting effect, the DC strength of the LRG was positively
correlated with the HAMD24 score, and the FCs between LCN and the
right and left middle frontal gyri were negatively correlated with
the alerting effect, while the other clinical parameters were not
affected by the abnormal brain regions. The correlation analysis
results demonstrated that abnormal DCs or FCs in affected brain
regions contribute to cognitive impairment or depression, as
indicated in our previous study (19). Our previous study demonstrated that
the alerting effect of patients with anti-NMDAR encephalitis was
decreased compared with that of HCs (19); however, no difference was found in
the alerting effect between patients and HCs in the present study,
which may be due to a gradual recovery of cognitive function over
time. The results of the correlation analysis suggested that the
alerting function was affected by the area of brain damage in
patients with anti-NMDAR encephalitis, and brain impairment could
be observed even when the alerting function was close to normal in
the late recovery period, which highlighted the importance of
rs-fMRI in the study of anti-NMDAR encephalitis.
MVPA can be used as a potential diagnostic approach
for categorizing individuals by investigating spatial and temporal
information from neuroimaging data in numerous neurological and
psychiatric diseases, such as mild cognitive impairment, major
depressive disorder, obsessive-compulsive disorder and temporal
lobe epilepsy (42-45).
In clinically atypical patients with anti-NMDAR encephalitis, the
clinical symptoms are mild, and there are no obvious abnormalities
on MRI, electroencephalogram or negative cerebrospinal fluid
anti-NMDAR antibody (56).
Multimodal imaging may be an auxiliary diagnostic method, which is
expected to have certain diagnostic value for the clinically
confirmed patients (57). Although
rs-fMRI needs more time to finish scanning and coordinate to keep
headless motion, it can be used as a potentially important
diagnostic method to neurological and psychotic disorders, such as
schizophrenia or attention deficit hyperactivity disorder (58). Therefore, multimodal imaging may
provide novel supporting evidence for the diagnosis of anti-NMDAR
encephalitis, particularly in undiagnosed patients or patients in
the convalescence period. In the present study, by performing MVPA
using DC maps, the regions that were more important in
discriminating between patients with anti-NMDAR encephalitis and
HCs were predominantly located in the cerebellum, prefrontal lobe,
parietal lobe and LCN. These brain regions were consistent with
those identified in the DC and FC analyses of the present study. As
aforementioned (39,53,59),
these regions are involved in cognitive function and psychosis, and
are associated with clinical symptoms, such as
psychiatric/behavioral abnormalities, cognitive impairment,
seizures and movement disorders, in patients with anti-NMDAR
encephalitis. In the present study, correlation analyses also
showed that abnormal DC and FC values in affected brain regions
were correlated with cognitive deficits, and further demonstrated
that these abnormalities in brain regions were involved in the
clinical disorder. In brief, DC analysis is conducive to revealing
the imaging parameters involved in the development of clinical
symptoms, and when combined with the MVPA approach, DC analysis may
be a powerful tool for diagnosing anti-NMDAR encephalitis,
especially in patients with no abnormalities on regular MRI or with
non-specific imaging findings.
Several limitations should be considered in the
present study. First, the small sample in our research maybe lack
representativeness and homogeneity, reduces statistical power and
can only partially reflect real-world evidence results (for
example, the alerting effect did not differ between patients and
HCs in the present study). Therefore, more participants in both
groups should be recruited to establish the stability and
reliability of the research results. Furthermore, obtaining data
from patients with anti-NMDAR encephalitis during the acute stage
may be important for exploring potential imaging alterations that
illustrate clinical features. In addition, as patients may present
various symptoms, subgroup analysis based on different clinical
symptoms may be helpful for elucidating the pathogenesis of the
corresponding symptoms. Patients may have a favorable prognosis
after early and comprehensive treatment; however, most patients
still suffer from long-term deficits in different aspects (5). Therefore, a longitudinal study may be
useful for identifying vulnerable brain regions responsible for
persistent neuropsychological dysfunction.
In summary, the current study revealed the presence
of disrupted DC and FC in the entire brain, which were
predominantly located in the cerebellar network, DMN and ECN in
patients with anti-NMDAR encephalitis. Furthermore, it was
demonstrated that by combining DC maps with MVPA and disrupted
functional activity may yield high accuracy, sensitivity and
specificity for the primary diagnosis of anti-NMDAR encephalitis.
These abnormal functional activities may be associated with severe
and complex clinical symptoms, and could provide pathological and
compensatory imaging evidence for a deeper understanding of
anti-NMDAR encephalitis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National Natural
Science Foundation of China (grant no. 81560223), the Natural
Science Foundation of Guangxi Province (grant no.
2016GXNSFAA380182) and the Medical and Health Appropriate
Technology Development and Application Project of Guangxi Province
(grant no. S2023017).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ and BF conceived the study, designed the
methodology, analyzed data and wrote the manuscript. XZ, LP, LQ and
CL analyzed data. XZ wrote the manuscript. BF and LP interpreted
data and edited the manuscript. All authors have read and approved
the final manuscript. JZ and BF confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
All subjects were informed in detail about the study
and provided written informed consent. The study was approved by
the Medical Research Ethics Committee of The First Affiliated
Hospital of Guangxi Medical University (approval no.
2015-KY-National Fund-064; Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vitaliani R, Mason W, Ances B, Zwerdling
T, Jiang Z and Dalmau J: Paraneoplastic encephalitis, psychiatric
symptoms, and hypoventilation in ovarian teratoma. Ann Neurol.
58:594–604. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi
JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, et al:
Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis
associated with ovarian teratoma. Ann Neurol. 61:25–36.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Staley EM, Jamy R, Phan AQ, Figge DA and
Pham HP: N-Methyl-d-aspartate receptor antibody encephalitis: A
concise review of the disorder, diagnosis, and management. ACS Chem
Neurosci. 10:132–142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neyens RR, Gaskill GE and Chalela JA:
Critical care management of Anti-N-Methyl-D-aspartate receptor
encephalitis. Crit Care Med. 46:1514–1521. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heine J, Kopp UA, Klag J, Ploner CJ, Prüss
H and Finke C: Long-Term cognitive outcome in
Anti-N-Methyl-D-Aspartate receptor encephalitis. Ann Neurol.
90:949–961. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dalmau J, Lancaster E, Martinez-Hernandez
E, Rosenfeld MR and Balice-Gordon R: Clinical experience and
laboratory investigations in patients with anti-NMDAR encephalitis.
Lancet Neurol. 10:63–74. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Titulaer MJ, McCracken L, Gabilondo I,
Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I,
Martinez-Hernandez E, et al: Treatment and prognostic factors for
long-term outcome in patients with anti-NMDA receptor encephalitis:
An observational cohort study. Lancet Neurol. 12:157–165.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nosadini M, Eyre M, Molteni E, Thomas T,
Irani SR, Dalmau J, Dale RC and Lim M: International NMDAR Antibody
Encephalitis Consensus Group. Anlar B, et al: Use and safety of
immunotherapeutic management of N-Methyl-d-Aspartate receptor
antibody encephalitis: A meta-analysis. JAMA Neurol. 78:1333–1344.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang H and Xiao Z: Current progress on
assessing the prognosis for Anti-N-Methyl-D-aspartate receptor
(NMDAR) encephalitis. Biomed Res Int. 2020(7506590)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo Y, Lv X, Wei Q, Wu Y, Chen Y, Ji Y,
Hou Q, Lv H, Zhou N, Wang K and Tian Y: Impaired neurovascular
coupling and cognitive deficits in anti-N-methyl-D-aspartate
receptor encephalitis. Brain Imaging Behav. 16:1065–1076.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Peer M, Prüss H, Ben-Dayan I, Paul F, Arzy
S and Finke C: Functional connectivity of large-scale brain
networks in patients with anti-NMDA receptor encephalitis: An
observational study. Lancet Psychiatry. 4:768–774. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu J, Guo Y, Li J, Lv X, Zhang J, Zhang J,
Hu Q, Wang K and Tian Y: Progressive cortical and sub-cortical
alterations in patients with anti-N-methyl-D-aspartate receptor
encephalitis. J Neurol. 269:389–398. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Finke C, Kopp UA, Scheel M, Pech LM,
Soemmer C, Schlichting J, Leypoldt F, Brandt AU, Wuerfel J, Probst
C, et al: Functional and structural brain changes in
anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol.
74:284–296. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liang Y, Cai L, Zhou X, Huang H and Zheng
J: Voxel-based analysis and multivariate pattern analysis of
diffusion tensor imaging study in anti-NMDA receptor encephalitis.
Neuroradiology. 62:231–239. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Phillips OR, Joshi SH, Narr KL, Shattuck
DW, Singh M, Di Paola M, Ploner CJ, Prüss H, Paul F and Finke C:
Superficial white matter damage in anti-NMDA receptor encephalitis.
J Neurol Neurosurg Psychiatry. 89:518–525. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kerik-Rotenberg N, Diaz-Meneses I,
Hernandez-Ramirez R, Muñoz-Casillas R, Reynoso-Mejia CA,
Flores-Rivera J, Espinola-Nadurille M, Ramirez-Bermudez J and
Aguilar-Palomeque C: A Metabolic brain pattern associated with
Anti-N-Methyl-D-Aspartate receptor encephalitis. Psychosomatics.
61:39–48. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leypoldt F, Buchert R, Kleiter I,
Marienhagen J, Gelderblom M, Magnus T, Dalmau J, Gerloff C and
Lewerenz J: Fluorodeoxyglucose positron emission tomography in
anti-N-methyl-D-aspartate receptor encephalitis: Distinct pattern
of disease. J Neurol Neurosurg Psychiatry. 83:681–686.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan J, Guan H, Zhou X, Niu N, Li F, Cui L
and Cui R: Changing brain metabolism patterns in patients With
ANMDARE: Serial 18F-FDG PET/CT Findings. Clin Nucl Med. 41:366–370.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fan B, Wu P, Zhou X, Chen Z, Pang L, Shi K
and Zheng J: Aberrant resting-state interhemispheric functional
connectivity in patients with anti-N-methyl-D-aspartate receptor
encephalitis. Neuroradiology. 64:2021–2030. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang JH, Zuo XN, Gohel S, Milham MP,
Biswal BB and He Y: Graph theoretical analysis of functional brain
networks: Test-retest evaluation on short- and long-term
resting-state functional MRI data. PLoS One.
6(e21976)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zuo XN, Ehmke R, Mennes M, Imperati D,
Castellanos FX, Sporns O and Milham MP: Network centrality in the
human functional connectome. Cereb Cortex. 22:1862–1875.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Van Den Heuvel MP and Hulshoff Pol HE:
Exploring the brain network: A review on resting-state fMRI
functional connectivity. Eur Neuropsychopharmacol. 20:519–534.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Stam CJ, De Haan W, Daffertshofer A, Jones
BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt
JP, de Munck JC, van Dijk BW, et al: Graph theoretical analysis of
magnetoencephalographic functional connectivity in Alzheimer's
disease. Brain. 132(Pt 1):213–224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song L, Peng Q, Liu S and Wang J: Changed
hub and functional connectivity patterns of the posterior fusiform
gyrus in chess experts. Brain Imaging Behav. 14:797–805.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dutra LA, Abrantes F, Toso FF, Pedroso JL,
Barsottini OGP and Hoftberger R: Autoimmune encephalitis: A review
of diagnosis and treatment. Arq Neuropsiquiatr. 76:41–49.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The montreal cognitive assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zigmond AS and Snaith RP: The hospital
anxiety and depression scale. Acta Psychiatr Scand. 67:361–370.
1983.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fan J, McCandliss BD, Sommer T, Raz A and
Posner MI: Testing the efficiency and independence of attentional
networks. J Cogn Neurosci. 14:340–347. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fonov V, Evans AC, Botteron K, Almli CR,
McKinstry RC and Collins DL: Brain Development Cooperative Group.
Unbiased average age-appropriate atlases for pediatric studies.
Neuroimage. 54:313–327. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Aglieri V, Cagna B, Velly L, Takerkart S
and Belin P: FMRI-based identity classification accuracy in left
temporal and frontal regions predicts speaker recognition
performance. Sci Rep. 11(489)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sheikh UA, Carreiras M and Soto D:
Decoding the meaning of unconsciously processed words using
fMRI-based MVPA. Neuroimage. 191:430–440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schrouff J, Rosa MJ, Rondina JM, Marquand
AF, Chu C, Ashburner J, Phillips C, Richiardi J and Mourão-Miranda
J: PRoNTo: Pattern recognition for neuroimaging toolbox.
Neuroinformatics. 11:319–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shine JM, Breakspear M, Bell PT, Ehgoetz
Martens KA, Shine R, Koyejo O, Sporns O and Poldrack RA: Human
cognition involves the dynamic integration of neural activity and
neuromodulatory systems. Nat Neurosci. 22:289–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baek EC, Hyon R, López K, Finn ES, Porter
MA and Parkinson C: In-degree centrality in a social network is
linked to coordinated neural activity. Nat Commun.
13(1118)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fuster JM: Frontal lobe and cognitive
development. J Neurocytol. 31:373–385. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Henri-Bhargava A, Stuss DT and Freedman M:
Clinical assessment of prefrontal lobe functions. Behav Neurol
Psychiatry. 24:704–726. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Paik E: Functions of the prefrontal cortex
in the human brain. J Korean Med Sci. 13:569–581. 1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Miller EK and Cohen JD: An integrative
theory of prefrontal cortex function. Annu Rev Neurosci.
24:167–202. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Miller EK: The prefrontal cortex and
cognitive control. Nat Rev Neurosci. 1:59–65. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Uemura K, Shimada H, Doi T, Makizako H,
Tsutsumimoto K, Park H and Suzuki T: Reduced prefrontal oxygenation
in mild cognitive impairment during memory retrieval. Int J Geriatr
Psychiatry. 31:583–591. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mace RA, Waters AB, Sawyer KS, Turrisi T
and Gansler DA: Components of executive function model regional
prefrontal volumes. Neuropsychology. 33:1007–1019. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Grahn JA, Parkinson JA and Owen AM: The
cognitive functions of the caudate nucleus. Prog Neurobiol.
86:141–155. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Çırak M, Yağmurlu K, Kearns KN, Ribas EC,
Urgun K, Shaffrey ME and Kalani MYS: The caudate nucleus: Its
connections, surgical implications, and related complications.
World Neurosurg. 139:e428–e438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mohan A, Roberto AJ, Mohan A, Lorenzo A,
Jones K, Carney MJ, Liogier-Weyback L, Hwang S and Lapidus KA: The
significance of the default mode network (DMN) in neurological and
neuropsychiatric disorders: A review. Yale J Biol Med. 89:49–57.
2016.PubMed/NCBI
|
|
45
|
Li J, Curley WH, Guerin B, Dougherty DD,
Dalca AV, Fischl B, Horn A and Edlow BL: Mapping the subcortical
connectivity of the human default mode network. Neuroimage.
245(118758)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Smallwood J, Bernhardt BC, Leech R, Bzdok
D, Jefferies E and Margulies DS: The default mode network in
cognition: A topographical perspective. Nat Rev Neurosci.
22:503–513. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tanglay O, Young IM, Dadario NB, Briggs
RG, Fonseka RD, Dhanaraj V, Hormovas J, Lin YH and Sughrue ME:
Anatomy and white-matter connections of the precuneus. Brain
Imaging Behav. 16:574–586. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hebscher M, Ibrahim C and Gilboa A:
Precuneus stimulation alters the neural dynamics of
autobiographical memory retrieval. Neuroimage.
210(116575)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Koziol LF, Budding D, Andreasen N,
D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C,
Parker K, et al: Consensus paper: The cerebellum's role in movement
and cognition. Cerebellum. 13:151–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Leggio M and Olivito G: Topography of the
cerebellum in relation to social brain regions and emotions. Handb
Clin Neurol. 154:71–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sbardella E, Upadhyay N, Tona F,
Prosperini L, De Giglio L, Petsas N, Pozzilli C and Pantano P:
Dentate nucleus connectivity in adult patients with multiple
sclerosis: Functional changes at rest and correlation with clinical
features. Mult Scler. 23:546–555. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhou X, Zhang Z, Liu J, Qin L, Pang X and
Zheng J: Disruption and lateralization of cerebellar-cerebral
functional networks in right temporal lobe epilepsy: A
resting-state fMRI study. Epilepsy Behav. 96:80–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ahmadian N, Van Baarsen K, Van Zandvoort M
and Robe PA: The cerebellar cognitive affective syndrome-a
meta-analysis. Cerebellum. 18:941–950. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cai L, Liang Y, Huang H, Zhou X and Zheng
J: Cerebral functional activity and connectivity changes in
anti-N-methyl-D-aspartate receptor encephalitis: A resting-state
fMRI study. Neuroimage Clin. 25(102189)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liang KJ and Carlson ES: Resistance,
vulnerability and resilience: A review of the cognitive cerebellum
in aging and neurodegenerative diseases. Neurobiol Learn Mem.
170(106981)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Flanagan EP, Geschwind MD, Lopez-Chiriboga
AS, Blackburn KM, Turaga S, Binks S, Zitser J, Gelfand JM, Day GS,
Dunham SR, et al: Autoimmune encephalitis misdiagnosis in adults.
JAMA Neurol. 80:30–39. 2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Beutler BD, Moody AE, Thomas JM, Sugar BP,
Ulanja MB, Antwi-Amoabeng D and Tsikitas LA:
Anti-N-methyl-D-aspartate receptor-associated encephalitis: A
review of clinicopathologic hallmarks and multimodal imaging
manifestations. World J Radiol. 16:1–8. 2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lam SL, Criaud M, Lukito S, Westwood SJ,
Agbedjro D, Kowalczyk OS, Curran S, Barret N, Abbott C, Liang H, et
al: Double-Blind, Sham-controlled randomized trial testing the
efficacy of fMRI neurofeedback on clinical and cognitive measures
in children with ADHD. Am J Psychiatry. 179:947–958.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dadario NB and Sughrue ME: The functional
role of the precuneus. Brain. 146:3598–3607. 2023.PubMed/NCBI View Article : Google Scholar
|