Exploring serum α‑synuclein and its autoantibodies in essential tremor: implications for diagnosis and symptom correlations
- Authors:
- Published online on: June 4, 2024 https://doi.org/10.3892/br.2024.1796
- Article Number: 108
-
Copyright: © Shalash et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Essential tremor (ET) is defined as a syndrome of isolated tremor of both upper limbs with a duration of at least 3 years, with or without tremor in other locations, such as the head, larynx (voice tremor), or lower limbs (1). Although the defining characteristic of ET has traditionally been its motor features, there is an increasing acknowledgment of additional NMSs associated with ET, such as cognitive decline, depression, anxiety and sleep disturbances (2-4). Currently, there is no definitive biomarker available for the diagnosis of ET. Therefore, the diagnosis relies heavily on the clinical presentation of the disorder (5).
Α-Synuclein (α-syn) is a small protein that is encoded by the SNCA gene and is located on the long arm of chromosome 4(6). The α-syn protein is considered a pivotal factor in the pathogenesis of a group of neurodegenerative conditions called synucleinopathies, which include Parkinson's disease (PD), dementia with Lewy bodies, and multiple system atrophy (7). Moreover, Lewy pathology has been detected in 25% of patients with ET, explaining the link between ET and PD (8).
Serum α-syn has been investigated and may serve as a potential biomarker for PD. However, investigating its level in patients with other parkinsonian syndromes and ET is important for confirming its value (9,10). Moreover, differentiating between ET and PD can be difficult, both in the early stages of these diseases and as they progress, since various types of tremors (such as rest, postural, kinetic and intention tremors) can be observed in both conditions. Therefore, investigating the α-syn protein in patients with ET could help differentiate between these two diseases (11). Interestingly, a recent study showed lower serum a-syn in ET and PD patients than in controls, with no difference between the two diseases (12).
The aim of the present study was to investigate the serum levels of the α-syn protein and its autoantibodies in patients with ET compared with those in healthy controls and its relation to tremor severity, non-motor symptoms (NMSs) and patient quality of life (QoL).
Materials and methods
In the present observational case-control study, patients were recruited from the outpatient movement disorders clinic at Ain Shams University Hospital from December 2021 to May 2023 (Cairo, Egypt). The present study was approved by the ethics committee of the Ain Shams faculty of medicine (approval no. FMASU MS 700/2021), and written informed consent was obtained from all patients.
Sample size calculations
Using an online sample size calculator (https://clincalc.com/stats/samplesize.aspx); using two independent study groups design, with a dichotomous primary endpoint (diagnosis or no diagnosis), 15 subjects per group will provide a power of 80% with an alpha error rate of 0.05.
A total of 32 patients with ET and 32 sex- and age-matched healthy controls were included in the present study. Patients were diagnosed with ET by history and examination by movement disorder experts who met the diagnostic criteria for ET according to the Consensus Statement of the Movement Disorder Society on Tremor (1). The exclusion criteria included patients with a diagnosis of tremors of other etiology, for example, PD or dystonia; the presence of known causes of enhanced physiological tremor; concurrent or recent exposure to tremorgenic drugs, the presence of a drug withdrawal tate, direct or indirect trauma to the nervous system within 3 months before the onset of tremor, a history or clinical evidence of psychogenic origins, mental retardation, and inability to perform the assessment. Matched healthy controls were chosen among subjects who showed no manifestations of ET or other neurodegenerative conditions, as excluded by a consulting neurologist.
Patients' tremors were assessed using the Fahn Tolosa Marin Tremor Rating Scale. NMSs, cognition, depression, anxiety, and QoL of patients and controls were evaluated using the non-motor symptoms scale (NMSS) (13), Montreal Cognitive Assessment (MoCA) - Arabic version (14), Beck Depression Inventory (BDI) - Arabic version (15), Hamilton Anxiety Rating Scale (HARS) - Arabic version (16), and the Short Form 36 Health Survey Questionnaire (SF-36) (17), respectively.
Other ancillary biochemical tests
It's important to note that while ancillary biochemical tests such as thyroid function tests can provide valuable information, they are not specific to ET and should be interpreted in conjunction with the clinical assessment and exclusion of other movement disorders. The final diagnosis of ET still heavily relies on the characteristic clinical features observed during examination and the absence of findings indicative of other neurological conditions. However, ancillary tests such as thyroid function tests can be useful in certain situations where there is a suspicion of secondary tremors due to thyroid dysfunction or other medical conditions that may mimic ET.
Laboratory testing of serum a-syn and its autoantibodies
Patients with ET and healthy controls who consented to participate in the present study underwent blood sample collection. Blood (3 ml) was withdrawn under sterile conditions and placed in serum separation tubes. The samples were then centrifuged at 3,000 x g for 15 min at 4˚C, after which they were stored in -80˚C freezers at Ain Shams Hospital, Ain Shams University (Cairo, Egypt).
ELISA tests were repeated in duplicates. The serum α-syn titers were expressed in picograms per milliliter (pg/ml) using a commercially available enzyme-linked immunosorbent assay (ELISA) kit purchased from Elabscience Biotechnology, Inc. (cat. No. E-EL-H0983). The sensitivity of the assay was 9.38 pg/ml. Serum anti-α-syn autoantibodies were tested using a commercially available ELISA kit purchased from MyBioSource, Inc. (cat. No. MBS 2086950). The titers were estimated on the basis of a calibration curve of autoantibody standards and are expressed in nanograms per milliliter (ng/ml). The sensitivity of the assay was 0.1 ng/ml. The test steps for both kits were carried out according to the manufacturer's protocols.
Statistical analysis
The data were analyzed using the SPSS software package version 20.0 (IBM Corp.) and expressed as the mean ± standard deviation. Statistical analysis was conducted using the unpaired Student's t-test, chi-square test and linear correlation coefficient. P<0.05 was considered to indicate a statistically significant difference. Receiver operating characteristic (ROC) curve analysis was also used to determine the sensitivity and accuracy of the suggested biomarkers.
Results
The mean ages of patients with ET and controls were 51.75±14.13 (32 to 77 years) and 51.34±13.22 (32 to 72 years), respectively. In each group, there were 10 women (31.25%) and 22 men (68.75%). The two groups were age- and sex-matched. A total of 21 patients (65.63%) had a positive family history of ET. Additionally, there were 19 ET-plus patients in the ET group, representing 59.38% of the patients. The demographic data and clinical characteristics of the patients are described in Table I. Compared with controls, patients with ET showed significantly worse cognitive impairment according to the MoCA (P=0.001), BDI, HARS (P<0.001), NMSS total (P<0.001) and subscale scores and all domains of the SF-36 (P=0.001 or <0.001) (Tables I and SI).
Table IComparison of demographics, NMS and alpha-synuclein levels between patients with essential tremor and controls. |
Correlations between NMS and QoL among patients with ET
The gastrointestinal and sexual domains were positively correlated with patient age (P=0.034 and 0.040, respectively), while the cardiovascular, perceptual problems/hallucinations, gastrointestinal and sexual functions domains were correlated with tremor severity (P=0.034, 0.026, 0.042 and 0.012, respectively). The NMSS total score was not correlated with the age of the patients, age of illness, illness duration or severity of tremor. The severity of tremor was negatively correlated with MoCA scores (P<0.001) but not with depression or anxiety (Table II). Only the vitality domain was negatively correlated with the severity of tremor (P=0.017). Role limitations due to physical health, vitality and bodily pain domains were positively correlated with the total MoCA score. All the QoL domains were negatively correlated with depression and anxiety, except for role limitations due to physical health and physical functioning domains, respectively. All SF-36 domains were negatively correlated with the total NMSS score, which means that increased severity of NMS is associated with greater impairment of QoL. All the SF-36 domains were negatively correlated with most of the NMSS domains (Table II).
Table IICorrelations between tremor severity and quality of life domains in patients with essential tremor. |
Serum α-syn and anti-α-syn autoantibody levels in ET patients and controls
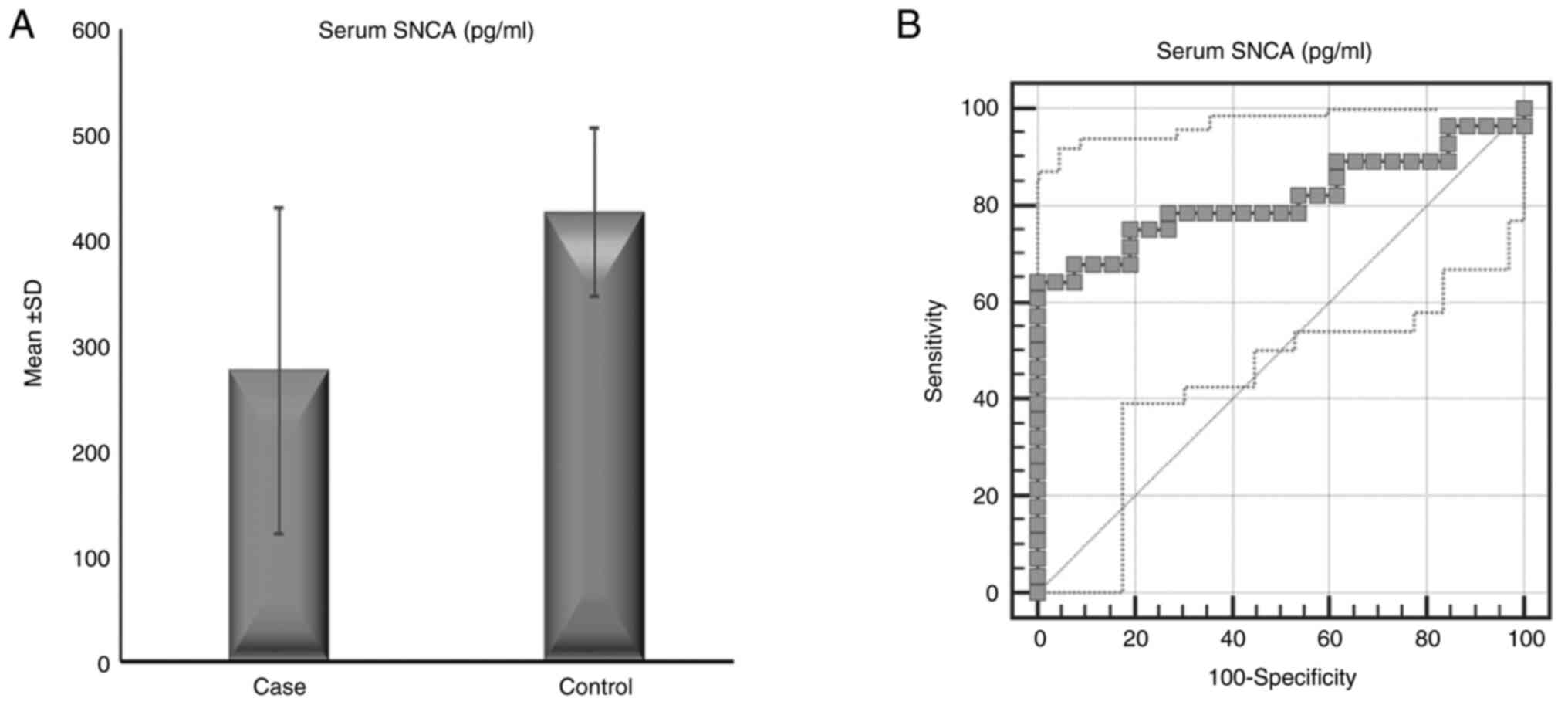
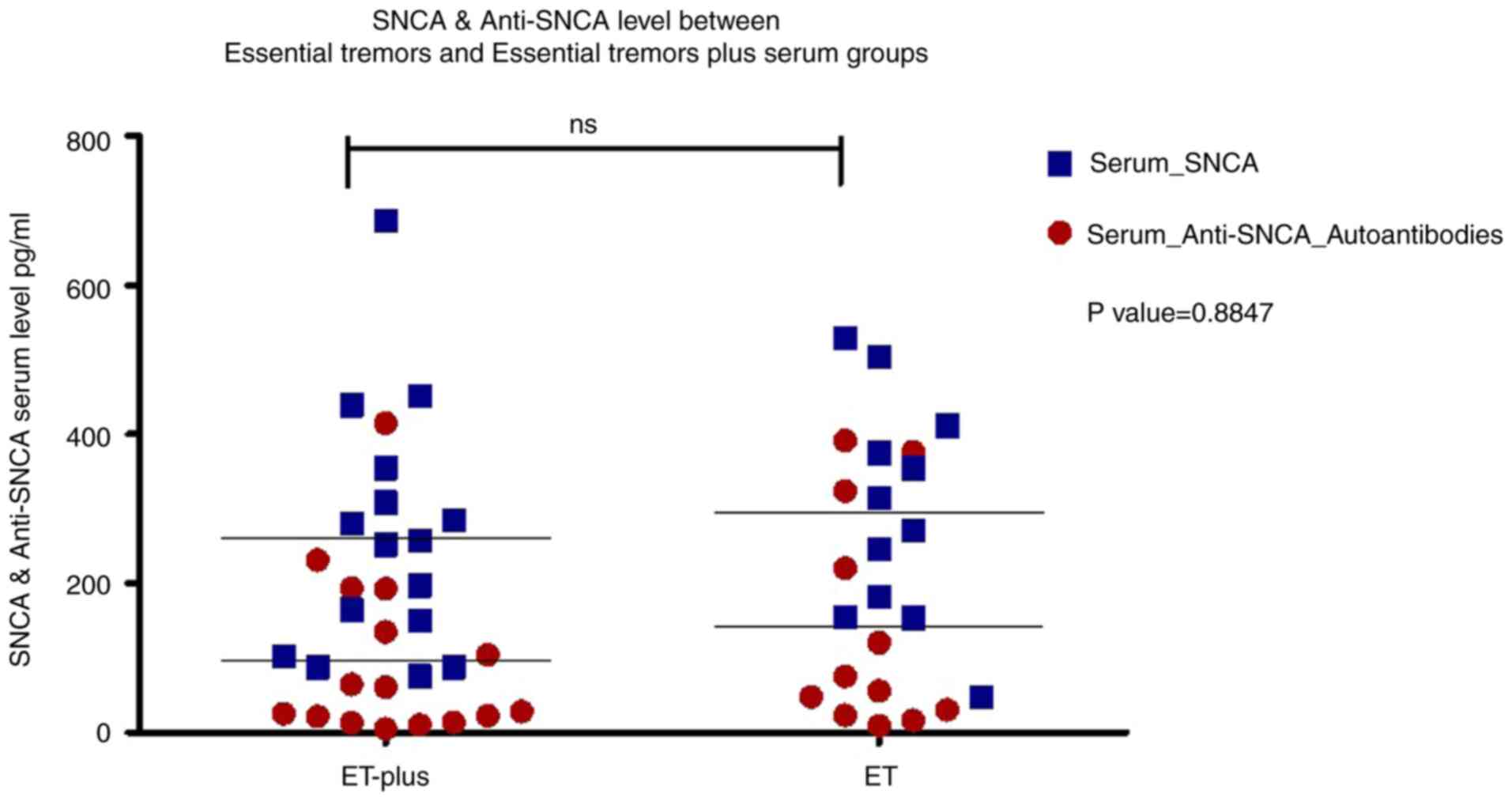
The serum α-syn concentration in patients with ET (28 patients, 275.36±155.36 pg/ml) was significantly lower than that in controls (26 subjects, 425.55±80.44 pg/ml) (P<0.001), while there was no significant difference in the serum anti-α-syn autoantibody concentration between patients with ET (115.12±128.22 ng/ml) and controls (110.55±44.76 ng/ml) (P=0.864) (Table I and Fig. 1). Correlation analysis revealed that serum α-syn and anti-α-syn antibody levels were not correlated with age, age of illness onset, illness duration or severity of tremor, NMSs or QoL (Table SII). There were no significant differences in serum α-syn or α-syn autoantibodies between patients with ET (12 patients) and patients with ET+ (16 patients) (P=0.338 and 0.574, respectively) as shown in Fig. 2.
ROC curve analysis
Serum α-syn levels were able to discriminate between controls and patients with ET, with an area under the curve (AUC)=0.815 (95% CI=0.692-0.937), a cutoff a-syn level ≤354.08 pg/ml, a positive predictive value (PPV) of 0.81, and a negative predictive value (NPV) of 0.75 (P<0.001). Serum anti-α-syn autoantibody levels were not able to discriminate between controls and patients with ET, with an AUC=0.632 (95% CI=0.463-0.801), a cutoff anti-α-syn autoantibody level ≤55.68 ng/ml, a PPV of 1, and an NPV of 0.65 (P=0.126) (Fig. 1). An analysis between the two subtypes of ET (ET and ET+) revealed no significant difference in the levels of serum α-syn or anti-α-syn autoantibodies with a P value of 0.8847 (Fig. 2).
Discussion
The present study explored serum α-syn and its autoantibodies in patients with ET and demonstrated lower values of serum α-syn among patients with ET than among controls, with a PPV of 0.81 and NPV of 0.75. Serum α-syn appears to differentiate between patients with ET and controls, with a cutoff value of 354.08 pg/ml. On the other hand, serum α-syn autoantibodies were not significantly different between the two groups involved in the present study. These findings are consistent with a study from Spain on 19 patients with ET, 19 patients with de novo PD, 35 patients with advanced PD and 35 healthy controls in which the serum α-syn levels in healthy controls were greater than those in patients with ET and PD (12) Another recent study investigated erythrocytic total and aggregated α-syn and showed increased concentrations in patients with ET and PD compared with controls. Remarkably, compared with patients with PD, patients with ET showed significantly greater erythrocytic total α-syn and lower ratios of erythrocytic aggregated to total α-syn, with a significant correlation between aggregated α-syn and disease duration (18). These studies demonstrated the potential role of α-syn and its variants in differentiating between ET and PD.
The lower α-syn values could be explained by reported Lewy pathology in the brains of patients with ET, particularly in the brainstem. Recently, ~25% of ET patients were reported to have Lewy pathology, which is heterogeneous and associated with conversion to PD (8). The findings of the present study support the possibility of a link between ET and PD (19). The increasing evidence of changes in α-syn levels in patients with ET is a confirmation of such discrepancies in the pathogenesis of both diseases (12,18). However, further studies are required to confirm the role of α-syn as a diagnostic biomarker of ET that might differentiate it from other diseases or as a biomarker for the conversion of ET to PD.
Interestingly, serum α-syn did not correlate with tremor severity or other clinical characteristics, indicating its minor role in the pathogenesis of the disease or disease progression. Some studies of serum α-syn in PD patients reported a moderate correlation with disease severity, indicating a different role of Lewy pathology in the two diseases (9).
Moreover, the present study revealed similar serum levels of anti-α-syn autoantibodies between patients with ET and controls. By contrast, patients with other neurodegenerative disorders, particularly PD, exhibit increased or decreased levels of anti-α-syn autoantibodies (9,10,20) suggesting the need for further studies to investigate the use of anti-α-syn as a differentiating biomarker between ET and PD. However, variable results of serum α-syn and its autoantibodies in PD patients have been reported, indicating the need for large studies with proper methodologies (9,20). To the best of the authors knowledge, no previous study has investigated serum anti-a-syn autoantibodies in patients with ET.
The present study confirmed worse NMSs, including cognitive impairment, depression and anxiety, and QoL, in patients with ET than in healthy controls, similar to the findings of previous studies (4,21-24). Moreover, motor severity was related to different NMSS domains but not depression or anxiety, while QoL domains were related to different NMSs, cognitive impairment, depression and anxiety. On the other hand, tremor severity was correlated with only the vitality domain of QoL.
Previous studies have reported variable correlations between tremor severity and ET, owing to variability in clinical characteristics, variable assessment tools and small numbers of different cohorts. Similarly, in the present study, cognitive impairment and depression were not correlated with tremor severity, similar to the findings of previous studies (23-25) but were consistent with the findings of other studies (4,26). Similarly, the relationship between anxiety and tremor severity was variable in previous studies (4,22). However, the findings of the present study are consistent with most studies showing that NMSs, including cognitive impairment, depression and anxiety, are the main determining factors of ET patients' QoL (4,21,22). The findings of the present study support that the associated NMSs of ET are inherent characteristics of the disease rather than secondary phenomena, which can be explained by dysfunction of the frontocerebellar circuits (4,25,27). The small sample size was the main limitation of the present study. In addition, adding a PD group is important for confirming the potential differentiating role of α-syn. Future larger studies are needed to confirm the role of the α-syn protein and its autoantibodies in ET.
In conclusion, the present study explored the serum levels of the α-syn protein and its autoantibodies in patients with ET and demonstrated that the serum α-syn level was lower in these patients than in controls, with no correlations with patient characteristics and similar anti-α-syn autoantibody levels. These findings suggest a potential role for serum α-syn as a biomarker for ET. Furthermore, profiling of proteins and their antibodies may provide a more holistic understanding of the disease process as well as the pathogenic involvement of the immune system. Additionally, the integral role of NMSs in ET and their negative impact on QoL was confirmed but no correlation with serum α-syn levels was observed.
The clinical implications of a study investigating the diagnostic value of serum alpha-synuclein (α-syn) to distinguish ET patients from healthy controls. This opens up several important possibilities: (i) Improved diagnosis: If α-syn levels are a reliable biomarker for ET, doctors could use them to diagnose ET sooner and more accurately. (ii) Improved treatment: Early diagnosis could allow doctors to start treatment sooner, which could improve outcomes for individuals with Et and Improved QoL: The present study also revealed that patients with ET had worse cognitive problems, emotional problems, and NMSs (such as fatigue and sleep problems), as well as a lower QoL. Knowing this could help doctors provide improved support and care for individuals with ET, improving their daily lives.
The present study is piloting the use of serum α-syn as a potential biomarker for ET. The results are still considered very early to reflect on clinical relevance, given absence of correlation to any of the clinical parameters measured. The present study was considered, as a trigger provider for future research to delve deeper into clinical and patho-mechanistic conclusions.
Supplementary Material
Comparison of quality of life between patients with essential tremor and controls.
Correlations of serum a-syn and anti- a-syn autoantibodies levels with demographics, tremor severity, non-motor symptoms and quality of life of essential tremor patients.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported (grant no. RSG-2022) by Faculty support grant [MS], The American University in Cairo.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
AS and EH conceptualized the present study. AS and AD organized the research project. AS, AD and MS executed the research project. AS and EH supervised and acquired the resources. AS, AD and MS designed the statistical analysis. AS and MS confirm the authenticity of all the raw data. AS and MS performed the statistical analysis. AS, AD, MB and EH reviewed and criticized the statistical analysis. AS and AD wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the ethics committee of the Ain Shams University faculty of medicine (Cairo, Egypt) (approval no. FMASU MS 700/2021), and written informed consent was obtained from all patients. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM and Deuschl G: Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 33:75–87. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lee SM, Kim M, Lee HM, Kwon KY and Koh SB: Nonmotor symptoms in essential tremor: Comparison with Parkinson's disease and normal control. J Neurol Sci. 349:168–173. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Shalash AS, Hamid E, Elrassas H, Bahbah EI, Mansour AH, Mohamed H and Elbalkimy M: Non-motor symptoms in essential tremor, akinetic rigid and tremor-dominant subtypes of Parkinson's disease. PLoS One. 16(e0245918)2021.PubMed/NCBI View Article : Google Scholar | |
|
Shalash AS, Mohamed H, Mansour AH, Elkady A, Elrassas H, Hamid E and Elbalkimy MH: Clinical profile of non-motor symptoms in patients with essential tremor: Impact on quality of life and age-related differences. Tremor Other Hyperkinet Mov (N Y). 9(10.7916/tohm.v0.736)2019.PubMed/NCBI View Article : Google Scholar | |
|
Espay AJ, Lang AE, Erro R, Merola A, Fasano A, Berardelli A and Bhatia KP: Essential pitfalls in ‘essential’ tremor. Mov Disord. 32:325–331. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Atik A, Stewart T and Zhang J: Alpha-Synuclein as a Biomarker for Parkinson's Disease. Brain Pathol. 26:410–418. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Altay MF, Liu AKL, Holton JL, Parkkinen L and Lashuel HA: Prominent astrocytic alpha-synuclein pathology with unique post-translational modification signatures unveiled across Lewy body disorders. Acta Neuropathol Commun. 10(163)2022.PubMed/NCBI View Article : Google Scholar | |
|
Louis ED, Iglesias-Hernandez D, Hernandez NC, Flowers X, Kuo SH, Vonsattel JPG and Faust PL: Characterizing lewy pathology in 231 essential tremor brains from the essential tremor centralized brain repository. J Neuropathol Exp Neurol. 81:796–806. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ganguly U, Singh S, Pal S, Prasad S, Agrawal BK, Saini RV and Chakrabarti S: Alpha-synuclein as a biomarker of Parkinson's disease: Good, but not good enough. Front Aging Neurosci. 13(702639)2021.PubMed/NCBI View Article : Google Scholar | |
|
Shalash A, Salama M, Makar M, Roushdy T, Elrassas HH, Mohamed W, El-Balkimy M and Abou Donia M: Elevated Serum α-Synuclein autoantibodies in patients with Parkinson's disease relative to Alzheimer's disease and controls. Front Neurol. 8(720)2017.PubMed/NCBI View Article : Google Scholar | |
|
Thenganatt MA and Jankovic J: The relationship between essential tremor and Parkinson's disease. Parkinsonism Relat Disord. 22 (Suppl 1):S162–S165. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Albillos SM, Montero O, Calvo S, Solano B, Trejo JM and Cubo E: Can Plasma α-synuclein help us to differentiate Parkinson's disease from essential tremor? Tremor Other Hyperkinet Mov (NY). 11(20)2021.PubMed/NCBI View Article : Google Scholar | |
|
Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, Ondo W, Abe K, Macphee G, Macmahon D, et al: The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Mov Disord. 22:1901–1911. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Abdel Rahman TT and El Gaafary MM: Montreal cognitive assessment arabic version: Reliability and validity prevalence of mild cognitive impairment among elderly attending geriatric clubs in Cairo. Geriatr Gerontol Int. 9:54–61. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Beck AT, Ward CH, Mendelson M, Mock J and Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry. 4:561–571. 1961.PubMed/NCBI View Article : Google Scholar | |
|
Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol. 32:50–55. 1959.PubMed/NCBI View Article : Google Scholar | |
|
Coons SJ, Alabdulmohsin SA, Draugalis JR and Hays RD: Reliability of an Arabic version of the RAND-36 Health Survey and its equivalence to the US-English version. Med Care. 36:428–432. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Yu Z, Liu G, Zheng Y, Huang G and Feng T: Erythrocytic alpha-synuclein as potential biomarker for the differentiation between essential tremor and Parkinson's disease. Front Neurol. 14(1173074)2023.PubMed/NCBI View Article : Google Scholar | |
|
Yoo SW, Ha S, Lyoo CH, Kim Y, Yoo JY and Kim JS: Exploring the link between essential tremor and Parkinson's disease. NPJ Parkinsons Dis. 9(134)2023.PubMed/NCBI View Article : Google Scholar | |
|
Garg P, Maass F, Sundaram SM, Mollenhauer B, Mahajani S, van Riesen C, Kugler S and Bahr M: The relevance of synuclein autoantibodies as a biomarker for Parkinson's disease. Mol Cell Neurosci. 121(103746)2022.PubMed/NCBI View Article : Google Scholar | |
|
Sengul Y, Sengul HS, Yucekaya SK, Yucel S, Bakim B, Pazarci NK and Ozdemir G: Cognitive functions, fatigue, depression, anxiety, and sleep disturbances: Assessment of nonmotor features in young patients with essential tremor. Acta Neurol Belg. 115:281–287. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Chandran V and Pal PK: Essential tremor: Beyond the motor features. Parkinsonism Relat Disord. 18:407–413. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Huey ED, Cosentino S, Chapman S, Azar M, Rohl B, Collins K, Morgan S, Liu X and Louis ED: Self-report depressive symptoms are dissociated from tremor severity in essential tremor. Parkinsonism Relat Disord. 50:87–93. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Musacchio T, Purrer V, Papagianni A, Fleischer A, Mackenrodt D, Malsch C, Gelbrich G, Steigerwald F, Volkmann J and Klebe S: Non-Motor symptoms of essential tremor are independent of tremor severity and have an impact on quality of life. Tremor Other Hyperkinet Mov (N Y). 6(361)2016.PubMed/NCBI View Article : Google Scholar | |
|
Lombardi WJ, Woolston DJ, Roberts JW and Gross RE: Cognitive deficits in patients with essential tremor. Neurology. 57:785–790. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Dogu O, Louis ED, Sevim S, Kaleagasi H and Aral M: Clinical characteristics of essential tremor in Mersin, Turkey-a population-based door-to-door study. J Neurol. 252:570–574. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Louis ED: Non-motor symptoms in essential tremor: A review of the current data and state of the field. Parkinsonism Relat Disord. 22 (Suppl 1):S115–S118. 2016.PubMed/NCBI View Article : Google Scholar |