Introduction
Nasopharyngeal carcinoma (NPC) is a highly
metastatic malignant tumor originating from the epithelium of the
nasopharynx (1). Genetic mutations,
Epstein-Barr virus infection, tobacco smoking and consumption of
alcohol and salted fish are risk factors for NPC (1). In 2019, age-standardized incidence
rate (ASIR) of NPC was 2.12 globally. However, certain regions have
higher ASIRs of NPC than the global average, including China,
Singapore and Taiwan, with rates of 5.7, 10.81 and 7.14,
respectively (2,3). The survival rate of patients with NPC
has been improved significantly by combining chemotherapy and
radiotherapy (4). However, ~30% of
patients still have a poor prognosis due to distant metastases
(5), suggesting that certain
patients do not receive adequate benefits from the current
treatment. Thus, improving the current treatments or developing new
therapeutic drugs is necessary to improve prognosis of patients
with NPC.
In recent years, increasing evidence (6-9)
has indicated that bioactive compounds from plants have promising
anticancer properties. For example, cordycepin and fucoidan are
natural compounds extracted from Cordyceps sinensis and
brown seaweed cell wall matrix, respectively (10,11).
These compounds can induce apoptosis in various cancer cell lines,
including esophageal, colorectal, liver, and non-small-cell lung
cancer (12-15).
Similarly, curcumin extracted from Curcuma longa inhibits
the survival of non-small-cell lung, papillary thyroid, and bone
cancer cells by inducing apoptosis (16-18)
in vitro. It also suppresses bone cancer cell growth in
vivo (18). Additionally,
curcumin can inhibit oral cancer cell survival by inducing
autophagy (19). Furthermore,
paclitaxel, an anti-microtubule agent extracted from Taxus
brevifolia, is used clinically to treat multiple cancers,
including ovarian, lung, and breast (20).
Apoptosis is a programmed cell death characterized
by specific biological features, such as plasma membrane blebbing,
apoptotic body formation, cytokeratin 18 fragment release, DNA
fragmentation and poly ADP-ribose polymerase (PARP) cleavage
(21-23).
In addition, caspases, a family of endoproteases, are divided into
initiator and effector caspases (23); they are involved in executing
apoptosis. Upon activation, initiator caspases such as caspase-8
can activate effector caspases, such as caspase-3. This leads to
the cleavage of PARP, a DNA repair enzyme, ultimately contributing
to apoptosis (23).
Autophagy, a regulated self-digestion mechanism, is
characterized by formation of double-membraned vesicles called
autophagosomes that engulf cytoplasmic contents and fuse with
lysosomes for degradation (24).
The formation of autophagosomes is regulated by autophagy-related
proteins, such as Beclin 1, LC3, and Atg12-Atg5 conjugate (25,26).
Beclin 1 initiates autophagy, while LC3-II contributes to
autophagosome formation, a key feature of autophagy (25-27).
Additionally, a previous study showed that the knockdown of Beclin
1 or LC3 decreases autophagic activity (28), indicating that Beclin 1 and LC3
serve essential roles in activating autophagy.
Certain anticancer substances, such as cisplatin and
curcumin, can simultaneously induce apoptosis and autophagy in
bladder and gastric cancers, respectively (29,30).
Studies show that inhibiting autophagy can enhance cisplatin or
curcumin-induced apoptosis (29,30),
suggesting that autophagy may play a protective role in cancer cell
survival.
Oligonol, a polyphenolic compound found in lychee
fruit (31), has antioxidant and
anti-inflammatory properties and can alleviate sarcopenia (32-34).
Additionally, previous studies have suggested that oligonol may
have anticancer activity, as it induces apoptosis in breast and
ovarian cancer cells (35,36). Therefore, the present study
evaluated the potential anticancer effects of oligonol in NPC
cells.
Materials and methods
Materials
Oligonol was provided by Toong Yeuan Enterprise Co.,
Ltd. DMSO, 3-methyladenine (3-MA), LY294002 and MTT were purchased
from Sigma-Aldrich (Merck KGaA). Fetal bovine serum (FBS), PBS and
RPMI-1640 medium were purchased from HyClone (Cytiva).
Cell culture
NPC-TW01 cells were provided by Dr Chin-Hwa Tsai
from the National Taiwan University in Taipei, Taiwan. These cells
were established from moderately differentiated NPC tissue
(37). The second cell line,
NPC/HK1, was purchased from Quantum Biotechnology, Inc. and
established from well-differentiated NPC tissues (38). Both cell lines were cultured in
RPMI-1640 medium supplemented with 10% FBS at 37˚C in a 5%
CO2 incubator.
MTT assay
NPC cells were seeded into a 6-well plate at a
density of 3x105 cells/well for 24 h. Once the cells
reached 80% confluence, they were treated with oligonol at
concentrations of 3.125, 6.25, 12.5, 25, and 50 µg/ml for 48 h at
37˚C with 5% CO2. Cells not treated with oligonol were
used as the control. Supernatant was removed, and 2 ml MTT reagent
(0.5 mg/ml in PBS) was added to each well. After incubation at 37˚C
and 5% CO2 for 4 h, supernatant was removed, and 1 ml
DMSO was added to each well to dissolve the crystals. Next, 100 µl
DMSO lysate was transferred from each well to a 96-well plate.
ELISA reader (BMG LABTECH) measured the optical density at 570 nm.
All experiments were carried out ≥3 times independently.
ELISA
The concentration of cytokeratin 18 fragment levels
in the cell culture supernatant was measured using the SimpleStep
ELISA kit (cat. no. ab254515; Abcam) according to the
manufacturer's instructions. Each experiment was conducted
independently ≥3 times.
Immunoblotting assay
Extraction of total protein from cells and
immunoblotting were performed as described previously (29). Primary antibodies for detecting PARP
(cat. no. 9532; 1:1,000), cleaved PARP (cat. no. 9541; 1:1,000),
caspase-8 (cat. no. 9746; 1:1,000), cleaved caspase-8 (cat. no.
9429; 1:1,000), caspase-3 (cat. no. 9662; 1:1,000), cleaved
caspase-3 (cat. no. 9661; 1:1,000), Beclin 1 (cat. no. 3738;
1:1,000), LC3-I/II (cat. no. 4108; 1:1,000) and GAPDH (cat. no.
97166; 1:5,000) were purchased from Cell Signaling Technology, Inc.
Horseradish peroxidase-conjugated secondary antibodies, including
goat anti-rabbit (cat. no. 111-035-144; 1:5,000) and anti-mouse IgG
(cat. no. 111-035-146; 1:5,000), were purchased from Jackson
ImmunoResearch, Inc. Protein bands were detected using an enhanced
chemiluminescence detection kit (SuperSignal™ West Pico PLUS
Chemiluminescent Substrate; cat. no. 34580; Thermo Fisher
Scientific, Inc.). All experiments were conducted independently ≥3
times. The intensities of protein bands were quantified using
ImageJ software (version 1.43, National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean of ≥3 independent experimental repeats. The statistical
analysis was performed using SPSS software (version 17.0; SPSS,
Inc.). An unpaired t-test was used to compare significant
differences between two groups. One-way analysis of variance and
Tukey's post hoc test were used to compare significant differences
between >2 groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
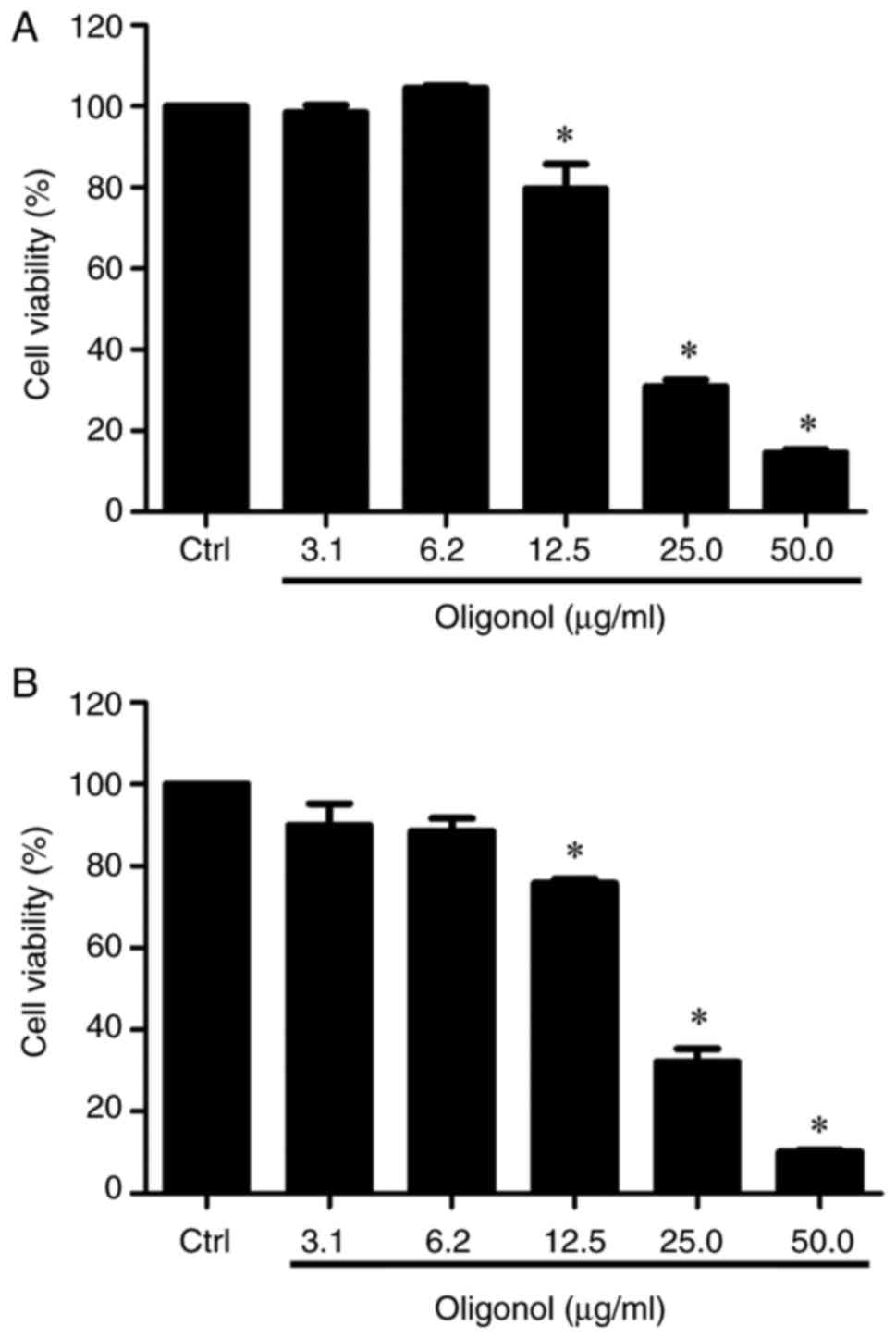
Oligonol inhibits viability of NPC
cells
NPC cell lines NPC-TW01 and NPC/HK1 were treated
with oligonol, and the cell viability was measured using an MTT
assay. Oligonol could significantly inhibit the viability of both
NPC cell lines at 12.5, 25.0 and 50.0 µg/ml compared with the
control (Fig. 1A and B). In addition, the half-maximal
inhibitory concentration of oligonol in both cell lines was 17
µg/ml.
Oligonol induces apoptotic effects in
NPC cells
Next, it was examined whether oligonol induces
apoptotic effects in NPC cells. NPC-TW01 and NPC/HK1 cells were
treated with 17 µg/ml oligonol. Immunoblotting assay was then
performed to detect protein expression of apoptotic markers, such
as cleaved caspase-8 and -3 and cleaved PARP (23) (Fig.
2A and F). Quantitative
evaluation of immunoblots demonstrated that in both cell lines,
oligonol significantly increased expression levels of cleaved
caspase-8 (Fig. 2B and G), cleaved caspase-3 (Fig. 2C and H) and cleaved PARP (Fig. 2D and I) compared with the control. In addition,
cytokeratin 18 is expressed in various types of epithelial cell
(22). During apoptosis, it is
cleaved by effector caspases and released from epithelial cells
(22). Therefore, cytokeratin 18
fragments serve as a biological marker of apoptosis (22). ELISA indicated that oligonol
significantly increased the release of cytokeratin 18 fragments
from NPC cells compared with the control (Fig. 2E and J), suggesting that oligonol could induce
apoptotic effects in NPC cells.
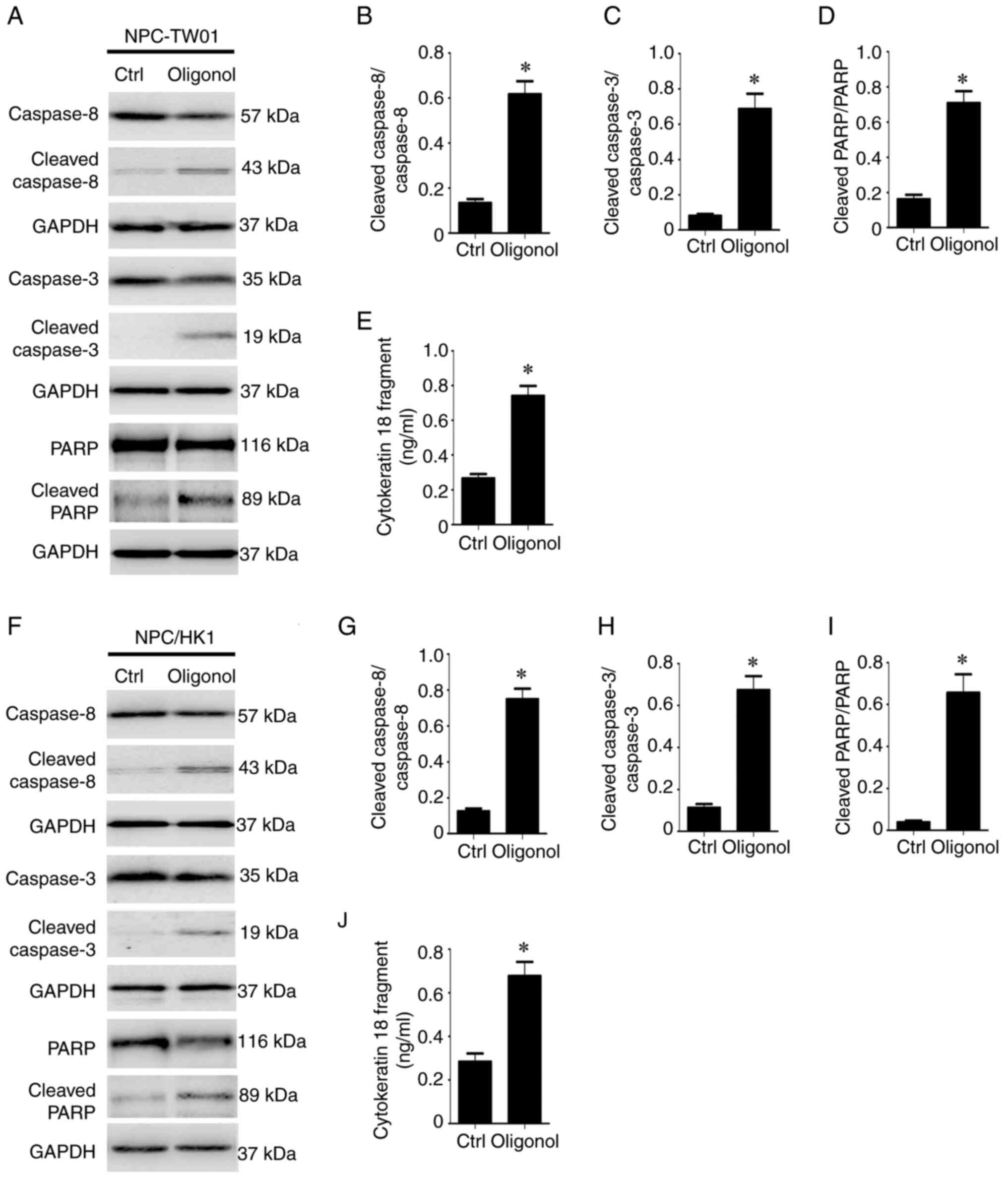 | Figure 2Oligonol induces apoptotic effects in
nasopharyngeal carcinoma cells. NPC-TW01 were treated with 17 µg/ml
oligonol for 48 h. (A) Protein expression of caspase-8, cleaved
caspase-8, caspase-3, cleaved caspase-3, PARP, cleaved PARP and
GAPDH was detected using an immunoblotting assay. The protein
expression levels of cleaved caspase-8 (B) cleaved caspase-3 (C)
and cleaved PARP (D) were quantified. (E) Concentration of
cytokeratin 18 fragment in the cell culture supernatants was
measured by ELISA. NPC/HK1 cells were treated with 17 µg/ml
oligonol for 48 h. (F) Protein expression of caspase-8, cleaved
caspase-8, caspase-3, cleaved caspase-3, PARP, cleaved PARP and
GAPDH was detected using an immunoblotting assay. The protein
expression levels of cleaved caspase-8 (G) cleaved caspase-3 (H)
and cleaved PARP (I) were quantified. (J) Concentration of
cytokeratin 18 fragment in the cell culture supernatants was
measured by ELISA. *P<0.05 vs. Ctrl. Ctrl,
control. |
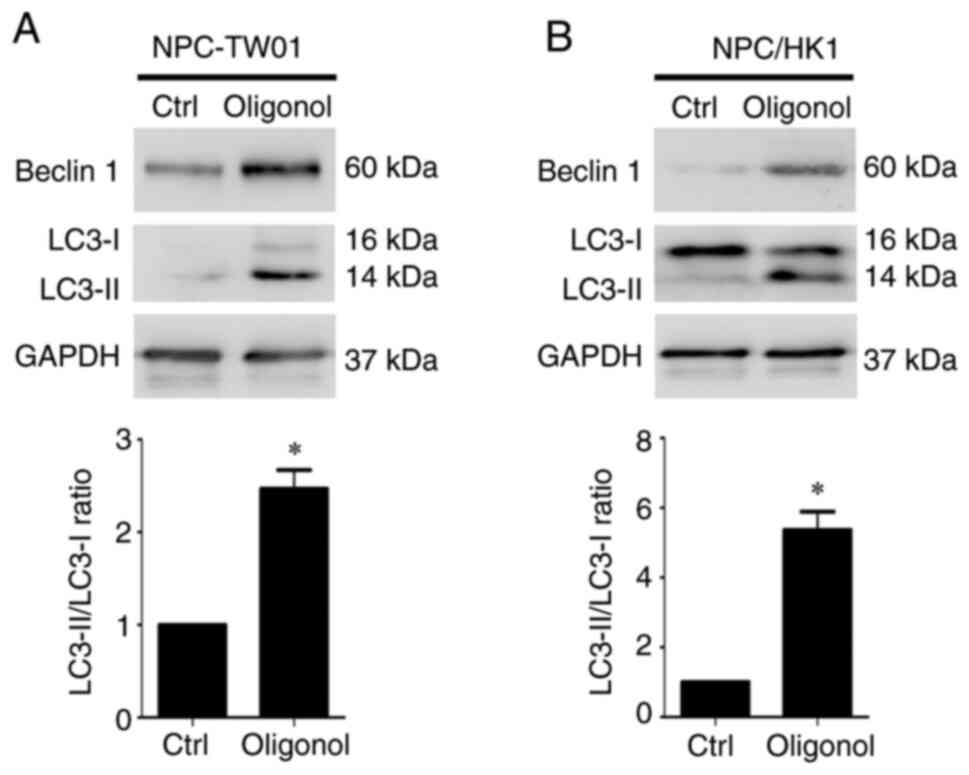
Oligonol induces autophagic effects in
NPC cells
As some natural extracts can simultaneously induce
apoptosis and autophagy in cancer cells (39,40),
it was investigated whether oligonol triggers autophagy in NPC
cells. Beclin 1 and LC3-II were used as the autophagy markers in
this study. Oligonol significantly induced protein expression of
Beclin 1 and LC3-II (Fig. 3A and
B). The conversion of LC3-I to
LC3-II is a reliable marker for autophagy activation (41). LC3-II/LC3-I ratio was significantly
increased by oligonol treatment compared with the control in both
NPC cell lines (Fig. 3A and
B), suggesting that oligonol also
induced autophagic effects in NPC cells.
Inhibition of autophagic effects
promotes oligonol-induced viability inhibition and apoptotic
effects in NPC cells
Because autophagy contributes to cancer cell
survival or death (42-44),
autophagy inhibitors 3-MA and LY294002(45) were used to investigate the effect of
oligonol-induced autophagic effects on NPC cell viability.
Treatment with 3-MA or LY294002 increased the viability inhibition
induced by oligonol in NPC cells (Fig.
4A). Since previous studies indicate that blocking autophagy
enhances the induction of apoptotic effects (29,46),
the present study examined protein expression levels of LC3-II and
apoptotic markers (cleaved caspase-8 and -3 and cleaved PARP) in
NPC cells after combination treatment with oligonol and LY294002.
LY294002 could suppress the oligonol-induced LC3-II expression
(Fig. 4B) and increase in
LC3-II/LC3-I ratio (Fig. 4C),
indicating that the autophagy inhibitor, LY294002, inhibited
oligonol-induced autophagy activation. Cotreatment of oligonol with
LY294002 could significantly enhance the protein expression of
cleaved caspase-8 (Fig. 4D),
cleaved caspase-3 (Fig. 4E) and
cleaved PARP (Fig. 4F) compared
with oligonol alone. Additionally, cotreatment of oligonol with
LY294002 significantly increased release of cytokeratin 18
fragments, another apoptotic marker, compared with oligonol alone
(Fig. 4G). These findings suggested
that inhibition of oligonol-induced autophagic effects by autophagy
inhibitors enhanced viability inhibition and apoptotic effects in
NPC cells.
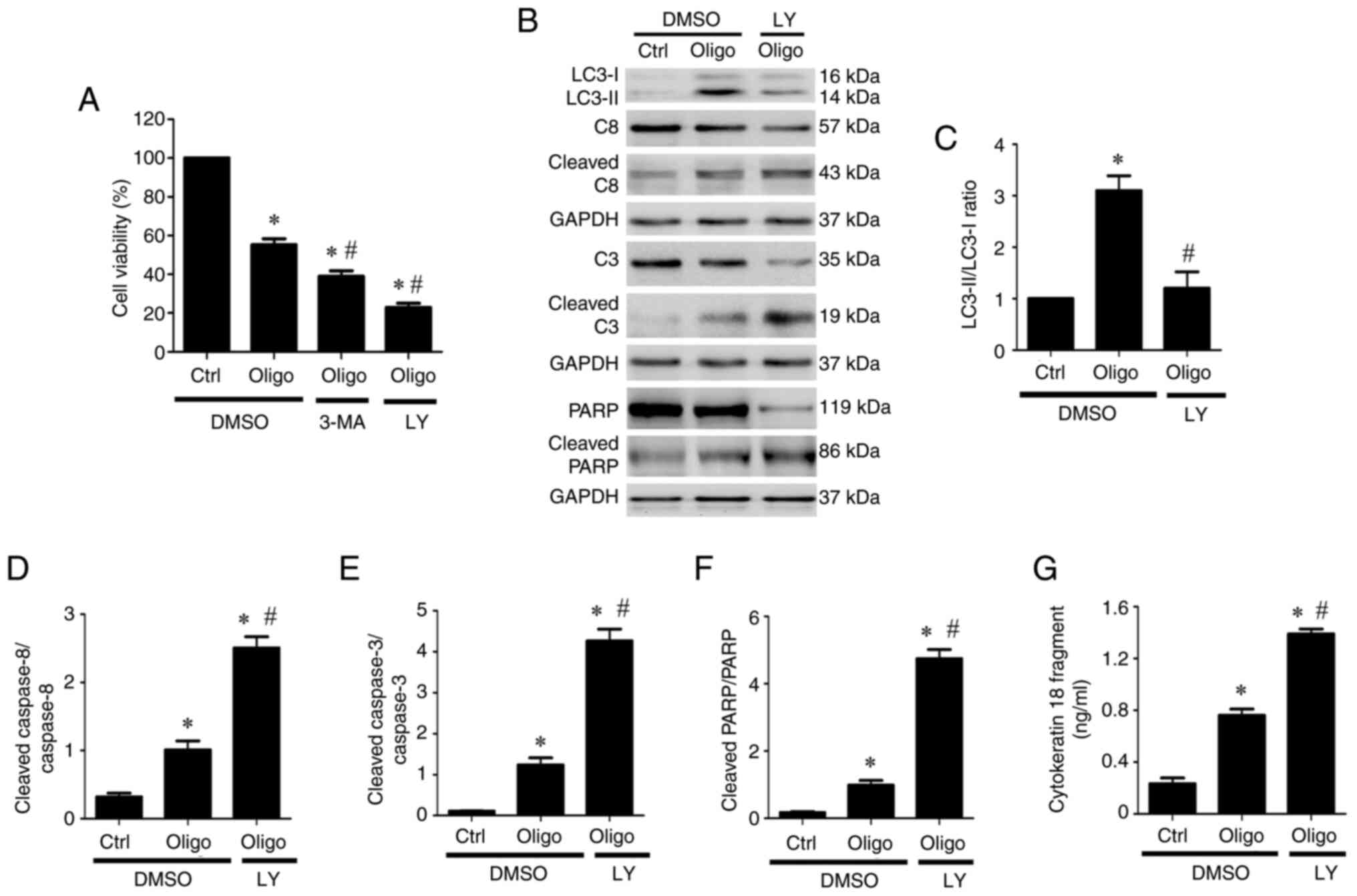 | Figure 4Inhibition of autophagic effects
promotes oligonol-induced viability inhibition and apoptotic
effects in nasopharyngeal cells. (A) NPC-TW01 cells were treated
with 17 µg/ml Oligo and DMSO, 3-MA or LY. Cell viability was
measured using MTT assay. (B) NPC-TW01 cells were treated with 17
µg/ml Oligo and DMSO or LY. Protein expression of LC3-I, LC3-II,
C8, cleaved C8, C3, cleaved C3, PARP, cleaved PARP and GAPDH was
examined by an immunoblotting assay. (C) Quantitative analysis of
LC3-II levels normalized using LC3-I. The protein expression levels
of cleaved C (D) 8 and (E) 3 and (F) cleaved PARP were quantified
using ImageJ software. (G) Concentration of cytokeratin 18 fragment
in cell culture supernatant was measured by ELISA.
*P<0.05 vs. Ctrl + DMSO; #P<0.05 vs.
oligo + DMSO. Ctrl, control; oligo, oligonol; 3-MA,
3-Methyladenine; LY, LY294002; C, caspase. |
Discussion
Plant extracts have been reported to serve a key
role in inhibiting the survival of cancer cells (12-15).
Oligonol is a polyphenolic compound primarily extracted from lychee
fruit that suppresses cancer cell viability (35,36).
However, whether it has anticancer activity in NPC cells is still
unknown. Here, oligonol effectively inhibited the viability of NPC
cell lines NPC-TW01 and NPC/HK-1, which suggested that oligonol
exerted anticancer effects in NPC cells.
As oligonol may induce apoptosis in cancer cells
(35,36), the present study investigated its
effects on NPC cells. Oligonol could stimulate the expression of
apoptosis markers, including cleaved caspase-8 and -3 and cleaved
PARP and induce the release of cytokeratin 18 fragments, suggesting
that oligonol has the ability to induce apoptotic effects in NPC
cells. Since caspase-8 can be cleaved (activated) through the
apoptotic death receptor pathway (23), oligonol may also have the ability to
activate the death receptor pathway. Previous studies have
indicated that inhibiting Akt or inducing AMP-activated protein
kinase (AMPK) activation promotes expression of Fas ligand (FasL)
(47,48), an inducer of the death receptor
pathway. As oligonol can inhibit Akt or promote AMPK activation
(49,50), oligonol may induce expression of
FasL by inhibiting Akt or inducing activation of AMPK in NPC cells.
This could initiate the death receptor pathway and ultimately
induce the activation of caspase-8. To determine if AMPK mediates
oligonol-induced FasL expression in NPC cells, an AMPK inhibitor
will be combined with oligonol to treat the NPC cells. The
expression of FasL will then be assessed using an immunoblotting
assay.
The present study examined whether oligonol induces
autophagy in NPC cells, as certain natural extracts have been
reported to simultaneously induce apoptosis and autophagy in cancer
cells (39,40). Oligonol induced autophagic effects
in NPC cells, as evidenced by increased expression of Beclin 1 and
LC3-II, two autophagy markers. According to previous studies,
inhibition of autophagy results in apoptosis in lung cancer cells
treated with cisplatin and endometrial cancer cells treated with
paclitaxel (29,44). The present study used two autophagy
inhibitors (3-MA and LY294002) to confirm the role of autophagy in
oligonol-induced viability inhibition and apoptotic effects.
Autophagy inhibitors enhanced oligonol-induced viability
inhibition. Expression of apoptosis markers, including cleaved
caspase-8 and -3 and cleaved PARP and the release of cytokeratin 18
fragments significantly increased following cotreatment of oligonol
with LY294002. These findings suggested that apoptotic effects were
promoted by inhibiting oligonol-induced autophagic effects, leading
to an increase in NPC cell viability inhibition. Therefore,
oligonol-induced autophagy may serve a cytoprotective role in NPC
cells.
It is unclear how the autophagic effects induced by
oligonol inhibit the apoptotic effects in NPC cells. Induction of
autophagy can cause mitochondria elongation (51,52),
which results in inhibition of cytochrome c release (51,52), a
pro-apoptotic factor associated with caspase-8 activation (51,53),
preventing apoptosis and maintaining cell viability (51). Additionally, autophagosome formation
proteins p62 and LC3-II interact with caspase-8, leading to its
degradation when the mature autophagosome fuses with the lysosome
(51,54). Therefore, oligonol-induced autophagy
may decrease expression of cleaved caspase-8, which can inhibit the
enhancement of apoptotic effects induced by oligonol in NPC cells.
Further experiments should confirm these possible mechanisms in the
future. For example, to investigate whether caspase-8 interacts
with p62 under oligonol treatment in the NPC cells, protein-protein
interaction techniques, such as co-immunoprecipitation and
fluorescence resonance energy transfer, will be utilized.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Science
and Technology Council (grant no. MOST-107-2320-B-471-001),
Pingtung Veterans General Hospital (grant nos. PTVGH-E-11305 and
PTVGH-11301) and I-Shou University (grant no. ISU111-S-06),
Taiwan.
Availability of data and materials
The datasets generated in the present study may be
requested from the corresponding author.
Author's contributions
YTW, YCC and YYL designed the study. CHL, WCC, TJH
and SJC performed experiments. YTW collected and analyzed the data.
YTW and YYL wrote the manuscript. YCC and YYL revised the
manuscript. YTW and YYL confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jicman Stan D, Niculet E, Lungu M, Onisor
C, Rebegea L, Vesa D, Bezman L, Bujoreanu FC, Sarbu MI, Mihailov R,
et al: Nasopharyngeal carcinoma: A new synthesis of literature data
(Review). Exp Ther Med. 23(136)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bai R, Sun J, Xu Y, Sun Z and Zhao X:
Incidence and mortality trends of nasopharynx cancer from 1990 to
2019 in China: An age-period-cohort analysis. BMC Public Health.
22(1351)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yu H, Yin X, Mao Y, Chen M, Tang Q and Yan
S: The global burden of nasopharyngeal carcinoma from 2009 to 2019:
An observational study based on the Global Burden of Disease Study
2019. Eur Arch Otorhinolaryngol. 279:1519–1533. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Blanchard P, Lee A, Marguet S, Leclercq J,
Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al:
Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An
update of the MAC-NPC meta-analysis. Lancet Oncol. 16:645–655.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu J, Zhou Q, Pan Z, Wang Y, Hu L, Chen G,
Wang S and Lyu J: Development and validation of a nomogram for
predicting long-term overall survival in nasopharyngeal carcinoma:
A population-based study. Medicine (Baltimore).
99(e18974)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shrihastini V, Muthuramalingam P, Adarshan
S, Sujitha M, Chen JT, Shin H and Ramesh M: Plant derived bioactive
compounds, their anti-cancer effects and in silico approaches as an
alternative target treatment strategy for breast cancer: An updated
overview. Cancers (Basel). 13(6222)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Asma ST, Acaroz U, Imre K, Morar A, Shah
SRA, Hussain SZ, Arslan-Acaroz D, Demirbas H, Hajrulai-Musliu Z,
Istanbullugil FR, et al: Natural Products/Bioactive Compounds as a
Source of Anticancer Drugs. Cancers (Basel).
14(6203)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yuan M, Zhang G, Bai W, Han X, Li C and
Bian S: The role of bioactive compounds in natural products
extracted from plants in cancer treatment and their mechanisms
related to anticancer effects. Oxid Med Cell Longev.
2022(1429869)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Esmeeta A, Adhikary S, Dharshnaa V,
Swarnamughi P, Ummul Maqsummiya Z, Banerjee A, Pathak S and
Duttaroy AK: Plant-derived bioactive compounds in colon cancer
treatment: An updated review. Biomed Pharmacother.
153(113384)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Weelden G, Bobinski M, Okla K, van
Weelden WJ, Romano A and Pijnenborg JMA: Fucoidan structure and
activity in relation to anti-cancer mechanisms. Mar Drugs.
17(32)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Su NW, Wu SH, Chi CW, Tsai TH and Chen YJ:
Cordycepin, isolated from medicinal fungus Cordyceps sinensis,
enhances radiosensitivity of oral cancer associated with modulation
of DNA damage repair. Food Chem Toxicol. 124:400–410.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu JC, Zhou XP, Wang XA, Xu MD, Chen T,
Chen TY, Zhou PH and Zhang YQ: Cordycepin induces apoptosis and
G2/M Phase Arrest through the ERK pathways in esophageal cancer
cells. J Cancer. 10:2415–2424. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li SZ, Ren JW, Fei J, Zhang XD and Du RL:
Cordycepin induces Bax-dependent apoptosis in colorectal cancer
cells. Mol Med Rep. 19:901–908. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
P A, K A, L S, M M and K M: Anticancer
effect of fucoidan on cell proliferation, cell cycle progression,
genetic damage and apoptotic cell death in HepG2 cancer cells.
Toxicol Rep. 6:556–563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X, Sun L, Wei X, Lu H, Tan Y, Sun Z
and Jiang J: Antitumor effect and molecular mechanism of fucoidan
in NSCLC. BMC Complement Med Ther. 21(25)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Endo H, Inoue I, Masunaka K, Tanaka M and
Yano M: Curcumin induces apoptosis in lung cancer cells by 14-3-3
protein-mediated activation of Bad. Biosci Biotechnol Biochem.
84:2440–2447. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Khan AQ, Ahmed EI, Elareer N, Fathima H,
Prabhu KS, Siveen KS, Kulinski M, Azizi F, Dermime S, Ahmad A, et
al: Curcumin-Mediated apoptotic cell death in papillary thyroid
cancer and cancer stem-like cells through targeting of the
JAK/STAT3 signaling pathway. Int J Mol Sci. 21(438)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun Y, Liu L, Wang Y, He A, Hu H, Zhang J,
Han M and Huang Y: Curcumin inhibits the proliferation and invasion
of MG-63 cells through inactivation of the p-JAK2/p-STAT3 pathway.
Onco Targets Ther. 12:2011–2021. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH,
Ryu MH and Park HR: Curcumin-induced autophagy contributes to the
decreased survival of oral cancer cells. Arch Oral Biol.
57:1018–1025. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sharifi-Rad J, Quispe C, Patra JK, Singh
YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, et
al: Paclitaxel: Application in modern oncology and
nanomedicine-based cancer therapy. Oxid Med Cell Longev.
2021(3687700)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ulukaya E, Karaagac E, Ari F, Oral AY,
Adim SB, Tokullugil AH and Evrensel T: Chemotherapy increases
caspase-cleaved cytokeratin 18 in the serum of breast cancer
patients. Radiol Oncol. 45:116–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khandia R, Dadar M, Munjal A, Dhama K,
Karthik K, Tiwari R, Yatoo MI, Iqbal HMN, Singh KP, Joshi SK and
Chaicumpa W: A comprehensive review of autophagy and its various
roles in infectious, non-infectious, and lifestyle diseases:
Current knowledge and prospects for disease prevention, novel drug
design, and therapy. Cells. 8(674)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wirth M, Joachim J and Tooze SA:
Autophagosome formation-the role of ULK1 and Beclin1-PI3KC3
complexes in setting the stage. Semin Cancer Biol. 23:301–309.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang T, Yu J, Cheng S, Zhang Y, Zhou CH,
Qin J and Luo H: Research progress on the anticancer molecular
mechanism of targets regulating cell autophagy. Pharmacology.
108:224–237. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci
B, Xie Y, Carlson D, Rothermel BA, Sun Y, et al: Beclin-1-Dependent
autophagy protects the heart during sepsis. Circulation.
138:2247–2262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hamurcu Z, Delibasi N, Gecene S, Şener EF,
Dönmez-Altuntaş H, Özkul Y, Canatan H and Ozpolat B: Targeting LC3
and Beclin-1 autophagy genes suppresses proliferation, survival,
migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin
β1/Src signaling in triple negative breast cancer cells. J Cancer
Res Clin Oncol. 144:415–430. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen J, Zhang L, Zhou H, Wang W, Luo Y,
Yang H and Yi H: Inhibition of autophagy promotes cisplatin-induced
apoptotic cell death through Atg5 and Beclin 1 in A549 human lung
cancer cells. Mol Med Rep. 17:6859–6865. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li W, Zhou Y, Yang J, Li H, Zhang H and
Zheng P: Curcumin induces apoptotic cell death and protective
autophagy in human gastric cancer cells. Oncol Rep. 37:3459–3466.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fujii H, Sun B, Nishioka H, Hirose A and
Aruoma OI: Evaluation of the safety and toxicity of the
oligomerized polyphenol Oligonol. Food Chem Toxicol. 45:378–387.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sakurai T, Nishioka H, Fujii H, Nakano N,
Kizaki T, Radak Z, Izawa T, Haga S and Ohno H: Antioxidative
effects of a new lychee fruit-derived polyphenol mixture, oligonol,
converted into a low-molecular form in adipocytes. Biosci
Biotechnol Biochem. 72:463–476. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee C, Bak J, Yoon S and Moon JO:
Protective effect of oligonol on dimethylnitrosamine-induced liver
fibrosis in rats via the JNK/NF-κB and PI3K/Akt/Nrf2 signaling
pathways. Antioxidants (Basel). 10(366)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chang YC, Chen YT, Liu HW, Chan YC, Liu
MY, Hu SH, Tseng WT, Wu HL, Wang MF and Chang SJ: Oligonol
alleviates sarcopenia by regulation of signaling pathways involved
in protein turnover and mitochondrial quality. Mol Nutr Food Res.
63(e1801102)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jo EH, Lee SJ, Ahn NS, Park JS, Hwang JW,
Kim SH, Aruoma OI, Lee YS and Kang KS: Induction of apoptosis in
MCF-7 and MDA-MB-231 breast cancer cells by Oligonol is mediated by
Bcl-2 family regulation and MEK/ERK signaling. Eur J Cancer Prev.
16:342–347. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim M, Park WH, Lee S, Suh DH, Kim K, No
JH and Kim YB: Oligonol, a low molecular weight polyphenol,
enhances apoptotic cell death in ovarian cancer cells via
suppressing NF-κB activation. Nutr Cancer. 71:141–148.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aimjongjun S, Mahmud Z, Jiramongkol Y,
Alasiri G, Yao S, Yagüe E, Janvilisri T and Lam EW: Lapatinib
sensitivity in nasopharyngeal carcinoma is modulated by
SIRT2-mediated FOXO3 deacetylation. BMC Cancer.
19(1106)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang DP, Ho JH, Poon YF, Chew EC, Saw D,
Lui M, Li CL, Mak LS, Lai SH and Lau WH: Establishment of a cell
line (NPC/HK1) from a differentiated squamous carcinoma of the
nasopharynx. Int J Cancer. 26:127–132. 1980.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li Y, Li R, Zhu S, Zhou R, Wang L, DU J,
Wang Y, Zhou B and Mai L: Cordycepin induces apoptosis and
autophagy in human neuroblastoma SK-N-SH and BE(2)-M17 cells. Oncol
Lett. 9:2541–2547. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gong X, Jiang L, Li W, Liang Q and Li Z:
Curcumin induces apoptosis and autophagy inhuman renal cell
carcinoma cells via Akt/mTOR suppression. Bioengineered.
12:5017–5027. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fang S, Wan X, Zou X, Sun S, Hao X, Liang
C, Zhang Z, Zhang F, Sun B, Li H and Yu B: Arsenic trioxide induces
macrophage autophagy and atheroprotection by regulating
ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell
Death Dis. 12(88)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang CY, Tsai SW, Chien HH, Chen TY, Sheu
SY, So EC and Huang BM: Cordycepin inhibits human gestational
choriocarcinoma cell growth by disrupting centrosome homeostasis.
Drug Des Devel Ther. 14:2987–3000. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang Y, Ji F, Liu Y, He M, Zhang Z, Yang
J, Wang N, Zhong C, Jin Q, Ye X and Chen T: Cisplatin-induced
autophagy protects breast cancer cells from apoptosis by regulating
yes-associated protein. Oncol Rep. 38:3668–3676. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu S and Li X: Autophagy inhibition
enhances sensitivity of endometrial carcinoma cells to paclitaxel.
Int J Oncol. 46:2399–2408. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pasquier B: Autophagy inhibitors. Cell Mol
Life Sci. 73:985–1001. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8(e56679)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sayed D, He M, Hong C, Gao S, Rane S, Yang
Z and Abdellatif M: MicroRNA-21 is a downstream effector of AKT
that mediates its antiapoptotic effects via suppression of Fas
ligand. J Biol Chem. 285:20281–20290. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tsai MC, Chen CC, Tseng TH, Chang YC, Lin
YJ, Tsai IN, Wang CC and Wang CJ: Hibiscus anthocyanins extracts
induce apoptosis by activating AMP-Activated protein kinase in
human colorectal cancer cells. Nutrients. 15(3972)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Park JY, Kim Y, Im JA, You S and Lee H:
Inhibition of adipogenesis by oligonol through Akt-mTOR inhibition
in 3T3-L1 adipocytes. Evid Based Complement Alternat Med.
2014(895272)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Park SK, Seong RK, Kim JA, Son SJ, Kim Y,
Yokozawa T and Shin OS: Oligonol promotes anti-aging pathways via
modulation of SIRT1-AMPK-Autophagy pathway. Nutr Res Pract.
10:3–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bata N and Cosford NDP: Cell survival and
cell death at the intersection of autophagy and apoptosis:
Implications for current and future cancer therapeutics. ACS
Pharmacol Transl Sci. 4:1728–1746. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gomes LC, Di Benedetto G and Scorrano L:
During autophagy mitochondria elongate, are spared from degradation
and sustain cell viability. Nat Cell Biol. 13:589–598.
2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pan J, Xu G and Yeung SC: Cytochrome c
release is upstream to activation of caspase-9, caspase-8, and
caspase-3 in the enhanced apoptosis of anaplastic thyroid cancer
cells induced by manumycin and paclitaxel. J Clin Endocrinol Metab.
86:4731–4740. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hou W, Han J, Lu C, Goldstein LA and
Rabinowich H: Autophagic degradation of active caspase-8: A
crosstalk mechanism between autophagy and apoptosis. Autophagy.
6:891–900. 2010.PubMed/NCBI View Article : Google Scholar
|