Introduction
Osteoclasts that first resorb calcified bone and
osteoblasts that form new bone to maintain homeostasis retain the
bone mass. The processes to maintain adequate bone mass are known
as bone remodeling. Disrupted bone remodeling, such as bone
formation and resorption dysregulation, causes metabolic bone
diseases such as osteoporosis (1).
Osteoblasts secrete osteoprotegerin (OPG) which is a member of the
TNF receptor family, along with receptor activator of NF-κB (RANK)
(2). OPG binds to the RANK ligand
(RANKL) on osteoblasts as a decoy receptor, prevents RANKL-RANK
binding on osteoclasts, and inhibits osteoclast formation and bone
resorption (2). IL-6, a
pro-inflammatory cytokine, is a bone resorption factor as it
promotes osteoclast development (3). The role of IL-6 in mouse osteoblasts
is to promote the differentiation of embryonic fibroblasts into
osteoblasts and induce mineralization (4), whereas another study reveals that IL-6
suppresses bone formation in mouse osteoblastic MC3T3-E1 cells and
primary mouse osteoblasts (5).
Therefore, OPG and IL-6 generally serve an important role in bone
remodeling that involves both osteoblasts and osteoclasts.
Prostaglandin F2α (PGF2α) serves different roles in
bone metabolism by regulating intracellular signal transduction
pathways within osteoblasts (6).
PGF2α has traditionally been seen as a dominant bone resorbing
agent, but it is now recognized as a bone remodeling regulator
(7). Our previous studies revealed
that PGF2α increases IL-6 secretion through p44/p42 MAPK and p38
MAPK pathways in the osteoblast-like MC3T3-E1 cell line (8,9). In
addition, PGF2α increases OPG synthesis via p44/p42 and p38 MAPKs
and stress-activated protein kinase/JNK pathways in the MC3T3-E1
cell line (10). Therefore,
PGF2α-induced cellular responses in osteoblasts involves activating
three major MAP kinases, including p44/p42 and p38 MAPKs and
JNK.
Gallein is a Gβγ subunit and G protein (Gs)
signaling inhibitor (11). Gallein
has been found in mouse in vivo studies to inhibit Gs
signaling, suppressing progression of heart failure progression
(12). In addition, gallein blocks
the chemotaxis of human promyelocytic leukemia cells and primary
human neutrophils. Systemic gallein administration inhibits paw
edema and neutrophil infiltration in a carrageenan-induced mouse
paw edema model, indicating that targeting Gβγ regulation may be an
effective anti-inflammatory strategy (13). Therefore, regulating OPG and IL-6
may improve our understanding of the benefits of gallein on bone
health, but the association of gallein with OPG and IL-6 synthesis
in osteoblasts and the molecular mechanisms behind it remains to be
elucidated. The present study aimed to investigate the role of
gallein in PGF2α-induced OPG and IL-6 secretion as well as the
mechanism in vitro in osteoblasts.
Materials and methods
Materials
Calbiochem (Merck KGaA) and MilliporeSigma provided
gallein and fluorescein, respectively. R&D Systems, Inc.
supplied PGF2α and mouse OPG (cat. no. MOP00) and IL-6 (cat. no.
M6000B) ELISA kits. Cell Signaling Technology, Inc. provided
antibodies, including phosphorylated (p-)p44/p42 MAPK (cat. no.
9101), p44/p42 MAPK (cat. no. 9102), p-p38 MAPK (cat. no. 4511),
p38 MAPK (cat. no. 9212), p-JNK (cat. no. 4668), and JNK (cat. no.
9252). Santa Cruz Biotechnology, Inc. supplied
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (cat.
no. sc-25778). Peroxidase-labeled anti-rabbit IgG antibodies (cat.
no. 5220-0336; SeraCare Life Sciences, Inc.) and peroxidase-labeled
anti-mouse IgG antibodies (cat. no. 7076; Cell Signaling
Technology, Inc.) were used as secondary antibodies. Cytiva
provided an ECL western blotting detection system.
Cell culture
The osteoblast-like MC3T3-E1 cells are naturally
immortalized and calvaria-derived cell lines (14). Dr M Kumegawa (Department of
Dentistry, Graduate School of Dentistry, Meikai University, Sakado,
Japan) donated these cells. Cells were maintained in minimal
essential medium (α-MEM; MilliporeSigma) supplemented with 10%
fetal bovine serum (FBS; Thermo Fisher Scientific Inc. and
incubated at 37˚C and 5% CO2 (15). Cells were plated on 90-mm diameter
plates (2x105 cells/plate) and 35-mm diameter plates
(5x104 cells/plate) in α-MEM supplemented with 10% FBS.
The incubation medium was replaced with α-MEM supplemented with
0.3% FBS 5 days after seeding. Cells were used for experiments
after 48 h.
Measurement of OPG and IL-6
Cultured cells were treated with 10 mM of PGF2α or
vehicle in 1 ml of α-MEM supplemented with 0.3% FBS for 48 h at
37˚C. These cells were pretreated with 1, 3, and 10 µM gallein or
fluorescein for 60 min. The conditioned medium was collected at the
end of the incubation, and the OPG and IL-6 concentrations in the
medium were measured by mouse OPG and IL-6 ELISA kits following the
manufacturer's protocol (16).
Reverse transcription-quantitative
(RT-q) PCR
Equal counts of cells (5x104 cells/35-mm
dish) were seeded and cultured through identical process among all
the experiments and used for the experiments 1 week after seeding.
Cultured cells were pretreated with 10 µM of gallein for 60 min and
then stimulated with 10 µM of PGF2α in α-MEM supplemented with 0.3%
FBS for 3 h. TRIzol reagent (Thermo Fisher Scientific, Inc.) was
used to isolate total RNA that was reverse transcribed into cDNA
using the Omniscript Reverse Transcriptase kit (Qiagen, Inc.). A
Light Cycler system with Fast Start DNA Master SYBR Green I (Roche
Diagnostics) was used for RT-qPCR. RNA extraction, cDNA synthesis,
and qPCR performed according to the manufacturer's protocols.
Samples were subjected to the following PCR thermocycling
conditions: Initial denaturation at 95˚C for 10 min, followed by 40
cycles of denaturation at 95˚C for 1 sec, annealing at 60˚C for 5
sec and elongation at 72˚C for 7 sec. Predesigned primer sets for
mouse OPG (cat. no. MA026526) and IL-6 (cat. no. MA039013) were
obtained from Perfect Real-Time Supporting System (Takara Bio
Inc.). For the GAPDH primer, the GAPDH sequence from a previous
article was adopted (16) and
MilliporeSigma synthesized the GAPDH primer (Forward primer ID:
5852-065, Reverse primer ID: 5852-066) with the same sequence. The
primer sequences were as follows: OPG forward,
5'-CAATGGCTGGCTTGGTTTCATAG-3' and reverse,
5'-CTGAACCAGACATGACAGCTGGA-3'; IL-6 forward,
5'-CCACTTCACAAGTCGGAGGCTTA-3' and reverse,
5'-GCAAGTGCATCATCGTTGTTCATAC-3'; and GAPDH forward,
5'-AACGACCCCTTCATTGAC-3' and reverse, 5'-TCCACGACATACTCAGCAC-3'.
All measurements were analyzed using the 2-ΔΔCq method
(17). All experiments were
performed and analyzed in triplicate.
Western blot analysis
Cultured cells were preincubated with 10, 30, and 50
µM of gallein and stimulated with 10 µM of PGF2α in α-MEM
containing 0.3% FBS for the indicated periods. Cells were rinsed
with phosphate-buffered saline and lysed with lysis buffer that
contained 62.5 mM of Tris/HCl, 50 mM of dithiothreitol, 2% sodium
dodecyl sulfate (SDS), and 10% glycerol for western blot analysis.
Proteins were separated on a 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gel and transferred to a
polyvinylidene difluoride (PVDF) membrane, followed by western blot
analysis (18). Although the mass
of protein per lane was not quantified, the same number of cells
(2x105 cells/dish) was seeded in each dish. The days of
treatment, conditions, and lysis buffer dose were also the same for
each dish. Lysates (10 µl) were applied per lane of all SDS-PAGE
gels. The membranes were blocked with 5% fat-free dry milk in
Tris-buffered saline-Tween (TBS-T; 20 mM Tris-HCl, pH7.6, 137 mM
NaCl, 0.1% Tween20) at room temperature for 2 h before incubation
with primary antibodies. The membrane was then incubated with
primary antibodies (1:1,000) against p38 MAPK, p-p38 MAPK, p44/p42
MAPK, p-p44/p42 MAPK, JNK, p-JNK and GAPDH in 5% fat-free dry
milk-TBS-T at 4˚C overnight. The membrane was incubated with
secondary antibodies (1:1,000) in 5% fat-free dry milk-TBS-T at
room temperature for 1 h. The same cell lysate was used for
phosphorylation, total, and GAPDH in the present study. The
peroxidase activity on the PVDF sheet was visualized on X-ray film
using the ECL western blotting detection reagents (cat. no.
RPN2109, Cytiva).
Densitometric analysis
A scanner and image analysis software (ImageJ
version 1.48; National Institutes of Health) was used for
densitometric analysis of western blots. Phosphorylated protein
levels were calculated as follows: The background-subtracted signal
intensity of each phosphorylation signal was normalized to the
respective intensity of total protein and plotted as a fold
increase compared to unstimulated control cells.
Statistical analysis
Mini StatMate (version 2.01; ATMS) was used for all
data analysis. Analysis of variance followed by the Bonferroni
significance test was used for multiple comparisons between the two
groups. All measurements were conducted and analyzed in triplicate
from dependent cell preparations. Data were expressed as mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Gallein, but not fluorescein,
increases PGF2α-induced OPG release using the MC3T3-E1 cell
line
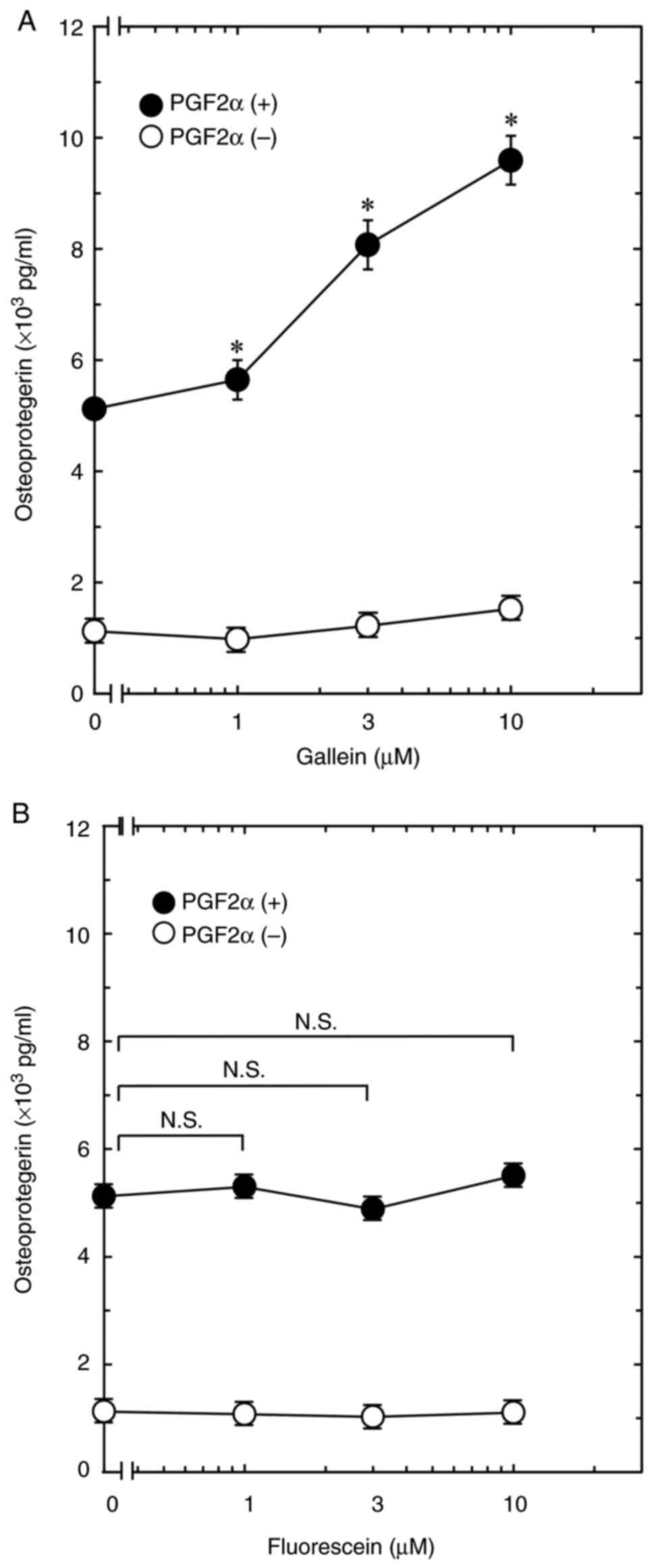
The present study first investigated the effect of
gallein on PGF2α-induced OPG secretion using an osteoblastic
MC3T3-E1 cell line. The result revealed that gallein did not affect
OPG release in MC3T3-E1 cells, but significantly increased
PGF2α-induced OPG secretion. The effect of gallein on OPG secretion
increased dose-dependently up to 10 µM (Fig. 1A). The association of fluorescein, a
gallein-like compound that does not bind Gβγ (13), with PGF2α-induced OPG secretion was
also investigated. The result indicated that fluorescein at
concentrations of 1-10 µM demonstrated no effect on OPG secretion
with or without PGF2α stimulation (Fig.
1B).
Gallein, but not fluorescein,
increases PGF2α-induced IL-6 release using MC3T3-E1 cell line
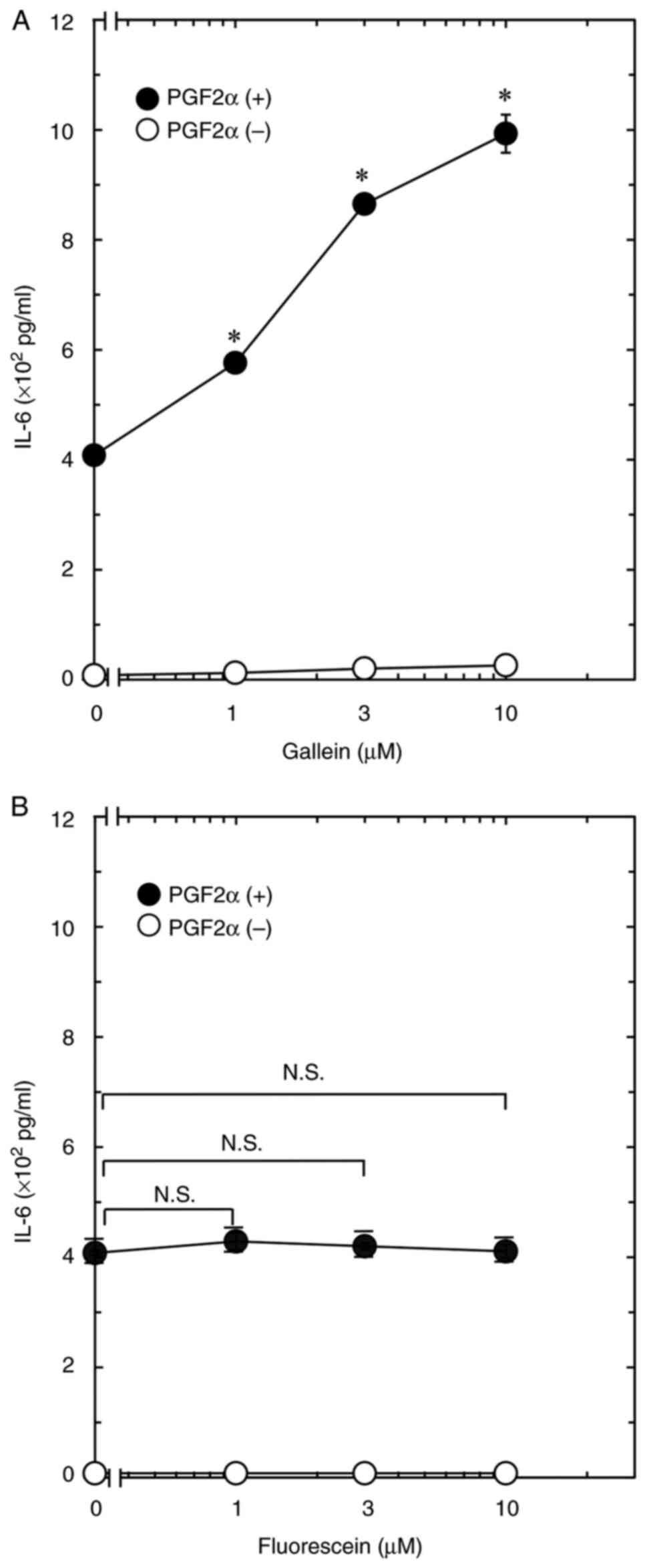
The present study next investigated the effect of
gallein on PGF2α-induced IL-6 secretion using the osteoblastic
MC3T3-E1 cell line. The result revealed that gallein did not affect
IL-6 release in the MC3T3-E1 cell line, but it significantly
increased PGF2α-induced IL-6 secretion. The effect of gallein on
IL-6 secretion increased dose-dependently at 1, 3, and 10 µM
(Fig. 2A). In addition, the
association of fluorescein with PGF2α-induced IL-6 secretion was
investigated. The result revealed that 1, 3, and 10 µM of
fluorescein did not affect IL-6 secretion with or without PGF2α
stimulation (Fig. 2B).
Gallein enhances PGF2α-induced OPG and
IL-6 mRNA expression using MC3T3-E1 cell line
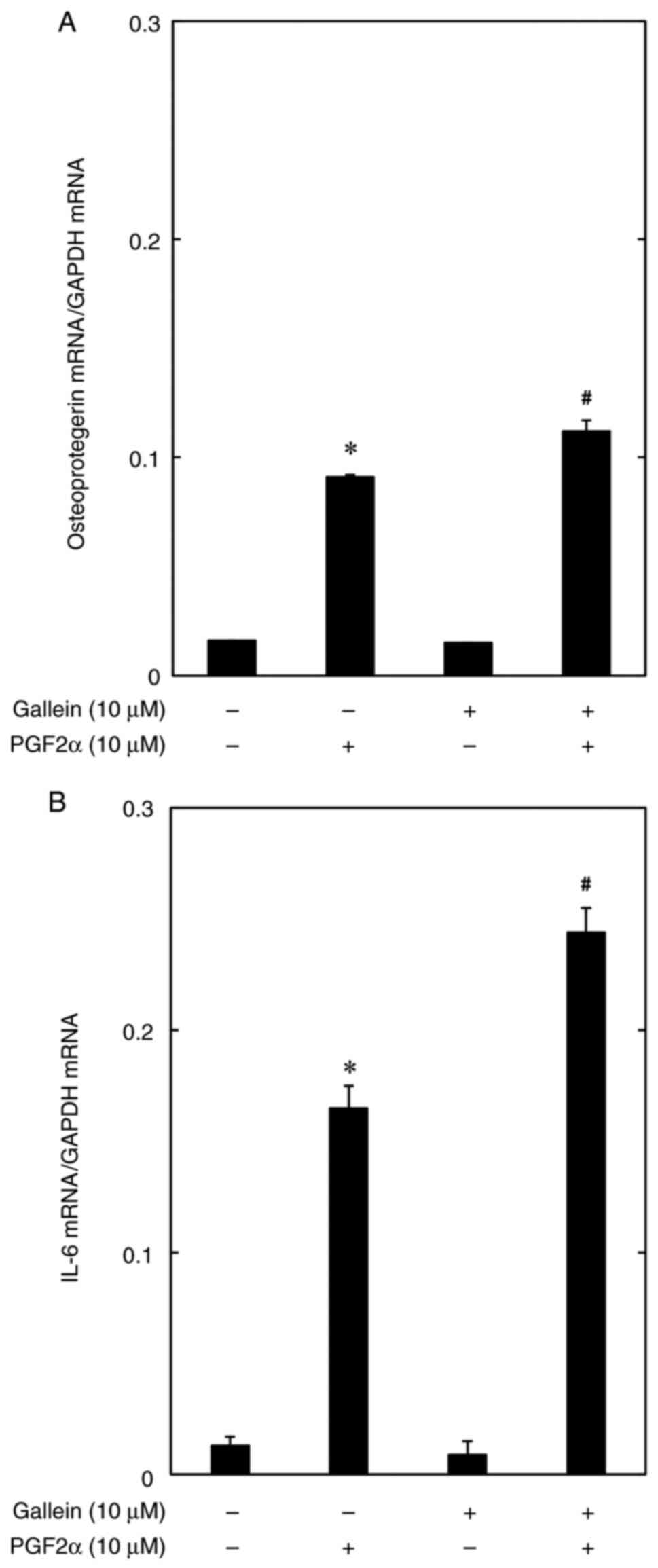
The aforementioned results reveal that gallein
improves OPG and IL-6 protein secretion; thus, the association of
gallein with PGF2α-induced OPG and IL-6 mRNA expression levels was
then investigated using the MC3T3-E1 cell line. The results
revealed that 10 µM of gallein significantly improved PGF2α-induced
OPG and IL-6 mRNA expression levels (Fig. 3).
Gallein does not affect
PGF2α-activated p44/p42 MAPK, p38 MAPK and JNK in the MC3T3-E1 cell
line
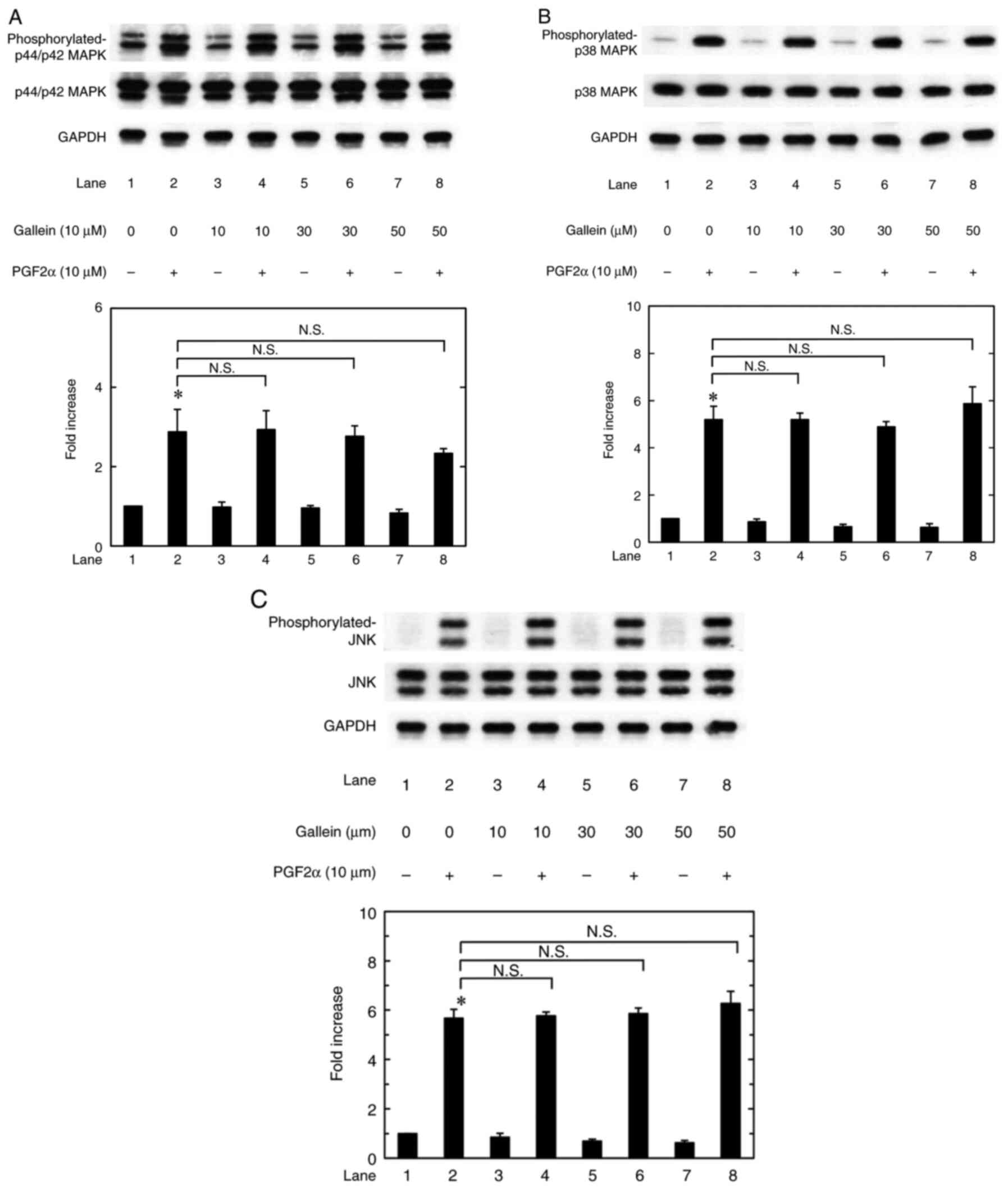
Regarding the signaling mechanism in osteoblasts,
the authors previously used osteoblast-like MC3T3-E1 cells to
demonstrate that PGF2α activates the phosphorylation of three major
MAPKs, including p44/p42 and p38 MAPKs and JNK (10). The present study investigated how
gallein affected p44/p42 and p38 MAPKs and JNK phosphorylation in
these cells with or without PGF2α stimulation. The result was that
gallein alone at 10, 30, and 50 µM did not affect p44/p42 and p38
MAPKs and JNK phosphorylation as well as PGF2α-activated p44/p42
and p38 MAPKs and JNK phosphorylation (Fig. 4).
Discussion
The present study revealed that gallein increased
PGF2α-induced OPG and IL-6 secretion in osteoblast-like MC3T3-E1
cells. In addition, gallein improved the mRNA expression levels of
PGF2α-induced OPG and IL-6. Therefore, gallein probably upregulated
PGF2α-induced OPG and IL-6 secretion and the improving effect of
gallein on PGF2α-induced OPG and IL-6 secretion may be mediated
through transcriptional events. We have previously shown that PGF2α
activates the phosphorylation of three major MAPKs, such as p44/p42
and p38 MAPKs and JNK, in osteoblast-like MC3T3-E1 cells regarding
intracellular signal transduction by PGF2α in osteoblasts (10). In addition, we revealed that PGF2α
increases OPG secretion by activating these three major MAPKs
(10). In addition, PGF2α induces
IL-6 secretion by activating p44/p42 and p38 MAPKs in these cells
(8,9). The present study showed that gallein
did not affect PGF2α-induced p44/p42 and p38 MAPKs and JNK
phosphorylation. Therefore, the intracellular signaling pathway may
not activate the three major MAPKs although gallein amplifies
PGF2α-induced OPG and IL-6 secretion in osteoblast-like MC3T3-E1
cells. To the best of the authors' knowledge, this is the first
study revealing the active involvement of gallein in PGF2α-induced
OPG and IL-6 secretion in osteoblasts.
Heterotrimeric G proteins are membrane-bound G
proteins that form heterotrimeric complexes. The heterotrimeric
protein directly binds to seven transmembrane G protein-coupled
receptors. Heterotrimeric proteins are composed of Gα, Gβ, and Gγ
subunits. The subunit binds either GTP or GDP and acts as an on/off
switch for G protein activation. A ligand that binds to a GPCR
catalyzes the exchange of GDP and GTP on the Gα subunit and is
released from the Gβγ subunit. Gα and Gβγ subunit dissociation
enables each subunit to bind directly to downstream signaling
proteins (19). Gallein generally
inhibits Gβγ signaling without affecting GPCR-dependent Gα
activation (20). The present study
used fluorescein, which has a structure similar to gallein but does
not bind to Gβγ (13), as a control
to investigate the association of Gβγ inhibition with the effects
of gallein. Fluorescein did not affect PGF2α-induced OPG and IL-6
secretion in osteoblast-like MC3T3-E1 cells at either the protein
or mRNA levels. Therefore, fluorescein did not inhibit Gβγ
signaling and was not involved in PGF2α-induced OPG and IL-6
secretion despite being a gallein-like compound. Therefore, the
amplifying effect of gallein on PGF2α-induced OPG and IL-6
secretion is mediated by its Gβγ inhibition in osteoblasts.
PGF2α regulates various physiological functions by
interacting with and activating the PGF2α receptor (FP), which
belongs to the G protein-coupled receptor family. Generally, PG
receptors activate different G protein subtypes, among which FP is
primarily coupled to the Gα subunit. Gαβγ subunits dissociate into
Gα and Gβγ components and cell signaling pathways are exerted when
PGF2α binds to FP (21). Therefore,
the present results indicated that gallein may act on FP, which has
a GPCR structure, and inhibit the Gβγ subunit activation, thereby
increasing PGF2α-induced OPG and IL-6 secretion in osteoblasts.
OPG binds to RANKL and inhibits RANK activation in
bone metabolism, thereby preventing bone resorption through
osteoclast differentiation and activation (2). The present study revealed that gallein
upregulates PGF2α-induced OPG secretion in osteoblasts. Therefore,
the positive result that gallein increases OPG secretion may
indicate that gallein suppresses bone resorption by osteoclasts by
regulating the RANK/RANKL/OPG axis. Conversely, the
pro-inflammatory cytokine, IL-6, is generally a bone resorption
factor. IL-6 can stimulate osteoclast formation in bone resorption
in conditions such as estrogen deficiency (3). However, IL-6 exhibits a positive
effect on bone formation and is considered an osteotropic factor
(22). Furthermore, osteoclast bone
resorption, followed by bone formation by osteoblasts, triggers
bone resorption in bone remodeling (1). PGF2α generally serves an important
role as a bone remodeling regulator (7). Considering these results, the findings
that gallein enhances IL-6 are not necessarily inconsistent with
the result that gallein promotes OPG. We have recently revealed
that oncostatin M, which serves an important role in fracture
healing, promotes PGD2-induced OPG and IL-6 secretion using the
osteoblastic MC3T3-E1 cell line (23). The amplifying effect of gallein on
OPG and IL-6 secretion may be an important discovery from the
perspective of the treatment of metabolic bone diseases,
considering that oncostatin M has favorable effects on bone
metabolism.
In conclusion, the results of the present study
revealed that gallein increased PGF2α-induced OPG and IL-6
secretion in osteoblasts, indicating that gallein may regulate bone
remodeling through OPG/IL-6 in bone metabolism.
Acknowledgements
The authors thank Ms. Yumiko Kurokawa, Department of
Pharmacology, Gifu University, for her skillful technical
assistance.
Funding
Funding: The present study was supported by Grants-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science, and Technology (grant nos. 22K09438 and 19K18471)
and from the National Center for Geriatrics and Gerontology, Japan
(grant no. 20-12, 21-1).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GK was responsible for data curation, formal
analysis, funding acquisition, writing-original draft, and approval
of final manuscript. TH, RM-N and TO were responsible for data
curation, formal analysis, writing the original draft and approval
of final manuscript. OK was responsible for conceptualization,
project administration, supervision, writing, reviewing and
editing, and approval of final manuscript. HT was responsible for
conceptualization, funding acquisition, project administration,
supervision, writing, reviewing and editing, and approval of final
manuscript. GK and HT confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ono T, Hayashi M, Sasaki F and Nakashima
T: RANKL biology: Bone metabolism, the immune system, and beyond.
Inflamm Regen. 40(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jilka RL, Hangoc G, Girasole G, Passeri G,
Williams DC, Abrams JS, Boyce B, Broxmeyer H and Manolagas SC:
Increased osteoclast development after estrogen loss: Mediation by
interleukin-6. Science. 257:88–91. 1992.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Taguchi Y, Yamamoto M, Yamate T, Lin SC,
Mocharla H, DeTogni P, Nakayama N, Boyce BF, Abe E and Manolagas
SC: Interleukin-6-type cytokines stimulate mesenchymal progenitor
differentiation toward the osteoblastic lineage. Proc Assoc Am
Physicians. 110:559–574. 1998.PubMed/NCBI
|
|
5
|
Kaneshiro S, Ebina K, Shi K, Higuchi C,
Hirao M, Okamoto M, Koizumi K, Morimoto T, Yoshikawa H and
Hashimoto J: IL-6 negatively regulates osteoblast differentiation
through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J
Bone Miner Metab. 32:378–392. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hikiji H, Takato T, Shimizu T and Ishii S:
The roles of prostanoids, leukotrienes, and platelet-activating
factor in bone metabolism and disease. Prog Lipid Res. 47:107–392.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Agas D, Marchetti L, Hurley MM and
Sabbieti MG: Prostaglandin F2α: A bone remodeling mediator. J Cell
Physiol. 228:25–29. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tokuda H, Kozawa O, Harada A and Uematsu
T: p42/p44 mitogen-activated protein kinase activation is involved
in prostaglandin F2α-induced interleukin-6 synthesis in
osteoblasts. Cell Signal. 11:325–330. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Minamitani C, Otsuka T, Takai S,
Matsushima-Nishiwaki R, Adachi S, Hanai Y, Mizutani J, Tokuda H and
Kozawa O: Involvement of Rho-kinase in prostaglandin F2α-stimulated
interleukin-6 synthesis via p38 mitogen-activated protein kinase in
osteoblasts. Mol Cell Endocrinol. 291:27–32. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuroyanagi G, Tokuda H,
Matsushima-Nishiwaki R, Kondo A, Mizutani J, Kozawa O and Otsuka T:
Resveratrol suppresses prostaglandin F2α-induced osteoprotegerin
synthesis in osteoblasts: Inhibition of the MAP kinase signaling.
Arch Biochem Biophys. 542:39–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Campbell AP and Smrcka AV: Targeting G
protein-coupled receptor signalling by blocking G proteins. Nat Rev
Drug Discov. 17:789–803. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Casey LM, Pistner AR, Belmonte SL,
Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O,
Matavel A, Lopes CM, et al: Small molecule disruption of G beta
gamma signaling inhibits the progression of heart failure. Circ
Res. 107:532–539. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lehmann DM, Seneviratne AM and Smrcka AV:
Small molecule disruption of G protein beta gamma subunit signaling
inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol.
73:410–418. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a
new clonal osteogenic cell line derived from newborn mouse
calvaria. J Cell Biol. 96:191–198. 1983.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kozawa O, Suzuki A, Tokuda H and Uematsu
T: Prostaglandin F2α stimulates interleukin-6 synthesis via
activation of PKC in osteoblast-like cells. Am J Physiol.
272:E208–E211. 1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Hurowitz EH, Melnyk JM, Chen YJ,
Kouros-Mehr H, Simon MI and Shizuya H: Genomic characterization of
the human heterotrimeric G protein alpha, beta, and gamma subunit
genes. DNA Res. 7:111–120. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koch WJ, Hawes BE, Inglese J, Luttrell LM
and Lefkowitz RJ: Cellular expression of the carboxyl terminus of a
G protein-coupled receptor kinase attenuates G beta gamma-mediated
signaling. J Biol Chem. 269:6193–6197. 1994.PubMed/NCBI
|
|
21
|
Lv X, Gao K, Nie J, Zhang X, Zhang S, Ren
Y, Sun X, Li Q, Huang J, Liu L, et al: Structures of human
prostaglandin F2α receptor reveal the
mechanism of ligand and G protein selectivity. Nat Commun.
14(8136)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Franchimont N, Wertz S and Malaise M:
Interleukin-6: An osteotropic factor influencing bone formation?
Bone. 37:601–606. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kuroyanagi G, Hioki T, Tachi J,
Matsushima-Nishiwaki R, Iida H, Kozawa O and Tokuda H: Oncostatin M
stimulates prostaglandin D2-induced osteoprotegerin and
interleukin-6 synthesis in osteoblasts. Prostaglandins Leukot
Essent Fatty Acids. 192(102575)2023.PubMed/NCBI View Article : Google Scholar
|