Introduction
Mesenteric panniculitis (MP) is a rare idiopathic
condition characterised histologically by chronic inflammatory and
fibrosis of mesenteric adipose tissue (1-2). Ogden
et al (3) introduced the
term MP to encompass sclerosing mesenteritis and mesenteric
lipodystrophy. While these conditions share the same features, they
differ in the relative predominance of chronic inflammation or
fibrosis Pathological features predominant in inflammation are
termed MP, whereas those predominant in fibrosis are termed
sclerosing mesenteritis (1-2). A systematic review showed that MP
primarily affects middle-aged men, presenting most commonly with
abdominal pain (78.1%), followed by fever (26.0%), weight loss
(22.9%), diarrhoea (19.3%) and vomiting (18.2%) (1). Abdominal tenderness (38.0%) and masses
(34.4%) are observed on physical examination (1). MP diagnosis is difficult due to the
presentation of non-specific symptoms and unclear findings. Chylous
ascites has been reported in two case series of patients with MP,
demonstrating frequencies of 18.5% (minimal chylous ascites)
(3) and 14.1% (no information
regarding amount of ascites) (4).
To the best of our knowledge, few case reports describe massive
chylous ascites (5-7).
Similarly, chylous pleural effusion, a rare complication in
patients with MP, has only been documented in two reports, to the
best of our knowledge (8-9).
Protein-losing enteropathy (PLE) is characterised by
uncompensated loss of plasma protein in the intestines, leading to
panhypoproteinemia in the absence of liver or kidney dysfunction
(10,11). Studies have identified mucosal
damage and lymph drainage failure as primary mechanisms underlying
PLE, with several factors contributing to the development of these
mechanisms, including inflammatory conditions (such as Crohn's
disease and ulcerative colitis), cardiovascular disorders (such as
chronic congestive heart failure) and widespread abdominal
malignant neoplasms (such as malignant lymphoma and multiple
myeloma) (10-11). To the best of our knowledge,
however, only six cases of MP-associated PLE have been published
(12-17).
The present study reported a case of MP complicated
by massive chylous ascites, pleural effusion and PLE and reviewed
clinicopathological characteristics of these conditions.
Case report
Case presentation
A 56-year-old male presented to Osaka Medical and
Pharmaceutical University Hospital (Osaka, Japan, in May 2023 for
dyspnoea. The patient had no history of diabetes mellitus,
allergies, malignancy or thoracic/abdominal surgery, but showed a
significant smoking history of 10 cigarettes/day for 34 years. The
patient initially noted the development of systemic oedema,
transient abdominal pain and fever and weight loss (approximately
20 kg) in June 2018; consultation revealed hypoalbuminemia (1.6
g/dl, reference range: 4.1-5.1). The patient was subsequently
referred to Osaka Medical and Pharmaceutical University Hospital,
Osaka, Japan, in November 2018. Physical examination revealed
massive ascites, which was confirmed to be chylous on abdominal
paracentesis. Endoscopy of the small intestine and duodenum
revealed mucosal oedema. Histopathological specimens were fixed in
10% formalin at room temperature for 24 h, dehydrated in ethanol
and xylene at room temperature, and embedded in paraffin (60˚C).
The 4-micrometer sections were stained with haematoxylin and eosin
for 5 min each at room temperature. Histopathological study using
light microscope of duodenal mucosa showed lymphangiectasia of the
lamina propria (magnification, x400). Therefore, the patient was
diagnosed with PLE with massive chylous ascites. Prednisolone (20
mg/day for 3 years, per oral), albumin preparation (Albuminar 25%,
twice, per intravenous injection), and diuretic agents
(Spironolactone, 100 mg/day for 3 years, per oral)were
administered, resulting in the improvement of oedema. During the 3
years following initial presentation, the patient reported gradual
and uncontrolled fluid accumulation. A peritovenous shunt for
peritoneal fluid drainage into the internal jugular vein was placed
in December 2020, which stabilised symptoms.
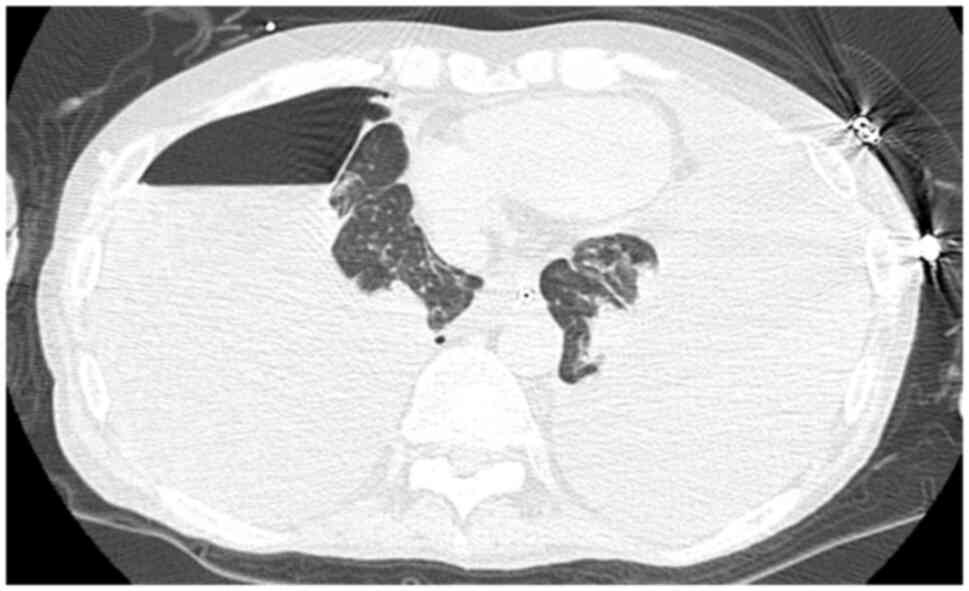
Computed tomography (CT) (Aquilion-ONE, Canon
Medical Systems, Japan) without contrast agent revealed
pneumothorax of the right lung, massive bilateral pleural effusion
and shrunken bilateral lungs (Fig.
1) in March 2023. Drainage of the right thoracic cavity was
attempted but the right lung failed to expand. Elevated levels of
white blood cells (13.63x103/µl; range:
3.3-8.6x103) and C-reactive protein (13.63 mg/dl, range:
<0.14), along with ground-glass opacity on CT, indicated
pulmonary infection, prompting the initiation of various antibiotic
regimens [Voriconazole (300 mg x 2/day), Ampicillin/Sulbactam (3 g
x 4/day), and Vancomycin (1,000 mg x 2/day) per intravenous
injection for three weeks (all)]. However, due to elevated liver
enzyme levels [aspartate aminotransferase, 1,467 U/l (reference
range 13-30) and alanine aminotransferase 829 U/l (reference range
10-42)], antibiotics were discontinued in April 2023. Respiratory
failure worsened due to increasing pleural effusion and persistent
lung collapse, leading to death in April 2023.
Autopsy findings
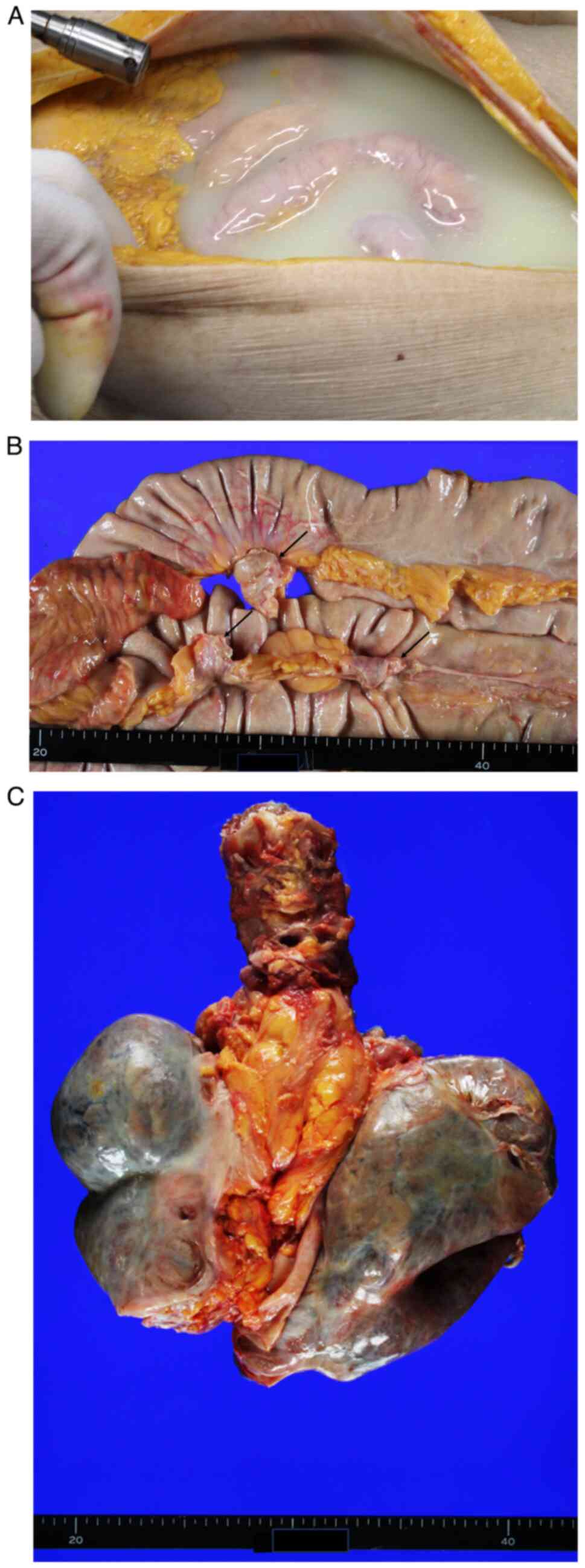
Upon opening the abdominal wall, massive chylous
ascites (3680 ml) was observed (Fig.
2A). Massive chylous pleural effusion in bilateral pleural
cavities (left, 1,650; right, 2,160 ml) was also present. The
mesentery of the small intestine was thickened, hardened and
appeared slightly whitish in colour (Fig. 2B). Similar thickening was observed
in the mesocolon. Calcified nodules, measuring 2-3 cm in diameter,
were present in the small bowel mesentery (Fig. 2B), with a few identified in the
mesocolon. Bilateral lungs were shrunken and their surfaces were
covered with dense, white fibrous tissue (Fig. 2C). Examination of the diaphragm
revealed no visible defects.
Histopathological and
immunohistochemical findings
Autopsy specimens were fixed in 10% formalin at room
temperature for 48 h, dehydrated in ethanol and xylene at room
temperature and embedded in paraffin (60˚C). The 4-micrometer
tissue sections were stained with haematoxylin and eosin for 5 min
each at room temperature. Immunohistochemical analyses were
performed using an autostainer (Leica Bond-III; Leica Biosystem
GmbH). The 4-micrometer tissue sections were incubated with mouse
monoclonal antibody against IgG4 (clone HP6025, cat. no. 605-900;
1:400 dilution, Thermo Fisher Scientific Inc.) and mouse monoclonal
antibody against podoplanin (clone D2-40, cat. No. M361901-2,
pre-diluted, Agilent Technologies Inc.) for 20 min at room
temperature. Secondary antibodies were pre-diluted and were used to
incubate the sections for 8 min at room temperature (Novolink Max
Polymer Detection System (cat. No. RE7140-L; Leica Biosystems
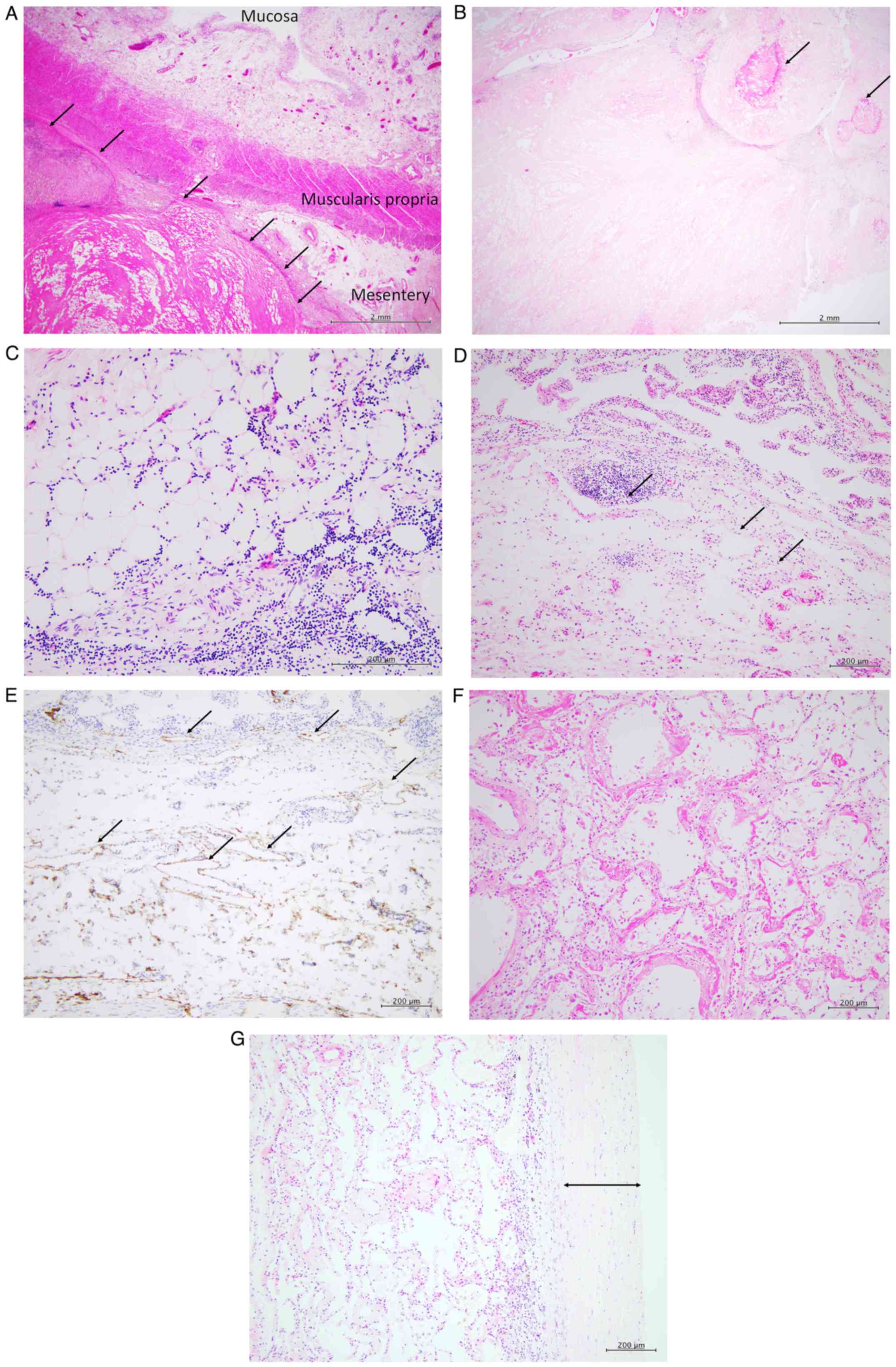
GmbH). Microscopic examination showed diffuse fat necrosis and
fibrosclerosis in the mesentery of the small intestine (Fig. 3A), with calcification within the
necrotic and fibrotic areas (Fig.
3B). Small lymphocytic infiltration without plasma cells was
observed within the mesenteric adipose tissue surrounding necrotic
areas (Fig. 3C).
Immunohistochemical staining showed no IgG4-positive plasma cells.
Collectively, these features supported the diagnosis of MP.
Examination of small intestine confirmed
lymphangiectasia in the lamina propria of the mucosa and submucosa
(Fig. 3D). This was confirmed by
positive podoplanin immunostaining, a marker for lymphatic
endothelial cells (18) (Fig. 3E).
Microscopic examination of bilateral lungs showed
diffuse alveolar damage, characterised by hyaline membrane
formation (Fig. 3F). Diffuse
fibrosclerotic thickening of the visceral pleura was observed,
corresponding to the gross findings of shrunken lungs covered with
dense, white fibrous tissue (Fig.
3G).
Discussion
To the best of our knowledge, the present study is
the first to report MP accompanied by massive chylous ascites,
pleural effusion and PLE. Although a premortem diagnosis of MP was
not made, histopathological examination during autopsy revealed
findings that were consistent with MP. Excessive fluid buildup and
protein loss were likely attributed to intestinal lymph drainage
obstruction caused by mesenteric inflammation, fibrosis and fat
necrosis in MP.
MP is an idiopathic condition characterised by
chronic inflammation and fibrosis of mesenteric adipose tissue
(1-2). MP is associated with infection,
autoimmune disease and abdominal trauma (1). Certain cases of MP result from
IgG4-associated diseases (19).
although most cases showed no such associations (20). Therefore, the absence of
IgG4-positive plasma cells in the present cased ruled out
differential diagnoses for IgG4-related diseases. Since MP is
predominant in middle-aged male patients presenting with
non-specific symptoms, such as abdominal pain, fever, weight loss
and diarrhoea (1), its diagnosis is
often difficult. Previous studies have reported a postmortem
diagnosis of MP, similar to the present case (9,14).
Imaging studies may be valuable diagnostic tools in visualizing
certain MP features. CT demonstrates hyperattenuating fat with
tumoural pseudocapsules and soft tissue nodules in the mesentery
(1,4,21).
Magnetic resonance imaging demonstrates an ill-defined,
hyperintense signal in mesenteric fat on T2-weighted imaging,
reflecting early-stage oedema, followed by a hypointense signal in
later stages (1). Calcification,
which is a potential consequence of fat necrosis, may be observed
in the mesentery (4,7,14), as
in the present case. Notably, the presence of calcification might
be a characteristic finding on CT scans for MP. Furthermore, while
small intestinal mesentery is the most commonly affected site
(1), lesions in both the small
intestine and colon are possible, as in the present case. The
treatment strategy for MP typically involves administration of
steroids, colchicine, tamoxifen and antibiotics, with over 70% of
patients showing symptom resolution (1,4,21).
However, non-responders have been documented (1,4,14,15),
with some developing fatal outcomes on follow-up (1,9,14).
Here, the patient succumbed to respiratory failure despite medical
treatment.
Massive chylous pleural effusion and PLE were rare
features in the present case. Table
I summarises clinicopathological characteristics of MP cases
with chylous pleural effusion or PLE, including the present case
(8,9,12-17).
To the best of our knowledge, only two prior cases of MP with
massive pleural effusion have been documented (8,9).
Kobayashi et al (9) reported
an autopsy-confirmed case of MP with relatively minimal chylous
ascites (200 ml) and massive pleural effusion (left, 1,300; right,
1,400 ml). Although the mechanism underlying chylous effusion in
bilateral thoracic cavities remains unclear, transfer from the
abdomen via a diaphragmatic defect is hypothesized (8,9).
Similarly, bilateral pleural effusion may be present in patients
with hepatic hydrothorax and diaphragmatic defect (22,23).
In the present case, massive chylous ascites and pleural effusion
were observed. While no macroscopic diaphragmatic defect was
observed during autopsy, negative intrathoracic pressure may have
facilitated transfer of chylous fluid from the abdomen to the
pleural cavity. Additionally, autopsy revealed shrunken lungs
covered with dense, white fibrous tissue. This feature resembles
‘trapped lung’, which is a consequence of dysfunctional healing
following pleural injury (24).
This condition progresses from lung entrapment to formation of a
visceral pleural peel, persistent pleural effusion and inability of
the lung to expand (24). These
changes are marked by fibrosis and scarring of visceral pleura,
resulting in a peel-like appearance and trapped lung (24). While infection is the most common
cause, pneumothorax, haemothorax, thoracic intervention and
rheumatoid pleuritis can also lead to trapped lungs (24). Moreover, Owen et al (25) reported complications of trapped
lungs and chylothorax in a patient with long-standing cirrhosis due
to hepatitis C. Considering the aforementioned findings, the
persistent massive chylous pleural effusion in the present case may
have contributed to the development of trapped lungs.
 | Table IClinicopathological features of
mesenteric panniculitis with chylous pleural effusion and/or
protein-losing enteropathy. |
Table I
Clinicopathological features of
mesenteric panniculitis with chylous pleural effusion and/or
protein-losing enteropathy.
| Patient no. | Age | Sex | Symptoms | Ascites | Pleural effusion | Protein-losing
enteropathy | Treatment | Outcome | First author,
year | (Refs.) |
|---|
| 1 | 81 | Male | Dyspnea, fatigue,
abdominal pain, diarrhea | Yes | Yes | No | Pleurodesis | Lost to
follow-up | Rice et al,
2010 | (8) |
| 2 | 81 | Female | Edema, fatigue,
dyspnea | Yes | Yes | No | Azosemide (60
mg/day), heparin (10,000 IU/day) | Died of respiratory
failure | Kobayashi et
al, 2018 | (9) |
| 3 | 54 | Male | Diarrhea | No | No | Yes | Prednisolone | Improved | Höring | (12) |
| 4 | 68 | Male | Hypoalbuminemia,
edema | Yes | No | Yes | Prednisolone 60
mg/day for six weeks and tapered to10 mg/day), Colchicine (0.6
mg/day), Azathioprine (from 100 mg/day to 200 mg/day),
Cyclophosphamide and thalidomide (no detailed information were
available) | No improvement of
serum albumin | Rajendran and
Duerksen. 2006 | (13) |
| 5 | 77 | Male | Abdominal distention,
diarrhea | Yes | No | Yes | Albumin
preparation | Died | Kida et al.
2011 | (14) |
| 6 | 28 | Male | Abdominal pain | No | No | Yes | Prednisolone (30
mg/day) Colchicine and Azathioprine | No improvement of
symptom | Endo et al.
2014 | (15) |
| 7 | 59 | Male | Abdominal pain,
vomiting, diarrhea | No | No | Yes | Prednisolone (60
mg/day) and tamoxifen (20 mg/day) | Died of ischemic
ileo-colitis | Rispo et al,
2015 | (16) |
| 8 | 75 | Male | Abdominal
distention | Yes | No | Yes | Prednisolone (40
mg/day) | No improvement | Saito et al.
2017 | (17) |
| Present | 56 | Male | Edema, dyspnea | Yes | Yes | Yes | Prednisolone (20
mg/day per oral), Albumin preparation (Albuminar 25%, twice,
perintravenous injection),and Spironolactone (100 mg/day for 3
years, per oral), Voriconazole (300 mg x 2/day per intravenous
injection for 3 weeks), Ampicillin/Sulbactam (3 g x 4/day, per
intravenous injection for 3 weeks), and Vancomycin (1,000 mg x
2/day, per intravenous injection for 3 weeks) | Died of respiratory
failure | Nakazawa et
al, 2024 | - |
Despite its rare occurrence, PLE can be a symptom of
MP (Table I). Chylous ascites have
been noted in four of seven patients with MP and PLE including the
present patient (13,14,17).
Among MP patients with PLE, only one showed symptom improvement
with steroid therapy (12). In the
present case, the patient initially showed improvement of oedema,
although worsening ascites and pleural effusion were observed. The
occurrence of PLE in MP is likely due to lymphangiectasia of the
small intestinal mucosa caused by lymph drainage obstruction
(14). Autopsy findings in the
present case suggested MP as the underlying cause of PLE. Although
rare, PLE may be recognised as a possible complication of MP.
Examination including imaging studies and histological examination
of the affected mesentery are therefore warranted for diagnosis of
MP.
To the best of our knowledge, the present study is
the first to describe MP with massive ascites, pleural effusion and
PLE, highlighting the potential of MP changes in obstructing lymph
drainage in the mesentery. Despite its rarity and non-specific
symptoms, MP should be included as a differential diagnosis in
patients presenting with the aforementioned features.
Acknowledgements
The authors would like to thank Ms Shizuka Ono, Mr
Yusuke Ohnishi and Mr Naoto Kohno (all Department of Pathology,
Osaka Medical and Pharmaceutical University, Osaka, Japan) for
technical assistance.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and tables of this article.
Authors' contributions
MI conceived and designed the study. YN, MI, KS, FS,
TS and YH interpreted clinical findings. YN, MI, KS, FS, TS and YH
confirm the authenticity of all the raw data. YN and MI wrote the
manuscript. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The patient's family provided written informed
consent for participation.
Patient consent for publication
Written informed consent for publication was
obtained from the patient's family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma P, Yadav S, Needham CM and
Feuerstadt P: Sclerosing mesenteritis: A systematic review of 192
cases. Clin J Gastroenterol. 10:103–111. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Emory TS, Monihan JM, Carr NJ and Sobin
LH: Sclerosing mesenteritis, mesenteric panniculitis and mesenteric
lipodystrophy: A single entity? Am J Surg Pathol. 21:392–398.
1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ogden WW II, Bradburn DM and Rives JD:
Mesenteric panniculitis: Review of 27 cases. Ann Surg. 161:864–875.
1965.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akram S, Pardi DS, Schaffner JA and Smyrk
TC: Sclerosing mesenteritis: Clinical features, treatment, and
outcome in ninety-two patients. Clin Gastroenterol Hepatol.
5:589–596. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arora M and Dubin E: A clinical case
study: Sclerosing mesenteritis presenting as chylous ascites.
Medscape J Med. 10(30)2008.PubMed/NCBI
|
|
6
|
Ehrenpreis ED, Boiskin I and Schaefer K:
Chylous ascites in a patient with mesenteric panniculits. J Clin
Gastroenterol. 42:327–328. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lim HW and Sultan KS: Sclerosing
mesenteritis causing chylous ascites and small bowel perforation.
Am J Case Rep. 18:696–699. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rice BL, Stoller JK and Heresi GA:
Transudative chylothorax associated with sclerosing mesenteritis.
Respir Care. 55:475–477. 2010.PubMed/NCBI
|
|
9
|
Kobayashi H, Notohara K, Otsuka T,
Kobayashi Y, Ujita M, Yoshioka Y, Suzuki N, Aoyagi R, Ohashi R and
Suzuki T: An autopsy case of mesenteric panniculitis with massive
pleural effusions. Am J Case Rep. 19:13–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ozen A and Lenardo MJ: Protein-losing
enteropathy. N Engl J Med. 389:733–748. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Strober and Fuss IJ: Protein-losing
enteropathies. In: Mestecky J, Strober W, Russell MW, eds. Mucosal
Immunology. 4th ed. Academic Press, Boston, 1667-1694, 2015.
|
|
12
|
Höring E, Hingerl T, Hens K, von Gaisberg
U and Kieninger G: Protein-losing enteropathy: First manifestation
of sclerosing mesenteritis. Eur J Gastroenterol Hepatol. 7:481–483.
1995.PubMed/NCBI
|
|
13
|
Rajendran B and Duerksen DR: Retractile
mesenteritis presenting as protein-losing gastroenteropathy. Can J
Gastroenterol. 20:787–789. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kida T, Suzuki K, Matsuyama T, Okita M,
Isozaki Y, Matsumoto N, Miki S, Nagao Y, Kawabata K, Kohno M and
Oyamada H: Sclerosing mesenteritis presenting as protein-losing
enteropathy: A fatal case. Intern Med. 50:2845–2849.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Endo K, Moroi R, Sugimura M, Fujishima F,
Naitoh T, Tanaka N, Shiga H, Kakuta Y, Takahashi S, Kinouchi Y and
Shimosegawa T: Refractory sclerosing mesenteritis involving the
small intestinal mesentery: A case report and literature review.
Intern Med. 53:1419–1427. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rispo A, Sica M, Bucci L, Musto D, Camera
L, Ciancia G, Luglio G and Caporaso N: Protein-losing enteropathy
in sclerosing mesenteritis. Eur Rev Med Pharmacol Sci. 19:477–480.
2015.PubMed/NCBI
|
|
17
|
Saito Y, Hiramatsu K, Nosaka T, Ozaki Y,
Takahashi K, Naito T, Ofuji K, Matsuda H, Ohtani M, Nemoto T, et
al: A case of protein-losing enteropathy caused by sclerosing
mesenteritis diagnosed with capsule endoscopy and double-balloon
endoscopy. Clin J Gastroenterol. 10:351–356. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ordóñez NG: Podoplanin: A novel diagnostic
immunohistochemical marker. Adv Anat Pathol. 13:83–88.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Z, Jiao Y, He L, Wang H and Wang D: A
rare case report of immunoglobulin G4-related sclerosing
mesenteritis and review of the literature. Medicine (Baltimore).
99(e22579)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Avincsal MO, Otani K, Kanzawa M, Fujikura
K, Jimbo N, Morinaga Y, Hirose T, Itoh T and Zen Y: Sclerosing
mesenteritis: A real manifestation or histological mimic of
IgG4-related disease? Pathol Int. 66:158–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang H, Zhao Z, Cao Q and Ning J: A review
of 17 cases of mesenteric panniculitis in Zhengzhou ninth people's
hospital in China. BMC Gastroenterol. 24(48)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cardenas A, Kelleher T and Chopra S:
Review article: Hepatic hydrothorax. Aliment Pharmacol Ther.
20:271–279. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roussos A, Philippou N, Mantzaris GJ and
Gourgouliannis KI: Hepatic hydrothorax: Pathophysiology diagnosis
and management. J Gastroenterol Hepatol. 22:1388–1393.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huggins JT, Sahn SA, Heidecker J, Ravenel
JG and Doelken P: Characteristics of trapped lung: Pleural fluid
analysis, manometry, and air-contrast chest CT. Chest. 131:206–213.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Owen SC, Bersabe DR, Skabelund AJ, McCann
ET and Morris MJ: Transudative chylothorax from cirrhosis
complicated by lung entrapment. Respir Med Case Rep.
28(100243)2016.PubMed/NCBI View Article : Google Scholar
|