1. Introduction
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a
bioactive compound of considerable importance among natural
constituents found in Capsicum plants (1-3).
Capsaicin, present in the Capsicum annuum L. plant, imparts
the characteristic spiciness to this species. This plant is a
member of the Solanaceae family, which is one of the earliest
cultivated crops in the Western hemisphere (4). The growing societal consumption of
Capsicum annuum is underscored by its substantial
nutritional value, which serves as a rich source of essential
vitamins such as C, E and provitamin A (carotene), renowned for
their antioxidant properties (5).
In addition, it offers a plentiful supply of neutral phenolic
compounds, including luteolin, quercetin and capsaicinoids
(6,7).
Capsaicin is acknowledged for its potential
analgesic properties and therapeutic applications in the management
of inflammation and inflammatory diseases. The underlying mechanism
predominantly centers on the interaction between capsaicin and its
receptor, transient receptor potential vanilloid 1 (TRPV1). Its
molecular basis was elucidated by Caterina et al (8) in 1997, igniting significant interest
in manipulating capsaicin and its receptor pharmacologically
(9). Clinical studies have explored
capsaicin as a topical treatment for various pain conditions, such
as osteoarthritis, rheumatoid arthritis, postherpetic neuralgia,
psoriasis, and diabetic neuropathy (10,11).
Furthermore, capsaicin exhibits in vitro
antibacterial activity against a spectrum of pathogens, such as
Streptococcus pyogenes (12), Porphyromonas gingivalis
(13), Vibrio cholerae
(14) and Staphylococcus
aureus (15,16), reflecting its potential in the
treatment of pathogenic bacterial infections and alleviation of
antimicrobial resistance. In addition, capsaicin has exhibited
promise as a chemopreventive agent for cancer. Its combination with
radiotherapy and chemotherapy drugs shows the potential to enhance
patient sensitivity to these treatments, reduce required dosages
and improve overall tolerance to cancer therapy (17,18).
The multifaceted attributes of capsaicin,
encompassing its analgesic, anti-inflammatory, antimicrobial and
anticancer properties, hold significant promise in an oral health
context. This review aimed to explore relevant publications that
investigate the utilization of capsaicin as a therapeutic agent for
oral conditions and the preservation of oral well-being.
2. Capsaicin biosynthesis
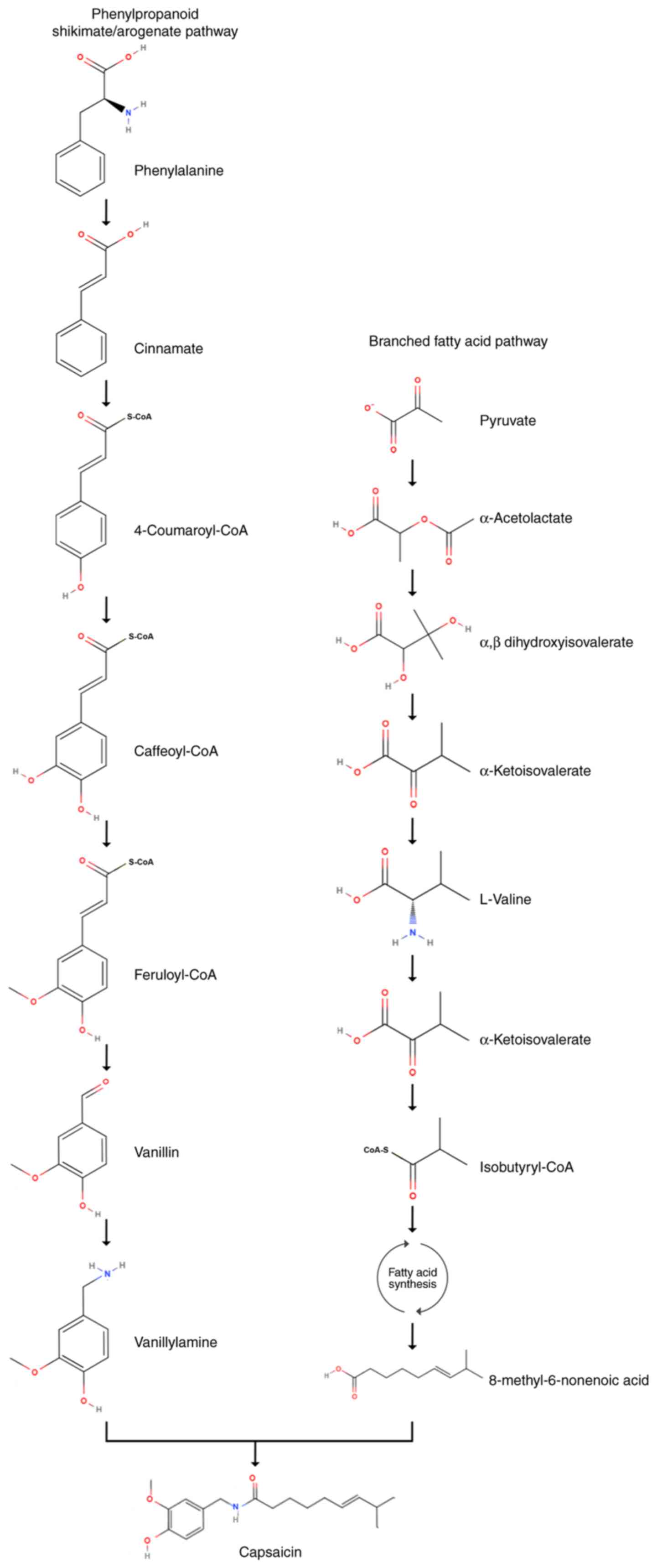
The biosynthesis pathway of capsaicin (Fig. 1) involves two distinct routes: i)
Through the synthesis of vanillylamine via the phenylpropanoid
shikimate/arogenate pathway; and ii) through the branched fatty
acid derived from valine (19-21).
Key enzymes proposed to participate in the phenylpropanoid pathway
include phenylalanine ammonia lyase, cinnamate 4-hydroxylase,
4-coumaroyl-CoA ligase, coumarate 3-hydroxylase, hydroxycinnamoyl
transferase, caffeoyl-coenzyme A (CoA) O-methyltransferase,
hydroxycinnamoyl-CoA hydratase/lyase, putative aminotransferase and
acyltransferase (21-26).
For the mechanisms underlying the synthesis of branched-chain fatty
acids, studies have hypothesized that a desaturase is involved in
the conversion of 8-methylnonanoic acid into 8-methyl-6-nonenoic
acid (24,27). Mazourek et al (21) proposed the inclusion of the
biosynthesis of amino acids that leads to capsacinoids in the
branched-chain fatty acid biosynthetic pathway.
Researchers have employed various techniques to
manipulate culture strategies and thus enhance capsaicinoid
biosynthesis. Among these strategies, osmotic stress has become an
effective method, resulting in the highest product accumulation,
followed by precursor feeding (20). In addition, the duration of exposure
to these treatments significantly influences the level of capsaicin
biosynthesis (20).
3. Analgesic and anti-inflammatory
properties
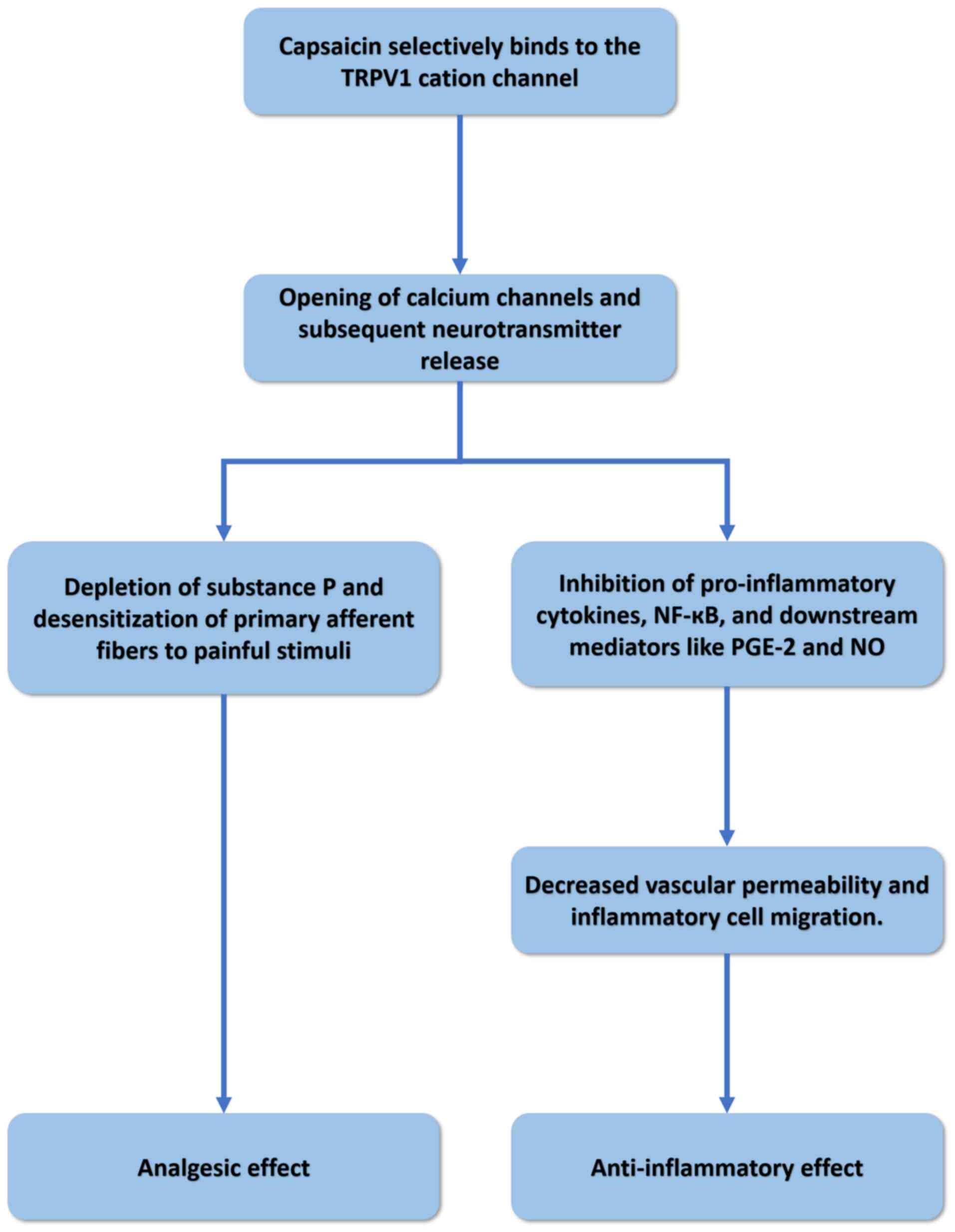
Studies have comprehensively elucidated the
foundational mechanism underlying the analgesic and
anti-inflammatory properties of capsaicin (Fig. 2), whereby capsaicin selectively
interacts with the TRPV1 cation channel (28). This channel exhibits high
permeability to calcium (Ca2+) ions and detects
potentially noxious stimuli. This interaction results in the
opening of Ca2+ channels and the subsequent
neurotransmitter release. This calcium-dependent process culminates
in the depletion of substance P and desensitization of primary
afferent fibers to painful stimuli, inducing analgesia (28). Concurrently, capsaicin exhibits
anti-inflammatory properties by reducing proinflammatory cytokines
and vascular permeability (29).
Capsaicin can deactivate nuclear transcription factor κB, thereby
inhibiting prostaglandin E-2 and nitric oxide production,
subsequently attenuating vascular leakage and modulating
inflammatory cell migration mediated by tumor necrosis factor-α and
interleukin (IL)-1(30).
Orofacial neuropathic pain
Capsaicin is commonly administered topically to
treat chronic pain associated with osteoarthritis, rheumatoid
arthritis, diabetic neuropathy and nondiabetic peripheral
neuropathy (10,31). The pain-relieving effects of
low-dose topical capsaicin (0.025, 0.050 and 0.075%) were also
demonstrated in conditions such as oral neuropathic pain and
trigeminal neuralgia (32-34).
These capsaicin cream formulations are also available over the
counter (35). However, the
efficacy of lower doses appears to be moderate and patient
compliance with this therapy is often hindered by the need for
daily repetitive application and the potential for irritation,
which can manifest as sensations of burning, stinging or itching
(31,36). Therefore, products containing higher
concentrations of capsaicin were suggested to relieve pain after a
single topical application (37).
Higher capsaicin doses may desensitize cutaneous and subcutaneous
receptors, resulting in reduced responsiveness to various sensory
stimuli (3).
Several studies have tested the effectiveness of 8%
capsaicin patch in managing orofacial neuropathic pain. In a case
report, Sayanlar et al (38)
revealed that a single application of the 8% capsaicin topical
patch on a patient diagnosed with trigeminal postherpetic neuralgia
demonstrated a substantial effect on reducing the pain level and
area. Gaul and Resch (39) reported
the effectiveness and safety of the application of 8% capsaicin
patch in the treatment of four cases of neuropathic pain in the
head and facial region caused by surgery or herpes zoster
infection. Sustained reduction in pain was noted in three of the
patients; however, two of them required repeated applications of
capsaicin (39). Similarly,
Martinez et al (40)
reported the success of repeated applications of 8% capsaicin patch
in managing pain in two patients with trigeminal neuralgia. These
three studies have used a similar method, which was applying 8%
capsaicin for 60 min in the painful area and ensuring eye
protection such as using safety goggles, a compress and plaster,
and eye dressing and cream (38-40).
Burning mouth syndrome (BMS) is a chronic
neuropathic pain common in post-menopausal women (41). Several studies have explored the
effectiveness of capsaicin in managing BMS symptoms using capsaicin
therapies in different durations (ranging from 1 month to 1 year),
concentrations (0.01, 0.02, 0.025 and 0.25%), and forms (capsule,
gel and mouth rinse). All of these studies have reported that
capsaicin successfully relieved BMS-related pain and discomfort
(42-45).
A study found no significant difference in the effectiveness of
0.01 and 0.025% capsaicin gels in reducing BMS symptoms, which
indicates that the 0.01% gel is adequate to activate the analgesic
effect of capsaicin for BMS (44).
Oral ulcers
Capsaicin in chili peppers was once proposed as a
component that could cause ulcers, particularly in the
gastrointestinal tract (46,47).
However, later studies have found that it acted contrarily, i.e.,
capsaicin helps in preventing and relieving ulcers by inhibiting
gastric acid secretion and stimulating mucus secretions and blood
flow (47).
Despite studies discussing the effect of capsaicin
on gastric or intestinal ulcers, published studies involving
capsaicin and oral ulcers are limited. A study on 11 patients who
underwent chemotherapy or radiotherapy reported the significant
analgesic effect of orally administered capsaicin on oral mucositis
pain; however, the effect was temporary in most patients (48). In an animal study, Jiang et
al (49) reported a healing
rate of 97.8% on the oral ulcer model in rats after 7 days of
treatment with 0.05% capsaicin candy, which was significantly
higher than those in groups receiving a placebo and dexamethasone.
The study also reported a high inflammatory effect of capsaicin, as
it reduces the expression of TNF-α and IL-6(49). This finding holds significance in
the treatment of oral ulcers and needs further investigation.
Temporomandibular disorders
(TMDs)
TMDs are considered neuropathic and idiopathic pain
disorders (50,51). In a randomized controlled study
involving 30 patients with unilateral pain in the temporomandibular
joint area, Winocur et al (52) found no significant difference in the
pain relief effect between the group using 0.025% capsaicin cream
four times a day and the placebo group, despite the significant
improvement in pain parameters throughout the experiment (4 weeks).
Later, Campbell et al (53)
demonstrated that a higher concentration of capsaicin (8% cream)
was effective in relieving pain in patients with TMDs, despite the
shorter experiment duration (1 week). However, the authors also
reported that the finding may be biased by the small sample size
and inclusion of female subjects only due to funding and difficulty
in participant recruitment (53).
4. Anticancer properties
In experimental studies utilizing cell cultures and
animal models, capsaicin consistently demonstrated the capacity to
inhibit oral cancer cell growth and induce apoptosis (54). Table
I lists several in vitro and in vivo
investigations that explored the potential of capsaicin as a
treatment agent for oral cancer (55-61).
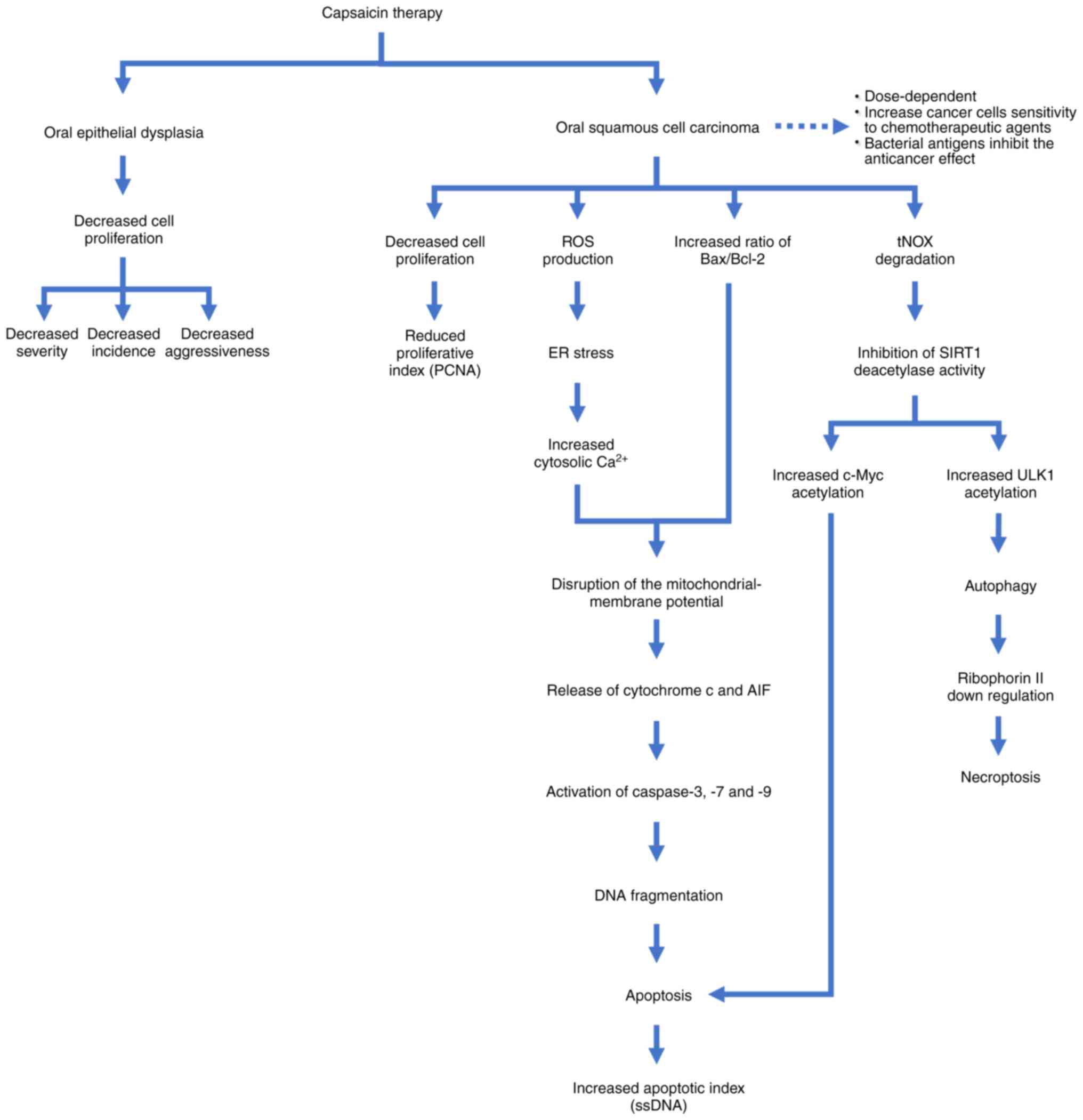
The proposed mechanism underlying the anticancer activity of
capsaicin on oral cancer from several studies is shown in Fig. 3 (54-61).
Capsaicin exerts anti-proliferative effects on oral epithelial
dysplasia, leading to a reduction in its incidence, severity and
aggressiveness (55,58). Capsaicin disrupts the mitochondrial
membrane potential in oral squamous cell carcinomas by triggering
endoplasmic reticulum (ER) stress and increasing the ratio of
Bax/Bcl-2, leading to the release of cytochrome c and
apoptosis-inducing factor from mitochondria (56,57).
This process activates caspase-3, -7 and -9, resulting in apoptosis
(56,57). Furthermore, capsaicin interacts with
tumor-associated NADH oxidase (tNOX), promoting both autophagy and
apoptosis in cancer cells (59).
Capsaicin also enhances the sensitivity of cancer cells to
anticancer drugs by increasing autophagy and reducing ribophorin II
protein levels (60). However,
studies suggest that capsaicin therapy for oral cancer is
dose-dependent and its efficacy may be compromised by bacterial
antigens (56,61). Considering its multifaceted effects
on cellular pathways and its potential implications for developing
therapeutic strategies for oral cancer management, further
investigations are warranted to truly understand the anti-oral
cancer mechanisms of capsaicin.
 | Table IStudies on capsaicin treatment for
oral cancer. |
Table I
Studies on capsaicin treatment for
oral cancer.
| Author(s),
year | Cell line(s)/test
species | Capsaicin
treatment | Effects | (Refs.) |
|---|
| Tanaka et
al, 2002 | 4-NQO-induced
tongue tumorigenesis in four-week-old male F344 rats | Diets containing
500 ppm capsaicin for 10 and 28 weeks | Reduced incidence
of carcinoma and severe dysplasia, as well as increased apoptotic
index and proliferative index of squamous cell carcinoma | (55) |
| Ip et al,
2012 | Human
nasopharyngeal carcinoma (NPC-TW 039) | Various doses of
capsaicin (0, 200, 250, 300 and 400 µM) for different incubation
times (12, 24, 36 and 48 h) | Induced ER stress,
increased ROS production, enhanced apoptosis induction with higher
doses and longer incubation periods | (56) |
| Kamaruddin et
al, 2019 | Oral squamous cell
carcinoma of Asian origin (ORL-48) | Various doses of
capsaicin (50, 100, 150, 200, 250, 300 and 350 µM) for different
incubation times (24, 48 and 72 h) | Induced apoptosis
via disruption of mitochondrial membrane potential, activation of
caspase-3, -7 and -9, intrinsic apoptotic pathway activation,
lowest cell viability and highest apoptosis percentage with longer
incubation periods | (57) |
| Mohamed and
AlQarni, 2019 | Experimentally
induced buccal pouch carcinogenesis in five-week-old golden
(Syrian) male hamsters | Water containing 10
ppm capsaicin for 9 weeks | Slower cell
proliferation, reduced tumor aggressiveness and lower oral
epithelial dysplasia | (58) |
| Chang et al,
2020 | p53-mutated HSC-3
and p53-functional SAS cells | Different
concentrations of capsaicin for 24 or 48 h; 2 mM capsaicin for 1 h
incubation at the end of treatment | Induced both
autophagy and apoptosis in p53-mutated HSC-3 cells, but only
autophagy in p53-functional SAS cells | (59) |
| Huang et al,
2021 | Oral squamous cell
carcinoma (HSC-3 and SAS) | 200 µM capsaicin
for 30 min | Induced ER stress
and autophagy by suppressing ribophorin II, increased sensitivity
of cancer cells to chemotherapeutic agents, inhibited viability by
increasing necroptosis markers (phospho-MLKL and phospho-RIP3) | (60) |
| Chakraborty et
al, 2021 | Oral cancer cells
Cal 27 | Capsaicin
treatments ranged from 0 to 150 µM with a 24-h incubation time.
Bacterial antigens, LPS and LTA, were introduced to cancer cells
before and/or during capsaicin administration | Exposure to
bacterial antigens resulted in reduced death and metabolic
inhibition of cancer cells, as well as decreased SOCS3 gene
expression, compared to capsaicin treatment alone | (61) |
Oral epithelial dysplasia
Tanaka et al (55) found that a 500-ppm capsaicin diet
reduced the incidence and multiplicity of tongue dysplasia on
4-NQO-induced tongue tumorigenesis in male rats. In another in
vivo study, Mohamed and AlQarni (58) experimentally induced hamster buccal
pouch carcinogenesis and demonstrated that capsaicin-treated
hamsters exhibited slower cell proliferation and reduced incidence
and severity of oral epithelial dysplasia.
Oral squamous cell carcinoma
Ip et al (56) found that increasing capsaicin doses
and longer incubation periods enhanced the induction of apoptosis
in NPC-TW 039 cells. Among the doses tested (0, 200, 250, 300 and
400 µM) and incubation times (12, 24, 36 and 48 h), treatment with
400 µM capsaicin resulted in the most significant decrease in cell
viability, reaching nearly 65% after 48 h of treatment (56).
In another in vitro investigation focusing on
oral squamous cell carcinoma of Asian origin (ORL-48), Kamaruddin
et al (57) revealed that
capsaicin treatment induced apoptosis, leading to apoptotic DNA
fragmentation. In addition, the cell viability rate was the lowest,
whereas the apoptosis rate was the highest after 72 h of treatment
compared with that at 48 h (57).
Chang et al (59) investigated the interplay between
apoptosis and autophagy in p53-mutated HSC-3 and p53-functional SAS
cells treated with different concentrations of capsaicin. They
revealed that capsaicin engaged with tumor-associated NADH oxidase
(tNOX) to cause its degradation, and inhibition of sirtuin 1
(SIRT1) deacetylase activity, which enhanced unc-51-like autophagy
activating kinase 1 (ULK1) acetylation and autophagy activation in
p53-functional SAS cells (59).
Capsaicin induced autophagy and apoptosis in p53-mutated HSC-3
cells, with autophagy inhibiting but later facilitating apoptosis.
Reduced tNOX and SIRT1 levels, combined with high levels of ULK1
and c-Myc acetylation, reactivated the tumor necrosis
factor-related apoptosis-inducing ligand pathway, resulting in
apoptosis (59).
Huang et al (60) investigated capsaicin-induced
sensitization to four chemotherapeutic agents (5-fluorouracil,
cisplatin, docetaxel and doxorubicin) in oral squamous cell
carcinoma (HSC-3 and SAS) and discovered that 200 µM capsaicin did
not significantly induce apoptosis but caused ER stress and
autophagy by suppressing ribophorin II. Furthermore, capsaicin in
combination with anticancer agents sensitizes cancer cells to these
agents and inhibits their viability by increasing necroptosis
markers such as mixed lineage kinase domain-like protein and
receptor-interacting protein kinase 3(60).
5. Antimicrobial properties
The chili fruit is rich in phenolic compounds,
predominantly flavonoids and capsaicin, alongside phenolic acids
such as tamarind ferulic, coumaric acid and cinnamic acid (62,63).
These secondary metabolites are positively associated with
antioxidant and antimicrobial activities, potentially interfering
with the synthesis of bacterial cell membranes (64). Numerous studies have proved the
antimicrobial properties of capsaicin, providing a promising
standpoint as an alternative strategy against antimicrobial
resistance (65).
Dental caries
Santos et al (66) evaluated the inhibitory effects of
capsaicin, dihydrocapsaicin and four synthetic capsaicinoid
derivatives against Streptococcus mutans, a key contributor
to cariogenic biofilm. They revealed that these compounds had a
minimum inhibitory concentration (MIC) ranging from 1.25 to 5.0
µg/ml for these bacteria (66).
Similarly, Gu et al (67)
demonstrated the potent action of capsaicin against cariogenic
bacterial strains, including S. mutans, Actinomyces
viscosus, Lactobacillus and Streptococcus
sanguis, by inhibiting acid production and biofilm formation.
The MIC values of capsaicin were 50 µg/ml for S. mutans,
A. viscosus and Lactobacillus and 25 µg/ml for S.
sanguis (67). However, Doğan
and Tunçer (68) reported
contrasting findings: Although capsaicin did not inhibit the growth
of S. mutans, it suppressed the growth of the oral probiotic
Streptococcus salivarius M18 at concentrations >100
µg/ml. These discrepancies underscore the need for further studies
into the nuanced effects of capsaicin, considering factors such as
compound nature and concentration, and its effect on nonpathogenic
oral bacteria.
Periodontal diseases
Previous studies have also highlighted the efficacy
of capsaicin against periodontitis-associated pathogens, notably
P. gingivalis. An in vitro study by Zhou et al
(13) demonstrated the inhibitory
effect of capsaicin on the growth of P. gingivalis and the
expression of NF-ĸB p65, indicating its potential to inhibit
alveolar bone resorption. In addition, animal experimental studies,
such as that by Cong et al (69), revealed that topical application of
0.075% capsaicin over the submandibular gland increased salivary
secretion, which could have a significant utility in the control of
microbial colonization.
Candidiasis
Investigations into the antifungal properties of
capsaicin, particularly against Candida albicans, a common
cause of oral candidiasis infections, have yielded promising
results (65). Nascimento et
al (70) found that at the MIC
of 25 µg/ml, capsaicin inhibited the growth of C. albicans.
Furthermore, Omolo et al (71) highlighted greater susceptibility of
C. albicans to capsaicin than certain bacterial strains.
Behbehani et al (72)
proposed the mechanism of capsaicin's antifungal activity,
suggesting its ability to disrupt C. albicans cell wall
integrity by inhibiting ergosterol biosynthesis. In addition, the
combination of capsaicin and fluconazole exhibited enhanced
efficacy, potentially aiding in preventing fluconazole resistance
(72).
Viral infection of the oral
cavity
Despite studies investigating the potential
antiviral properties of capsaicin, particularly against Herpes
simplex virus (73), Lassa virus
(74,75) and severe acute respiratory syndrome
coronavirus 2(75), research on its
effects on oral viruses is limited. Nevertheless, considering
capsaicin's potential to inhibit the replication of certain viruses
because of its ability to modulate immune and inflammatory
responses (76), further related
research could provide valuable insight into its therapeutic
potential for viral infections in the oral cavity.
6. Side effects
Mild to moderate burning sensation, stinging,
itching, redness and pain in the treated area are among the main
reported side effects of topical treatment of capsaicin for
orofacial pain and disorders, which are self-limiting and short
term (37-39,43).
Studies have recommended administering a local anesthetic before
applying topical capsaicin to minimize pain perception and control
the initial burning sensation (32,35).
Ensuring the patch fits the contour of the affected skin and
avoiding contact with the eyes are also essential for the
treatment's safety (38).
However, topical therapy requires repeated
applications daily, which could expose patients to repeated
potential irritations from side effects, reducing the patient's
compliance with the therapy (31,36).
Furthermore, capsaicin's bitter taste and unpleasant consistency
contributed to lower compliance with the therapy, particularly when
applied on the tongue (42).
In addition, further research on the intraoral use
of capsaicin is warranted to investigate potential side effects on
the gastrointestinal system. Petruzzi et al (42) reported mild gastric pain in patients
treated with oral systemic capsaicin for BMS symptoms. Jørgensen
and Pedersen (44) reported that
several patients discontinued the capsaicin gel therapy that
required application on the tongue for treating BMS because of
nausea and sore throat.
7. Conclusion and future perspectives
Capsaicin holds significant promise for enhancing
oral health owing to its analgesic, anti-inflammatory, anticancer
and antimicrobial effects. However, more studies are necessary to
determine the optimal dosage of capsaicin for alleviating oral pain
while minimizing adverse effects. Further investigations on the
effect of capsaicin on nonpathogenic oral bacteria and oral viruses
are also warranted. Human-based research is also needed to gain a
deeper understanding of the biomolecular mechanisms underlying the
properties of capsaicin. These research advancements could lead to
the development of more effective and targeted interventions for
oral health issues.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
The study was conceptualized by WY. WY and AR
significantly contributed to data collection, manuscript drafting,
reviewing and editing. Each author has thoroughly reviewed and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reyes-Escogido Mde L, Gonzalez-Mondragon
EG and Vazquez-Tzompantzi E: Chemical and pharmacological aspects
of capsaicin. Molecules. 16:1253–1270. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adetunji TL, Olawale F, Olisah C, Adetunji
AE and Aremu AO: Capsaicin: A Two-Decade Systematic Review of
Global Research Output and Recent Advances Against Human Cancer.
Front Oncol. 12(908487)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fattori V, Hohmann MS, Rossaneis AC,
Pinho-Ribeiro FA and Verri WA: Capsaicin: Current Understanding of
Its Mechanisms and Therapy of Pain and Other Pre-Clinical and
Clinical Uses. Molecules. 21(844)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chapa-Oliver AM and Mejía-Teniente L:
Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules.
21(931)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alonso-Villegas R, González-Amaro RM,
Figueroa-Hernández CY and Rodríguez-Buenfil IM: The Genus Capsicum:
A Review of Bioactive Properties of Its Polyphenolic and
Capsaicinoid Composition. Molecules. 28(4239)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bal S, Sharangi AB, Upadhyay TK, Khan F,
Pandey P, Siddiqui S, Saeed M, Lee HJ and Yadav DK: Biomedical and
Antioxidant Potentialities in Chilli: Perspectives and Way Forward.
Molecules. 27(6380)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Allam AE: Suppression of cytokine
production by newly isolated flavonoids from pepper. Fitoterapia.
151(104903)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Basith S, Cui M, Hong S and Choi S:
Harnessing the Therapeutic Potential of Capsaicin and Its Analogues
in Pain and Other Diseases. Molecules. 21(966)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Persson MS, Fu Y, Bhattacharya A, Goh SL,
van Middelkoop M, Bierma-Zeinstra SM, Walsh D, Doherty M and Zhang
W: OA Trial Bank Consortium. Relative efficacy of topical
non-steroidal anti-inflammatory drugs and topical capsaicin in
osteoarthritis: Protocol for an individual patient data
meta-analysis. Syst Rev. 5(165)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Srinivasan K: Biological Activities of Red
Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A
Review. Crit Rev Food Sci Nutr. 56:1488–1500. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marini E, Magi G, Mingoia M, Pugnaloni A
and Facinelli B: Antimicrobial and Anti-Virulence Activity of
Capsaicin Against Erythromycin-Resistant, Cell-Invasive Group A
Streptococci. Front Microbiol. 6(1281)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou Y, Guan X, Zhu W, Liu Z, Wang X, Yu H
and Wang H: Capsaicin inhibits Porphyromonas gingivalis growth,
biofilm formation, gingivomucosal inflammatory cytokine secretion,
and in vitro osteoclastogenesis. Eur J Clin Microbiol Infect Dis.
33:211–219. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chatterjee S, Asakura M, Chowdhury N,
Neogi SB, Sugimoto N, Haldar S, Awasthi SP, Hinenoya A, Aoki S and
Yamasaki S: Capsaicin, a potential inhibitor of cholera toxin
production in Vibrio cholerae. FEMS Microbiol Lett. 306:54–60.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qiu J, Niu X, Wang J, Xing Y, Leng B, Dong
J, Li H, Luo M, Zhang Y, Dai X, et al: Capsaicin protects mice from
community-associated methicillin-resistant Staphylococcus aureus
pneumonia. PLoS One. 7(e33032)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kalia NP, Mahajan P, Mehra R, Nargotra A,
Sharma JP, Koul S and Khan IA: Capsaicin, a novel inhibitor of the
NorA efflux pump, reduces the intracellular invasion of
Staphylococcus aureus. J Antimicrob Chemother. 67:2401–2408.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang S, Wang D, Huang J, Hu Y and Xu Y:
Application of capsaicin as a potential new therapeutic drug in
human cancers. J Clin Pharm Ther. 45:16–28. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nie J, Zhao C, Deng LI, Chen J, Yu B, Wu
X, Pang P and Chen X: Efficacy of traditional Chinese medicine in
treating cancer. Biomed Rep. 4:3–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Díaz J, Pomar F, Bernal A and Merino F:
Peroxidases and the metabolism of capsaicin in Capsicum annuum L.
Phytochem Rev. 3:141–157. 2004.
|
|
20
|
Kehie M, Kumaria S, Tandon P and Ramchiary
N: Biotechnological advances on in vitro capsaicinoids biosynthesis
in capsicum: a review. Phytochem Rev. 14:189–201. 2015.
|
|
21
|
Mazourek M, Pujar A, Borovsky Y, Paran I,
Mueller L and Jahn MM: A dynamic interface for capsaicinoid systems
biology. Plant Physiol. 150:1806–1821. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Suzuki T, Fujiwake H and Iwai K:
Intracellular localization of capsaicin and its analogues,
capsaicinoid, in Capsicum fruit 1. Microscopic investigation of the
structure of the placenta of Capsicum annuum var. annuum cv.
Karayatsubusa. Plant Cell Physiol. 21:839–853. 1980.
|
|
23
|
Sukrasno N and Yeoman MM: Phenylpropanoid
metabolism during growth and development of Capsicum frutescens
fruits. Phytochem. 32:839–844. 1993.
|
|
24
|
Stewart C Jr, Kang BC, Liu K, Mazourek M,
Moore SL, Yoo EY, Kim BD, Paran I and Jahn MM: The Pun1 gene for
pungency in pepper encodes a putative acyltransferase. Plant J.
42:675–688. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang ZX, Zhao SN, Liu GF, Huang ZM, Cao
ZM, Cheng SH and Lin SS: Discovery of putative capsaicin
biosynthetic genes by RNA-Seq and digital gene expression analysis
of pepper. Sci Rep. 6(34121)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arce-Rodríguez ML and Ochoa-Alejo N:
Biochemistry and molecular biology of capsaicinoid biosynthesis:
Recent advances and perspectives. Plant Cell Rep. 38:1017–1030.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Blum E, Mazourek M, O'Connell M, Curry J,
Thorup T, Liu K, Jahn M and Paran I: Molecular mapping of
capsaicinoid biosynthesis genes and quantitative trait loci
analysis for capsaicinoid content in Capsicum. Theor Appl Genet.
108:79–86. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clark R and Lee SH: Anticancer Properties
of Capsaicin Against Human Cancer. Anticancer Res. 36:837–843.
2016.PubMed/NCBI
|
|
29
|
Alawi K and Keeble J: The paradoxical role
of the transient receptor potential vanilloid 1 receptor in
inflammation. Pharmacol Ther. 125:181–195. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim CS, Kawada T, Kim BS, Han IS, Choe SY,
Kurata T and Yu R: Capsaicin exhibits anti-inflammatory property by
inhibiting IkB-a degradation in LPS-stimulated peritoneal
macrophages. Cell Signal. 15:299–306. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Derry S, Lloyd R, Moore RA and McQuay HJ:
Topical capsaicin for chronic neuropathic pain in adults. Cochrane
Database Syst Rev. CD007393, 2009.
|
|
32
|
Epstein JB and Marcoe JH: Topical
application of capsaicin for treatment of oral neuropathic pain and
trigeminal neuralgia. Oral Surg Oral Med Oral Pathol. 77:135–140.
1994.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ellison N, Loprinzi CL, Kugler J, Hatfield
AK, Miser A, Sloan JA, Wender DB, Rowland KM, Molina R, Cascino TL,
et al: Phase III placebo-controlled trial of capsaicin cream in the
management of surgical neuropathic pain in cancer patients. J Clin
Oncol. 15:2974–2980. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fusco BM and Alessandri M: Analgesic
effect of capsaicin in idiopathic trigeminal neuralgia. Anesth
Analg. 74:375–377. 1992.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Padilla M, Clark GT and Merrill RL:
Topical medications for orofacial neuropathic pain: A review. J Am
Dent Assoc. 131:184–195. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mason L, Moore RA, Derry S, Edwards JE and
McQuay HJ: Systematic review of topical capsaicin for the treatment
of chronic pain. BMJ. 328(991)2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Peppin JF and Pappagallo M: Capsaicinoids
in the treatment of neuropathic pain: A review. Ther Adv Neurol
Disord. 7:22–32. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sayanlar J, Guleyupoglu N, Portenoy R and
Ashina S: Trigeminal postherpetic neuralgia responsive to treatment
with capsaicin 8% topical patch: A case report. J Headache Pain.
13:587–589. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gaul C and Resch S: Application of the
capsaicin 8% cutaneous patch in neuropathic pain of the head and
face: A case series. Cephalalgia. 35:545–550. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Martinez MLC, Adan N, Carballude A,
Lamelas L, Villar E and Seoane PR: Capsaicin 8% patch in trigeminal
neuralgia: Case reports. Aust Med J. 12:98–102. 2019.
|
|
41
|
Jääskeläinen SK: Is burning mouth syndrome
A neuropathic pain condition? Pain. 159:610–613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Petruzzi M, Lauritano D, De Benedittis M,
Baldoni M and Serpico R: Systemic capsaicin for burning mouth
syndrome: Short-term results of a pilot study. J Oral Pathol Med.
33:111–114. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Silvestre FJ, Silvestre-Rangil J,
Tamarit-Santafé C and Bautista D: Application of a capsaicin rinse
in the treatment of burning mouth syndrome. Med Oral Patol Oral Cir
Bucal. 17:e1–e4. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jørgensen MR and Pedersen AM: Analgesic
effect of topical oral capsaicin gel in burning mouth syndrome.
Acta Odontol Scand. 75:130–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Azzi L, Croveri F, Pasina L, Porrini M,
Vinci R, Manfredini M, Tettamanti L, Tagliabue A, Silvestre Rangil
J and Spadari F: A ‘burning’ therapy for burning mouth syndrome:
Preliminary results with the administration of topical capsaicin. J
Biol Regul Homeost Agents. 31 (2 Suppl 1):89–95. 2017.PubMed/NCBI
|
|
46
|
Mann NS: Capsaicin induced acute erosive
gastritis: Its prevention by antacid, metiamide and cimetidine. J
Ky Med Assoc. 75:71–73. 1977.PubMed/NCBI
|
|
47
|
Satyanarayana MN: Capsaicin and gastric
ulcers. Crit Rev Food Sci Nutr. 46:275–328. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Berger A, Henderson M, Nadoolman W, Duffy
V, Cooper D, Saberski L and Bartoshuk L: Oral capsaicin provides
temporary relief for oral mucositis pain secondary to
chemotherapy/radiation therapy. J Pain Symptom Manage. 10:243–248.
1995.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jiang H, Yu X, Fang R, Xiao Z and Jin Y:
3D printed mold-based capsaicin candy for the treatment of oral
ulcer. Int J Pharm. 568(118517)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Diatchenko L, Nackley AG, Slade GD,
Fillingim RB and Maixner W: Idiopathic pain disorders - Pathways of
vulnerability. Pain. 123:226–230. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pedullà E, Meli GA, Garufi A, Mandalà ML,
Blandino A and Cascone P: Neuropathic pain in temporomandibular
joint disorders: Case-control analysis by MR imaging. AJNR Am J
Neuroradiol. 30:1414–1418. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Winocur E, Gavish A, Halachmi M, Eli I and
Gazit E: Topical application of capsaicin for the treatment of
localized pain in the temporomandibular joint area. J Orofac Pain.
14:31–36. 2000.PubMed/NCBI
|
|
53
|
Campbell BK, Fillingim RB, Lee S, Brao R,
Price DD and Neubert JK: Effects of High-Dose Capsaicin on TMD
Subjects:A Randomized Clinical Study. JDR Clin Trans Res. 2:58–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mosqueda-Solís A, Lafuente-Ibáñez de
Mendoza I, Aguirre-Urizar JM and Mosqueda-Taylor A: Capsaicin
intake and oral carcinogenesis: A systematic review. Med Oral Patol
Oral Cir Bucal. 26:e261–e268. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tanaka T, Kohno H, Sakata K, Yamada Y,
Hirose Y, Sugie S and Mori H: Modifying effects of dietary
capsaicin and rotenone on 4-nitroquinoline 1-oxide-induced rat
tongue carcinogenesis. Carcinogenesis. 23:1361–1367.
2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ip SW, Lan SH, Lu HF, Huang AC, Yang JS,
Lin JP, Huang HY, Lien JC, Ho CC, Chiu CF, et al: Capsaicin
mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039
cells through mitochondrial depolarization and endoplasmic
reticulum stress. Hum Exp Toxicol. 31:539–549. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kamaruddin MF, Hossain MZ, Mohamed Alabsi
A and Mohd Bakri M: The Antiproliferative and Apoptotic Effects of
Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin,
ORL-48. Medicina (Kaunas). 55(322)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Mohamed MA and AlQarni AA: Chemopreventive
effect of capsaicin in experimentally induced hamster buccal pouch
carcinogenesis (Immunohistochemical study Bcl-2). Egypt Dent J.
65:1237–1243. 2019.
|
|
59
|
Chang CF, Islam A, Liu PF, Zhan JH and
Chueh PJ: Capsaicin acts through tNOX (ENOX2) to induce autophagic
apoptosis in p53-mutated HSC-3 cells but autophagy in
p53-functional SAS oral cancer cells. Am J Cancer Res.
10:3230–3247. 2020.PubMed/NCBI
|
|
60
|
Huang YC, Yuan TM, Liu BH, Liu KL, Wung CH
and Chuang SM: Capsaicin Potentiates Anticancer Drug Efficacy
Through Autophagy-Mediated Ribophorin II Downregulation and
Necroptosis in Oral Squamous Cell Carcinoma Cells. Front Pharmacol.
12(676813)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chakraborty R, Vickery K, Darido C,
Ranganathan S and Hu H: Bacterial Antigens Reduced the Inhibition
Effect of Capsaicin on Cal 27 Oral Cancer Cell Proliferation. Int J
Mol Sci. 22(8686)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Materska M and Perucka I: Antioxidant
activity of the main phenolic compounds isolated from hot pepper
fruit (Capsicum annuum L). J Agric Food Chem. 53:1750–1756.
2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Shahidi F, Varatharajan V, Oh WY and Peng
H: Phenolic compounds in agri-food by-products, their
bioavailability and health effects. J Food Bioact. 5:57–119.
2019.
|
|
64
|
Romero-Luna HE, Colina J, Guzmán-Rodríguez
L, Sierra-Carmona CG, Farías-Campomanes ÁM, García-Pinilla S,
González-Tijera MM, Malagón-Alvira KO and Peredo-Lovillo A: C
apsicum fruits as functional ingredients with antimicrobial
activity: An emphasis on mechanisms of action. J Food Sci Technol.
60:1–11. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Periferakis AT, Periferakis A, Periferakis
K, Caruntu A, Badarau IA, Savulescu-Fiedler I, Scheau C and Caruntu
C: Antimicrobial Properties of Capsaicin: Available Data, Future
Research Perspectives. Nutrients. 15(4097)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Santos MM, Vieira-da-Motta O, Vieira IJ,
Braz-Filho R, Gonçalves PS, Maria EJ, Terra WS, Rodrigues R and
Souza CL: Antibacterial activity of Capsicum annuum extract and
synthetic capsaicinoid derivatives against Streptococcus mutans. J
Nat Med. 66:354–356. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gu H, Yang Z, Yu W, Xu K and Fu YF:
Antibacterial Activity of Capsaicin against Sectional Cariogenic
Bacteria. Pak J Zool. 51:681–687. 2019.
|
|
68
|
Doğan K and Tunçer S: Capsaicin Shows
Species and Strain-specific Activity: Investigation of the
Antibacterial Effects on the Oral Pathogen Streptococcus mutans and
the Oral Probiotics Streptococcus salivarius M18 and K12. Hacettepe
Biol Chem. 52:11–19. 2024.
|
|
69
|
Cong X, Zhang Y, Shi L, Yang NY, Ding C,
Li J, Ding QW, Su YC, Xiang RL, Wu LL and Yu GY: Activation of
transient receptor potential vanilloid subtype 1 increases
expression and permeability of tight junction in normal and
hyposecretory submandibular gland. Lab Invest. 92:753–768.
2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Nascimento PL, Nascimento TC, Ramos NS,
Silva GR, Gomes JE, Falcão RE, Moreira KA, Porto AL and Silva TM:
Quantification, Antioxidant and Antimicrobial Activity of Phenolics
Isolated from Different Extracts of Capsicum frutescens (Pimenta
Malagueta). Molecules. 19:5434–5447. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Omolo MA, Wong ZZ, Borh WG, Hedblom GA,
Dev K and Baumler DJ: Comparative analysis of capsaicin in twenty
nine varieties of unexplored Capsicum and its antimicrobial
activity against bacterial and fungal pathogens. J Med Plant Res.
12:544–556. 2018.
|
|
72
|
Behbehani JM, Irshad M, Shreaz S and
Karched M: Anticandidal Activity of Capsaicin and Its Effect on
Ergosterol Biosynthesis and Membrane Integrity of Candida albicans.
Int J Mol Sci. 24(1046)2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hafiz T, Mubaraki M, Dkhil M and
Al-Quraishy S: Antiviral Activities of Capsicum annuum Methanolic
Extract against Herpes Simplex Virus 1 and 2. Pakistan J Zool.
49:251–255. 2017.
|
|
74
|
Tang K, Zhang X and Guo Y: Identification
of the dietary supplement capsaicin as an inhibitor of Lassa virus
entry. Acta Pharm Sin B. 10:789–798. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gonzalez-Paz LA, Lossada CA, Moncayo LS,
Romero F, Paz JL, Vera-Villalobos J, Pérez AE, San-Blas E and
Alvarado YJ: Theoretical Molecular Docking Study of the Structural
Disruption of the Viral 3CL-Protease of COVID19 Induced by Binding
of Capsaicin, Piperine and Curcumin Part 1: A Comparative Study
with Chloroquine and Hydrochloroquine Two Antimalaric Drugs.
Preprint: Research Square, 2020. https://doi.org/10.21203/rs.3.rs-21206/v1.
|
|
76
|
Zhang MQ, Jia X, Cheng CQ, Wang YX, Li YY,
Kong LD, Li QQ, Xie F, Yu YL, He YT, et al: Capsaicin functions as
a selective degrader of STAT3 to enhance host resistance to viral
infection. Acta Pharmacol Sin. 44:2253–2264. 2023.PubMed/NCBI View Article : Google Scholar
|