Introduction
During adipocyte hypertrophy, macrophages infiltrate
adipose tissue following the biogenesis of adipocytes (1). Macrophages secrete proinflammatory
cytokines that induce adipose tissue inflammation, which causes
insulin resistance (2). Adipose
tissue-specific insulin-resistant mouse models accumulate
proinflammatory macrophages in the adipose tissue (3,4).
Nuclear receptor peroxisome proliferator-activated receptor
(PPAR)γ, which is abundantly expressed in adipose tissue and
macrophages, is a target receptor for thiazolidine antidiabetic
agents and contributes to adipogenesis and antiinflammation in
adipose tissue. The activation of PPARγ inhibits the secretion of
several cytokines, such as tumor necrosis factor-α (TNF-α),
interferon-γ, interleukin (IL)-1, IL-6 and transforming growth
factor-β (TGF-β), in adipose tissue (5). Foxp3+ regulatory T cells
(Tregs), which serve an important role in obesity, migrate to
inflamed non-lymphoid tissue, such as adipose tissue, and suppress
the function of proinflammatory T cells (6).
Flavonoids, which possess several hydroxyl groups in
their diphenylpropane structure, generally exert anti-inflammatory
effects due to multiple phenolic hydroxyl groups with
radical-scavenging ability (7).
However, their bioavailability is low, because of their hydrophilic
properties (8). Our unpublished
study found that prenylflavonoids, which possess a C5 isoprenoid
unit in a diphenylpropane structure, have a negligible effect on
radical scavenging activity but exhibit stronger PPARγ ligand
activity than general flavonoids such as quercetin, which is widely
found in plants. Moreover, increased prenylflavonoid accumulation
and decreased efflux from cells have been reported because the C5
isoprenoid unit increases hydrophobicity of the diphenylpropane
structure to enhance its affinity for the cell membrane (9) compared with non-prenylated flavonoids,
such as quercetin and naringenin. Prenylflavonoids are present in
the genus Lespedeza which belongs to the family Fabaceae and
is distributed throughout East Asia. The biological activities of
Lespedeza plants include anti-inflammatory (10), antidiabetic (11), anti-tyrosinase (12), antibacterial and antitumor
activities (13) and nitric oxide
production (14). Therefore, the
present study aimed to determine which Lespedeza sp. among
four species, namely L. buergeri (LB), L. homoloba
(LH), L. maximowiczii (LM) and L. thunbergii (LT),
that contain prenylflavonoids exhibit PPARγ ligand activity and the
accumulation of Tregs in adipose tissue to induce anti-inflammatory
effects and suppress blood glucose levels.
Materials and methods
Animals
A total of 54 5-week-old BALB/c male mice (weight
19-20 g) were purchased from Japan SLC, Inc. (Hamamatsu, Japan) and
housed under standardized conditions (temperature, 25±1˚C;
humidity, 55±5%) in a room with a 12/12-h light/dark cycle. The
mice were fed standard chow (cat. no. CE-2, CLEA Japan, Inc.) and
water and acclimatized to the room for 1 week. All animal
experiments were approved by Animal Experimental Committee of
Tohoku Medical and Pharmaceutical University (Sendai, Japan
(approval no. 23032-a) and performed in accordance with the ethical
guidelines of the university. Euthanasia of mice was performed to
adhere to the American Veterinary Medical Association Guidelines
for the Euthanasia of Animals (15). The mice were euthanized by excessive
inhalation of 5% isoflurane in a chamber; cardiac and respiratory
arrest were confirmed and then the tissues were resected for
analysis.
Extraction of samples
Lespedeza sp. were provided by Sendai Wild
Plants Garden under the jurisdiction of Sendai City Office
Construction Bureau Park Management Section. These plants were
air-dried and extracted with 20-fold methanol volume of plant
weight at room temperature for 7 days. The methanol extract was
filtered and concentrated by a rotary evaporator under reduced
pressure at 40˚C to obtain the dried extract. The yields of LB, LH,
LM and LT-dried extracts were 94.35, 64.13, 79.17 and 51.72 mg/g,
respectively.
PPARγ binding assay
PPARγ binding assay was conducted using the method
described by Konno et al (16). Briefly, 0.2 µg per well CREB binding
protein (CBP; BIOSS) was immobilized in the wells of a plastic
96-well plate at 37˚C for 1 h. After blocking with 5% skimmed milk
at 37˚C for 1 h, PPARγ (PPARγ Human Recombinant, ProSpec-Tany
TechnoGene Ltd.) was immobilized on the CBP in at 37˚C for 1 h.
After the washing with wash buffer (0.1 M PBS containing 0.05%
Tween 20), methanol extracts of Lespedeza sp. (the reaction
concentrations: 0.038, 0.075, 0.15, 0.3 mg/ml) or pioglitazone (the
reaction concentrations: 0.001, 0.01, 0.1, 1 µM) were added,
respectively, and the plate was incubated for 1 h at 37˚C. 500-fold
diluted PPARγ antibody (rabbit polyclonal, cat. No. AHP1269,
Bio-Rad Laboratories, Inc.) was added to the wells after washing
with the wash buffer and then incubated for 1 h at 37˚C. 40-fold
diluted alkaline phosphatase-conjugated IgG antibody (goat
anti-rabbit, catalog No. 170-6581, Bio-Rad Laboratories, Inc.) was
added after the wells washing with the wash buffer and then
incubated for 1 h at 37˚C. Following shaking with a microplate
shaker at room temperature in the dark for 20 min, absorbance of
the wells was measured at 405 nm with a spectrophotometer. The
PPARγ binding activity was calculated using the following formula:
PPARγ binding activity=absorbance of sample/absorbance of control.
Pioglitazone (FUJIFILM Wako Pure Chemical Corporation) was used as
a positive control.
DPPH radical scavenging assay
DPPH radical scavenging assay was conducted using
the method by Blois (17). Briefly,
100 mM (pH 5.5) acetate buffer, ethanol (95 vol%), 500 µM DPPH
ethanol solution and sample solution (6.25, 125, 250, 500, 1,000,
2,000 µg/ml) or Trolox (0.25, 0.5, 1.0, 2.0 µM), respectively, were
mixed in the same tube (1.00 and 1.25 ml and 250.00 and 50.00 µl,
respectively). The mixture was incubated at room temperature for 30
min in the dark and absorbance was measured at 517 nm with a
spectrophotometer. DPPH radical scavenging activity was calculated
using the following formula: [(Absorbance of control-absorbance of
sample)/absorbance of control] x100. Trolox (Tokyo Chemical
Industry Co., Ltd.) was used as a positive control.
Splenocyte preparation
The spleen of male BALB/c mice was extirpated under
sterile conditions following euthanasia via inhalation of
isoflurane. The spleen was minced by scissors and ground by slide
glass in RPMI-1640 medium (FUJIFILM Wako Pure Chemical Corporation)
and filtered through a 40-µM mesh. The cell suspension was
hemolyzed using 1X RBC Lysis Buffer, pluriSelect Life Science) to
obtain splenocytes. The splenocytes were suspended in RPMI-1640
containing 0.05 mM 2-mercaptoethanol (FUJIFILM Wako Pure Chemical
Corporation), 10% heat-inactivated FBS (Thermo Fisher Scientific,
Inc.) and Antibiotic-Antimycotic Mixed Stock Solution (100X;
Nacalai Tesque, Inc.).
Splenocyte toxicity
A total of 5x104 splenocytes and 4 µg
concanavalin A (derived from Canavalia esiformis; Merck
KGaA) were added to the individual wells of a 96-well plate and
incubated at 37˚C in a humidified atmosphere containing 5%
CO2 for 48 h. The medium was exchanged for RPMI-1640
medium containing LH and incubated at 37˚C in a humidified
atmosphere containing 5% CO2 for 48 h. The splenocyte
proliferative activity was determined by adding Cell Count Reagent
SF (Nacalai Tesque, Inc.) and incubating for 4 h at 37˚C in a
humidified atmosphere containing 5% CO2 and measuring
the absorbance at 450 nm with a spectrophotometer.
IL-2 secretion from cultured murine
splenocytes
A total of 5x106 splenocytes and 4 µg
concanavalin A were added to the individual wells of a 24-well
plate. The plate was incubated at 37˚C in a humidified atmosphere
containing 5% CO2 for 48 h. The medium was exchanged for
RPMI-1640 medium containing LH and incubated at 37˚C in a
humidified atmosphere containing 5% CO2 for 48 h. The
medium in the individual wells was collected and stored at -80˚C
until measurement of the IL-2 concentrations in the medium using an
ELISA kit (Mouse IL-2 Quantikine ELISA kit, cat. no. DY402-05,
R&D Systems, Inc.) according to the manufacturer's
instructions.
3T3-L1 cell culture
Medium-low-glucose DMEM (Merck KGaA) containing 10%
FBS, isobutyl-methylxanthine (IBMX), dexamethasone (DEX) and
insulin (all FUJIFILM Wako Pure Chemical Corporation) and
Antibiotic-Antimycotic Mixed Stock Solution were used for cell
culture at 37˚C in a humidified atmosphere containing 5%
CO2.
3T3-L1 cell differentiation
3T3-L1 cells, a fibroblast isolated from the embryo
of a mouse, were purchased from the Japanese Collection of Research
Bioresources Cell Bank (National Institutes of Biomedical
Innovation, Health, and Nutrition, Ibaraki, Japan). 3T3-L1 cell
cultivation and differentiation were performed using a previously
described method (18). 3T3-L1
cells at the 7th passage were cultured at 37˚C with 5%
CO2 in DMEM supplemented with 10% FBS and
antibiotic/antimycotic. To individual wells of a 24-well plate,
3x104 cells/well were seeded and the plate was incubated
for 48 h in maintenance medium (10% FBS and 1%
antibiotic/antimycotic in DMEM) at 37˚C in a humidified atmosphere
containing 5% CO2. After reaching 90% confluence, the
medium was changed to differentiation induction medium (0.5 mM IBMX
and 1.0 µM DEX in the maintenance medium) and incubated at 37˚C for
48 h in a humidified atmosphere containing 5% CO2. The
medium was replaced with differentiation medium (1.7 µM insulin in
the maintenance medium) and incubated at 37˚C for 48 h in a
humidified atmosphere containing 5% CO2.
Oil Red O (ORO) staining and
adiponectin secretion determination
3T3-L1-derived adipocytes were used to determine the
lipid accumulation and adiponectin secretion values. After the
3T3-L1 cell differentiation, the medium was replaced with
maintenance medium containing samples (0.00001, 0.0001, 0.001, 0.01
mg/ml) or pioglitazone (0.28, 2.8, 28, 280 µM), respectively, and
incubated for 96 h (medium was changed every 48 h) at 37˚C in a
humidified atmosphere containing 5% CO2. Medium in the
individual wells was collected and stored at -80˚C until use for
the determination of the adiponectin level using the ELISA kit
(Mouse adiponectin/Acrp30 DuoSet® ELISA, cat. No.
DY1119-05, R&D Systems, Inc.) according to the manufacturer's
instructions. Cells were fixed with 10% formaldehyde for 1 h at
room temperature. Following incubation in 2-propanol for 1 min at
room temperature, the lipid droplets in the cells were stained with
3 mg/ml ORO in 60% 2-propanol solution for 20 min at room
temperature. After washing with 60% 2-propanol, 4%
triton-X-100/2-propanol solution was added to the well for 5 min at
room temperature to extract ORO in lipid droplets of adipocytes.
The absorbance of the extracted solution was measured at 492 nm
with a spectrophotometer and the lipid accumulation value was
quantified using an ORO standard curve. Pioglitazone (FUJIFILM Wako
Pure Chemical Corporation) was used as a positive control.
Cytokine secretion assay
A total of 12 6-week-old male BALB/c mice were
divided into three groups (n=4/group) and allowed free access to
0.1% water solution of methanol extract of LH or LT for 14 days.
Four days later, mice were subcutaneously injected with 50 µl 1
mg/ml concanavalin A suspended in complete Freund's adjuvant (Merck
KGaA) through the tail to stimulate T cells. After 7 days,
splenocytes were prepared as aforementioned. A total of
5x106 splenocytes and 4 µg concanavalin A were added to
the individual wells of a 24-well plate. The plate was incubated at
37˚C in a humidified atmosphere containing 5% CO2. The
medium in the individual wells was collected following 25 and 50 h
of incubation and stored at -80˚C until measurement of the IL-10,
IL-17 and TNF-α concentrations in medium using an ELISA kit (Mouse
IL-10, cat. no. DY417-05, IL-17, cat. No. DY421-05, and TNF-α, cat.
No. DY410-05, Quantikine ELISA kits, respectively, R&D Systems,
Inc.) according to the manufacturer's instructions.
Fasting blood glucose and adiponectin
levels
A total of 15 6-week-old male BALB/c mice were
divided into three groups (n=5/group) and allowed free access to
0.1% or 0.2% water solution of methanol extract of LH to 0.1%
LH-loaded group or 0.2% LH-loaded group for 14 days, respectively,
to determine the dose-dependent effect of LH. To determine
low-(0.1%) and high-dose (0.2%) concentrations of LH, voluntary
water intake/day of mice was observed so that the mice ingested 100
or 200 mg/day sample extract. After fasting for 15 h, the blood
glucose levels of mice in the tail vein were measured using a blood
glucose meter and test paper (ACCU-CHECK® Aviva Strip,
Roche Diagnostics Co., Ltd.). After mouse euthanasia, the blood was
collected from the heart by heparin-treated syringe and centrifuged
at 15,000 x g for 5 min at room temperature to obtain plasma and
adiponectin concentration was measured using an ELISA kit (Mouse
adiponectin/Acrp30 DuoSet® ELISA, cat. No. DY1119-05,
R&D Systems, Inc.) according to the manufacturer's
instructions.
Flow cytometry sample preparation
A total of 15 6-week-old male BALB/c mice were
divided into three groups (n=5/group) and allowed free access to
0.1% or 0.2% water solution of LH for 14 days. Furthermore, obese
13-week-old male BALB/c mice established by the administration of
high-fat diet (D12492, RESEARCH DIETS Inc.) were divided into two
groups (n=6/group) and allowed free access to 0.1% water solution
of LH for 30 days. Four days later, the mice were subcutaneously
injected with 50 µl 1 mg/ml concanavalin A in complete Freund's
adjuvant (Merck KGaA) through the tail to stimulate T cells. After
7 days, spleen and subcutaneous fat were extirpated under sterile
conditions following mouse euthanasia via inhalation of isoflurane.
The splenocytes were prepared as aforementioned. The subcutaneous
fat was minced in RPMI-1640 medium containing 0.5% collagenase type
4 (Worthington Biochemical Corporation) using dissecting scissors.
After incubation at 37˚C for 30 min under shaking, DMEM containing
2% FBS was added to further mince the tissue by pipetting and
filtered through a 40-µM mesh. The tissue suspension was
centrifuged at 800 x g for 5 min at room temperature to separate
the adipocyte and the stromal vascular cell fraction. Lymphocytes
were isolated from stromal vascular cells using
Lympholyte®-M Cell Separation Media (Cedarlane).
Cell staining and flow cytometric
analysis
Splenocytes and lymphocytes isolated from stromal
vascular cell fraction were fixed and permeabilized using
Fixation/Permeabilization Concentrate and Diluent kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions and which divided into 100 µl to four tubes (tubes
A-D). To analyze the percentage of CD4+ cells in the
lymphocytes, the splenocytes or the lymphocytes in Tube B were
stained with 180-fold diluted Super Bright 600-conjugated CD4
antibody [monoclonal (RM4-5), Super Bright™ 600,
eBioscience™, cat. no. 63-0042-82, Thermo Fisher
Scientific Inc.]. To analyze the percentage of Tregs
(CD4+ CD25+ Foxp3+ T cells),
IL-10+ Tregs (CD4+ Foxp3+
IL-10+ T cells), cytotoxic T-lymphocyte antigen
(CTLA)-4+ Tregs (CD4+ Foxp3+
CTLA-4+ T cells) and T helper (Th)17 (CD4+
IL-17A+ T cells), the splenocytes or the lymphocytes in
Tube C were stained with 180-fold diluted Super Bright
600-conjugated CD4 (cat. no. 63-0042-82) and 160-fold diluted
PE-Cyanine5-conjugated CD25 antibody [CD25 monoclonal antibody
(PC61.5), PE-Cyanine5, cat. no. 15-0251-82)], 20-fold diluted Alexa
Fluor 488-conjugated Foxp3 [(cat. no. 53-4774-42)], 80-fold diluted
Alexa Fluor 700-conjugated IL-10 [IL-10 monoclonal antibody
(JES5-16E3), Alexa Fluor 700, cat. no.56-7101-82)], 80-fold diluted
phycoerythrin-conjugated CTLA-4 antibody [CD152 (CTLA-4 monoclonal
antibody (UC10-4B9), PE, cat. no. 12-1522-82] and 80-fold diluted
eFluor 450-conjugated IL-17A antibody [IL-17A monoclonal antibody
(eBio17B7), eFluor 450, cat. no. 48-7177-82; all
eBioscience™, Thermo Fisher Scientific, Inc.]. To
analyze the percentage of CD8+ T cells in the
lymphocytes, the splenocytes or the lymphocytes in Tube D were
stained with 80-fold diluted Super Bright 702-conjugated CD8
antibody (cat. no. 67-0081-82; Thermo Fisher Scientific, Inc.). The
splenocytes or the lymphocytes in Tube A were not stained. These
tubes were incubated on ice for 1 h. Flow cytometric analysis was
conducted using Attune NxT Acoustic Focusing Cytometer and the data
were analyzed using the Attune NxT Software version 2.6 (Thermo
Fisher Scientific, Inc.).
High performance liquid chromatography
(HPLC)
Methanol extracts of LH and LT were sonicated with
methanol at 40 kHz, 40˚C for 1 h and filtrated through a 0.45-µm
filter membrane to obtain solutions with a concentration of 1
mg/ml. Formononetin (0.05 mg/ml) was purchased from Tokyo Chemical
Industry Co., Ltd. Lespedezaflavanone H (0.025 mg/ml) was gifted by
Professor Toshio Miyase, School of Pharmaceutical Sciences,
University of Shizuoka (Shizuoka, Japan). HPLC analysis of methanol
extracts of LH and LT was performed by LC-20AD Solvent delivery
unit (Shimadzu Scientific Instruments) with COSMOSIL 5PE-MS packed
column (4.6x250.0 mm, 5 µm particle size), column oven (cat. no.
CO631C, GL Sciences) and UV detector (UV-VIS detector S-3170, Soma
Optics, Ltd.). Analysis was performed using the following
conditions: Mobile phase, acetonitrile:water=60:40; flow rate, 1
ml/min; detection wavelength, 214 and 280 nm; injection volume, 10
µl and column temperature, 40˚C. The peak areas were analyzed by
Chromato-PRO version 5.0.0.199 (Runtime Instruments Co., Ltd.).
Statistical analysis
Statistical analysis was conducted using the
SigmaStat statistical software ver. 2.03 (SPSS Inc.). Student's
unpaired t test, and one-way ANOVA followed by Dunnett's multiple
comparison or Bonferroni's test were performed for comparisons.
P<0.05 was considered to indicate a statistically significant
difference. Data are represented the mean ± SD of two independent
experiments.
Results
LH exhibits PPARγ binding and radical
scavenging activity
PPARγ binding activity of LB, LH, LM, and LT was
determined by ELISA. Pioglitazone, a PPARγ agonist, had significant
binding activity on PPARγ (Fig.
1A). LB had significantly reduced PPARγ binding activity at
0.038, 0.150, 0.300 mg/ml. LM exhibited no change in binding
activity; LH exhibited a significant increase at 0.300 but decrease
in 0.038 mg/ml compared with that of the control. LT had
significantly increased binding activity at 0.075-0.300 mg/ml
(Fig. 1B). Trolox exhibited a
dose-dependent increase in DPPH radical scavenging activity
(Fig. 1C). LH and LT exhibited a
dose-dependent increase in these DPPH scavenging, with LH having a
stronger radical scavenging activity than LT at 6.25-500.00 µg/ml
(Fig. 1D).
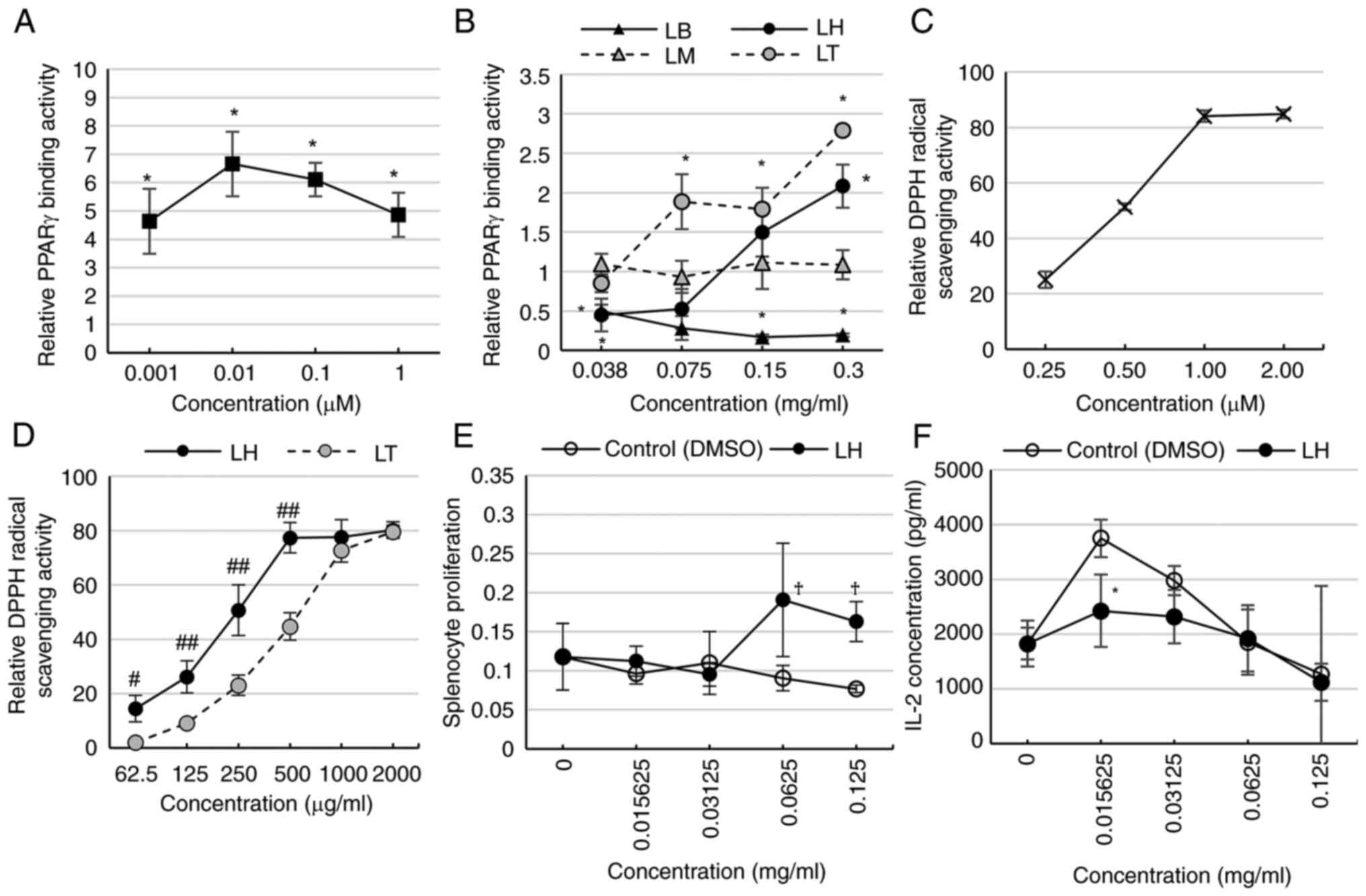 | Figure 1Comparison of PPARγ binding and
radical scavenging activity. (A) PPARγ binding activity of
pioglitazone (positive control). (B) Comparison of PPARγ binding
activity among LB, LH, LM, and LT. (C) DPPH radical scavenging
activity of Trolox (positive control). (D) Comparison of DPPH
radical scavenging activities between LH and LT. (E) Splenocyte
toxicity of LH. No significant differences between LH and the
control were observed. (F) IL-2 secretion of LH from splenocytes.
*P<0.05 vs. control, #P<0.05,
##P<0.01 vs. LT, †P<0.05 vs. 0 mg/ml.
LB, Lespedeza buergeri; LH, Lespedeza homoloba; LM,
Lespedeza maximowiczii; LT, Lespedeza thunbergii. |
LH suppresses IL-2 secretion from
splenocytes
PPARγ expression in T cells decreases the production
of inflammatory cytokines (19). To
investigate whether PPARγ-agonistic LH suppressed the production of
inflammatory cytokine IL-2, murine splenocytes were incubated with
LH, and the splenocyte toxicity and IL-2 secretion from splenocytes
were assessed. Splenocyte toxicity was not observed in the presence
of LH whereas LH showed significant proliferation of splenocytes at
0.0625 and 0.1250 compared with that at 0 mg/ml (Fig. 1E). IL-2 secretion from splenocytes
was evaluated following the addition of LH. LH significantly
suppressed IL-2 secretion at 0.15625 mg/ml compared with the
control (Fig. 1F).
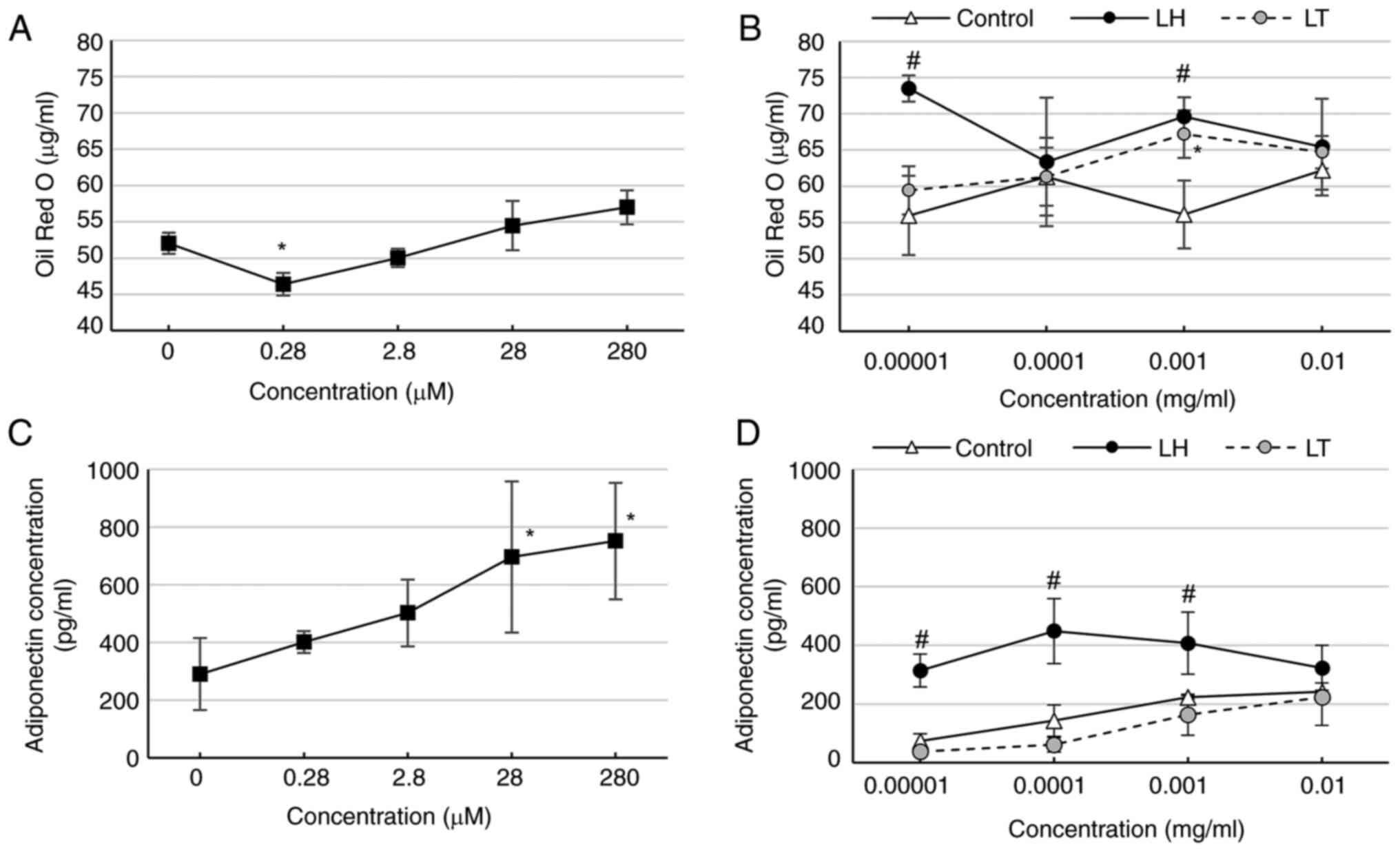
LH increases lipid accumulation and
adiponectin secretion of 3T3-L1-derived adipocytes
Activating of PPARγ expressed in adipocytes
increases lipid accumulation (20)
and adiponectin secretion (21).
Therefore, LH and LT, which showed PPARγ binding activity, were
evaluated for lipid accumulation and adiponectin secretion of
3T3-L1-derived adipocytes. Pioglitazone, significantly decreased
lipid accumulation at 0.28 µM (Fig.
2A). LH exhibited significantly increased lipid accumulation at
0.00001 and 0.00100 mg/ml, whereas LT showed significantly
increased accumulation at 0.00100 mg/ml (Fig. 2B). Pioglitazone significantly
increased adiponectin concentration at 28-280 µM (Fig. 2C). Further-more, LH showed increased
adiponectin concentration at 0.00001-0.00100 mg/ml (Fig. 2D).
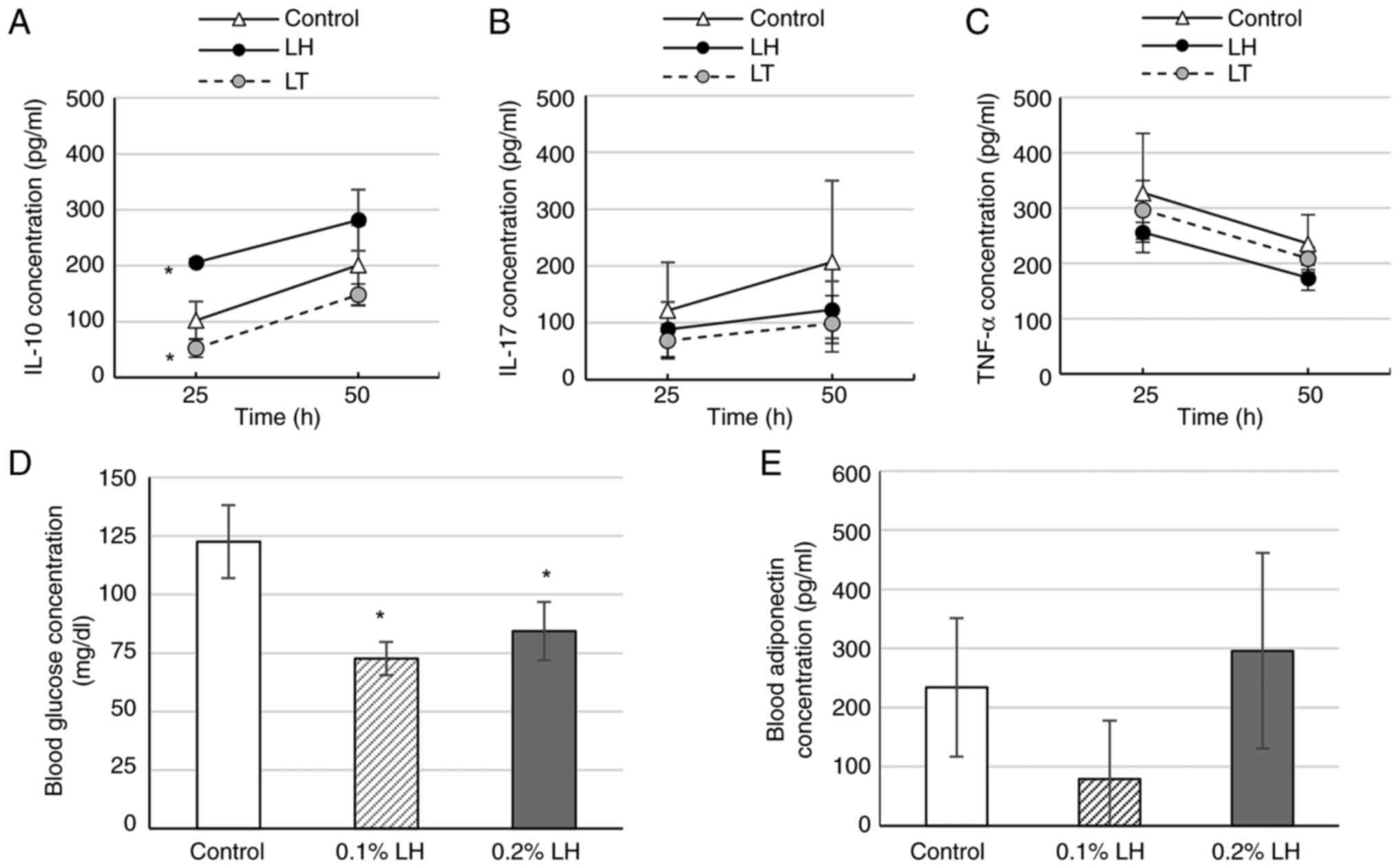
LH promotes IL-10secretion from
splenocytes
To determine whether LH and LT influence the levels
of anti-inflammatory cytokine IL-10 as well as proinflammatory
cytokines IL-17 and TNF-α secreted from the spleen, splenocytes of
0.1% LH- or LT-exposed mice were cultured. At 25 h, the splenocytes
of 0.1% LH-exposed mice exhibited significantly increased IL-10
levels, whereas those of 0.1% LT-exposed mice exhibited
significantly decreased IL-10 levels (Fig. 3A). Furthermore, splenocytes of LH-
and LT-exposed mice exhibited decreased IL-17 levels, but the
decrease was had not significant (Fig.
3B). Similarly, splenocytes of 0.1% LH-exposed mice decreased
exhibited TNF-α levels, but the decrease was not significant
(Fig. 3C).
LH suppresses fasting blood glucose
but does not affect blood adiponectin levels
PPARγ agonists increase levels of glucose
transporter (GLUT)-4 in adipocytes of peripheral tissues such as
muscle and liver to facilitate glucose uptake (22) and promote translocating fatty acids
in the peripheral tissues to adipose tissues, leading to
suppression of blood glucose levels (23). In white adipose tissue, PPARγ
agonists promote adiponectin transcription, synthesis and secretion
(21). Therefore, it was determined
whether LH, which exerted PPARγ agonistic activity, affects blood
glucose and adiponectin levels. Mice exposed to 0.1 and 0.2% LH
exhibited significantly decreased fasting blood glucose levels
compared with the control mice (Fig.
3D). 0.1% LH-exposed mice showed lower fasting adiponectin
levels whereas 0.2% LH-exposed mice showed higher fasting blood
adiponectin levels than the control mice, but these levels were not
significantly different (Fig.
3E).
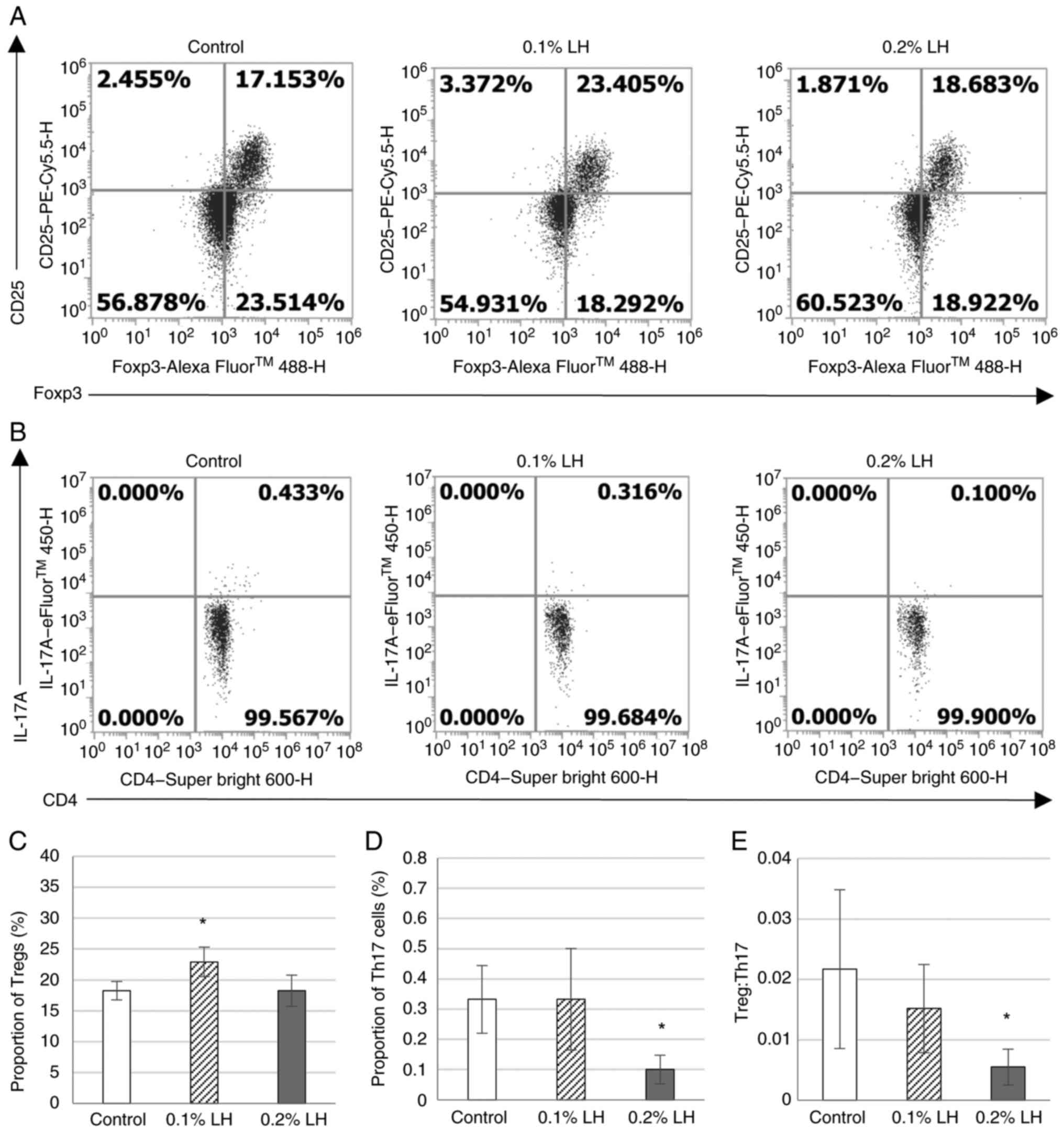
LH-exposed mice exhibit decrease
Th17/Treg in subcutaneous adipose tissue
To determine whether Tregs essential for suppressing
adipose tissue inflammation were increased in subcutaneous fat of
mice, the percentage of CD25+ Foxp3+ Tregs in
the CD4+ T cell population was analyzed via flow
cytometry (Fig. 4A). Th17 cells
mutually maintain a balance with Tregs to regulate the immune
system (24); therefore, the
percentage of IL-17A+ CD4+ Th17 cells in
CD4+ T cells was analyzed via flow cytometry (Fig. 4B). Mice exposed to 0.1% LH had
significantly increased Tregs but there was no effect in mice
exposed to 0.2% LH compared with the control (Fig. 4C). Although mice exposed to 0.2% LH
exhibited significantly decreased Th17 cells and Th17/Treg ratio,
0.1% LH exhibited no significant decrease in Th17 cells and
Th17/Treg ratio compared with control mice (Fig. 4D).
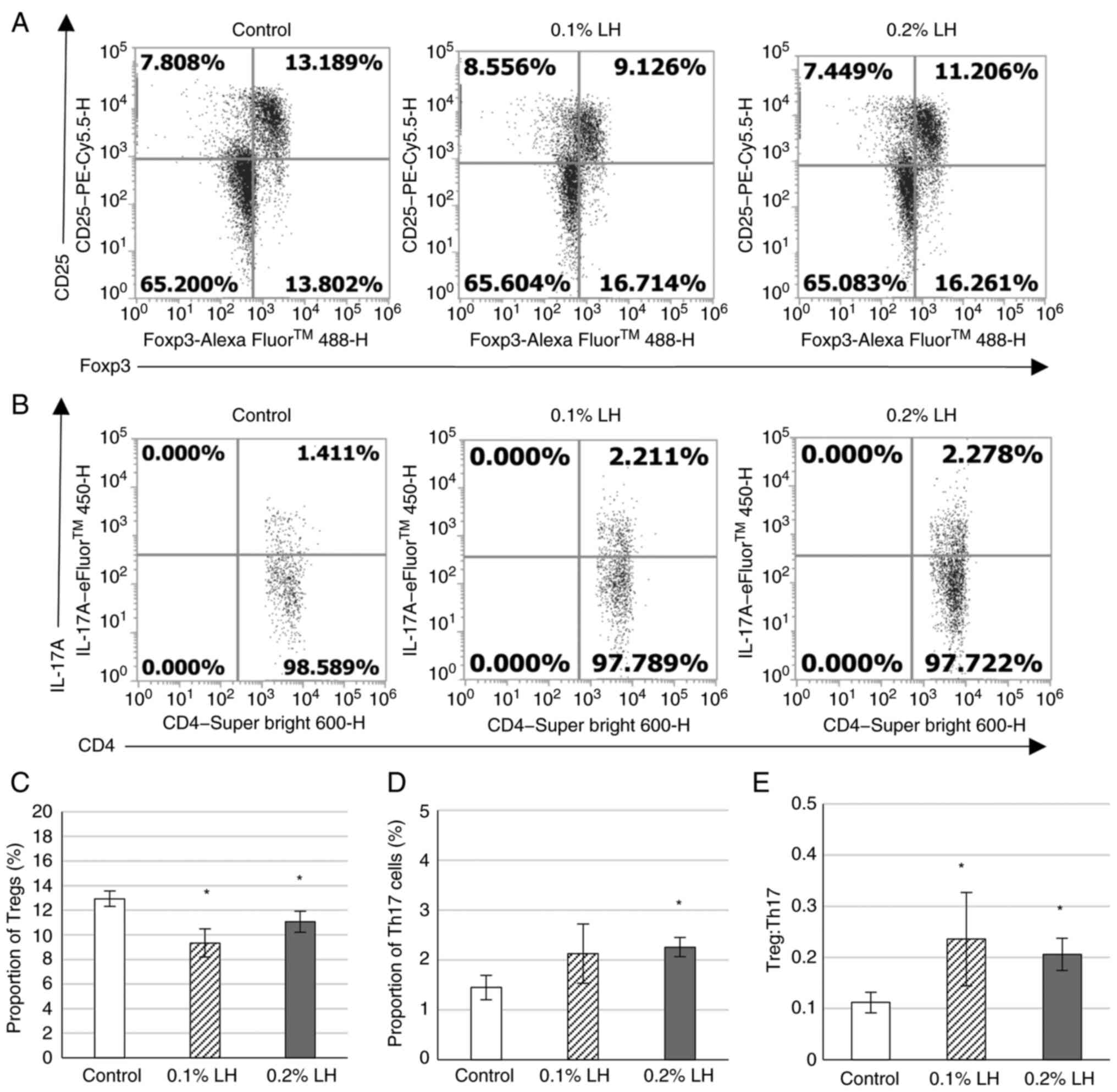
LH-exposed mice exhibit increase
Th17/Treg in the spleen
The proportion of CD25+ Foxp3+
and IL-17A+ CD4+ T cells was analyzed
(Fig. 5A and B). Mice exposed to 0.1 and 0.2% LH had
significantly decreased Tregs compared with the control (Fig. 5C). Mice exposed to 0.2% LH exhibited
significantly increased Th17 cells (Fig. 5D), whereas mice exposed to 0.1 and
0.2% LH had significantly increased Th17/Treg ratio (Fig. 5E).
LH-exposed mice exhibit suppress
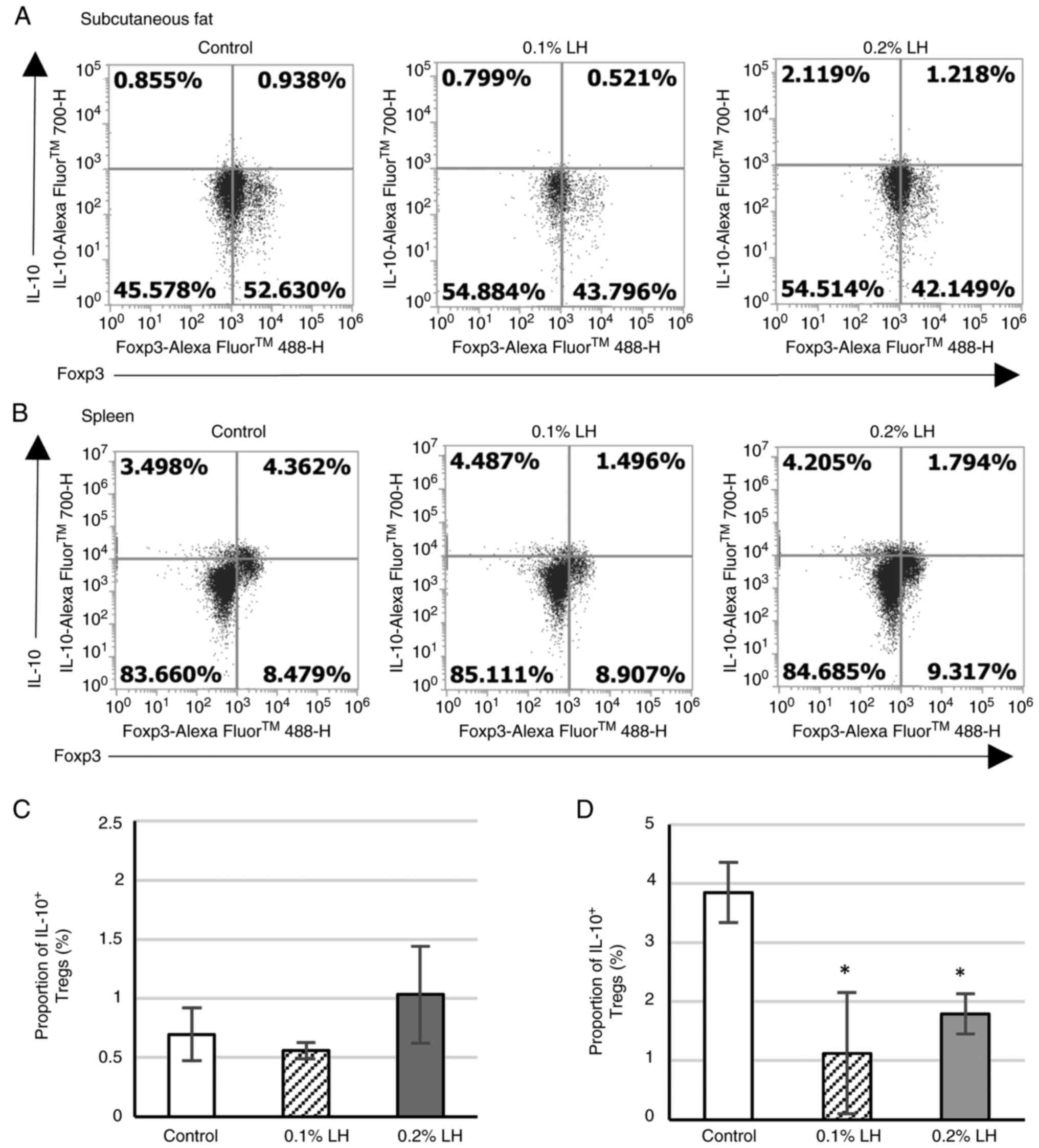
IL-10+ Tregs in the spleen
To determine whether LH creates an immunosuppressive
milieu in subcutaneous adipose tissue by increasing suppressive
cytokine IL-10 secretion from Tregs of the adipose tissue,
IL-10+ Tregs in subcutaneous adipose tissue and spleen
were analyzed via flow cytometry (Fig.
6A and B). No significant
difference was observed in IL-10+ Tregs in subcutaneous
adipose tissue (Fig. 6C); however,
in the spleen, mice exposed to 0.1 and 0.2% LH exhibited
significantly decreased IL-10+ Treg levels (Fig. 6D).
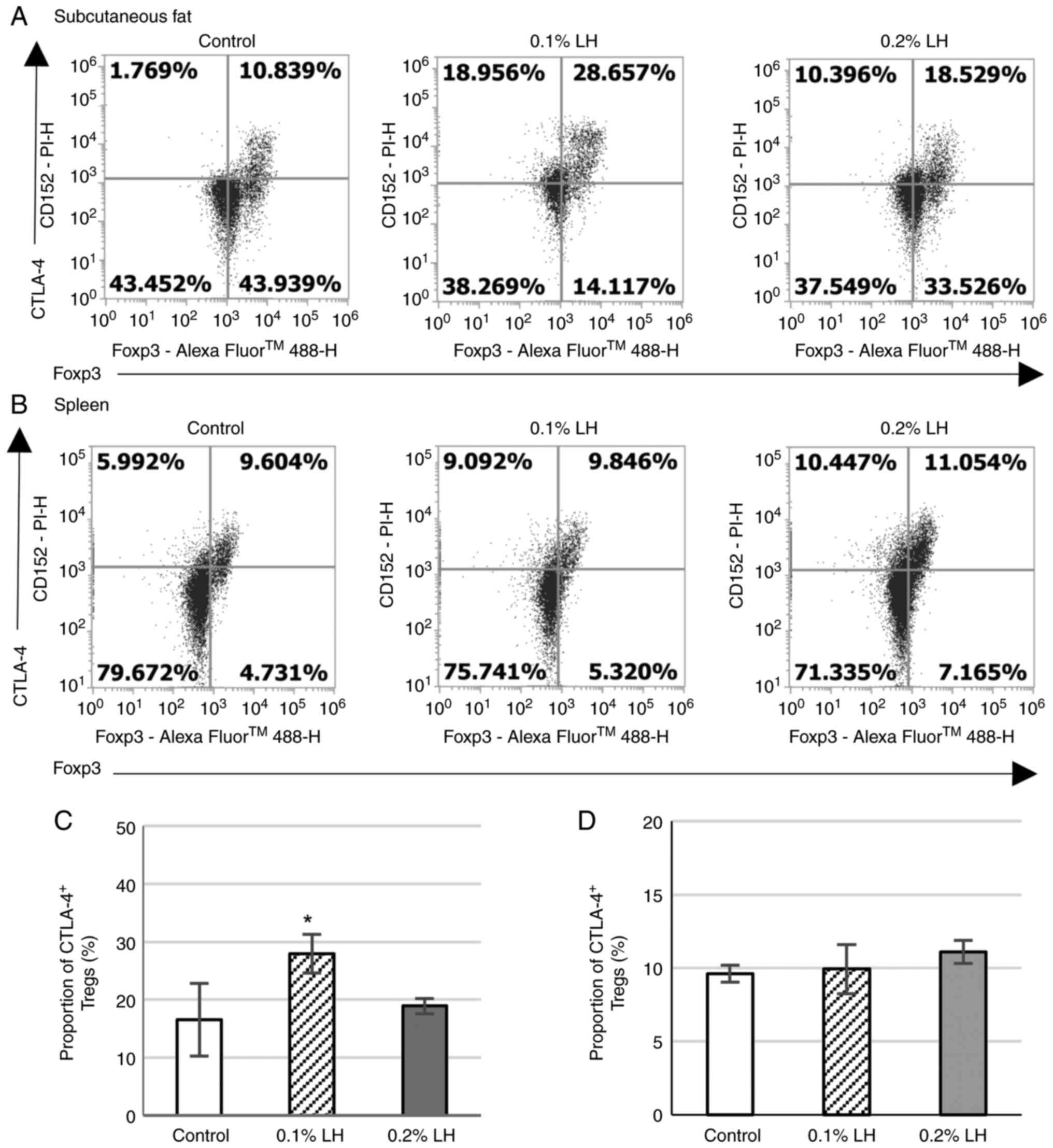
LH-exposed mice increase
CTLA-4+ Tregs in the subcutaneous adipose tissue
To determine whether LH creates an immunosuppressive
milieu in the subcutaneous adipose tissue by increasing CTLA-4
expression on Tregs to induce antigen-presenting cell (APC)
dysfunction, CTLA-4+ Tregs in the subcutaneous adipose
tissue and spleen were analyzed via flow cytometry (Fig. 7A and B). Mice exposed to 0.1% LH exhibited
significantly increased CTLA-4+ Treg levels but there
was no effect on mice exposed to 0.2% LH in the subcutaneous
adipose tissue (Fig. 7C); no
significant difference was observed in CTLA-4+ Tregs in
the spleen for either group (Fig.
7D).
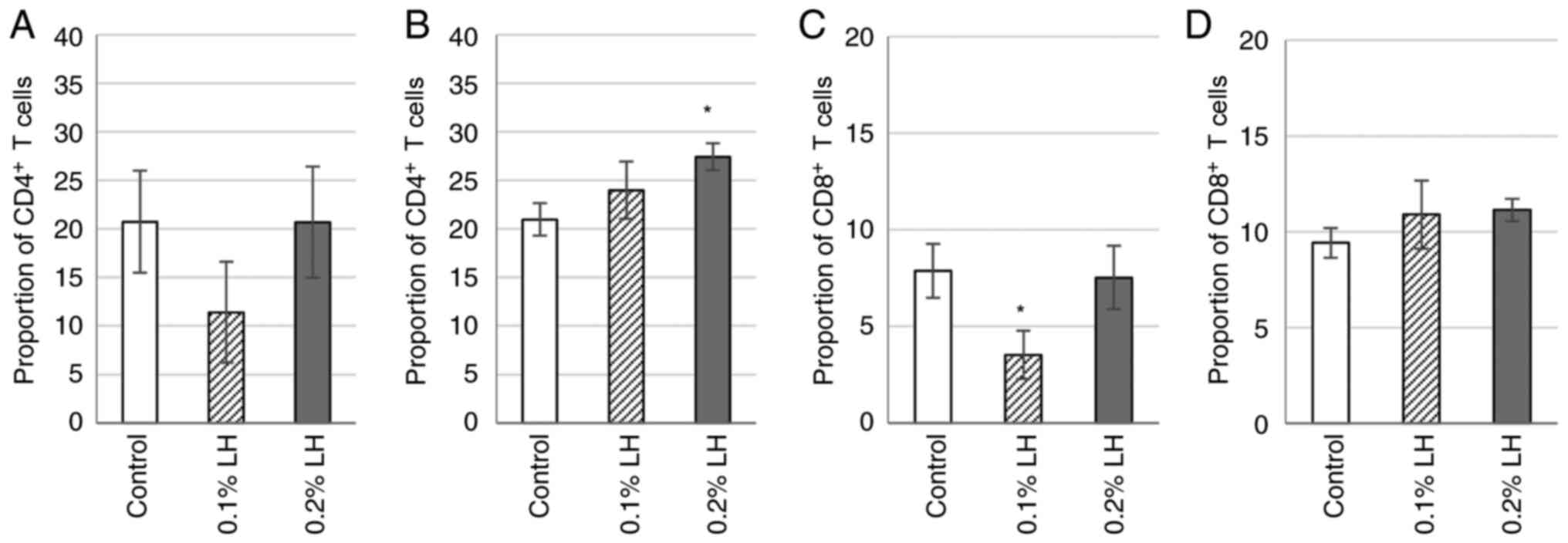
LH-exposed mice exhibit increase
CD4+ T cells in spleen and decrease CD8+ T
cells in subcutaneous fat
To determine whether the significant increase in
CTLA-4+ Tregs induced by LH in subcutaneous adipose
tissue affected expression of CD4+ and CD8+ T
cells, the proportion of CD4+ and CD8+ T
cells in was analyzed via flow cytometry. Mice exposed to 0.1% LH
showed decreased CD4+ T cells in the subcutaneous
adipose tissue (Fig. 8A); however,
in the spleen, CD4+ T cells were increased in LH-exposed
mice, significantly so in 0.2% LH-exposed mice (Fig. 8B). 0.1% LH-exposed mice had
significantly decreased CD8+ T cells in subcutaneous
adipose tissue (Fig. 8C); however,
in the spleen, no significant difference was observed in
CD8+ T cells (Fig.
8D).
Contents of LH with LT
(Fig. S1), peak
areas of No. 1 and 2, 4, 8, 10, and 11 of LH were significantly
higher than those of LT (Table
SI). Formononetin, which is hydroxylated at the 7-position and
methylated at 4'-position of isoflavone, showed approximately 4 min
of the retention time. Lespedezaflavanone H, which is prenylated at
the 6-, 8-, and 2'-positions of flavanone, showed approximately 12
min of the retention time. Based on these results, LH and LT might
contain low-polarity flavonoids, such as isoflavone or prenylated
flavonoids. Although LH and LT contain almost similar components in
the low-polarity flavonoids targeted in this study, the properties
are thought to differ because of the difference in the content of
these components.
Discussion
PPARγ is abundantly expressed in adipose tissue and
modulates expression of proteins involved in lipid metabolism in
adipocytes and adipogenesis. Thiazolidinediones (TZDs), including
pioglitazone and rosiglitazone (PPARγ agonists), promote expression
of adipogenic genes to differentiate preadipocytes into adipocytes
(25), leading to adipocyte
maturation (26). During adipocyte
maturation, PPARγ activation increases expression of fatty acid
transport protein (FATP) 1 and fatty acid-binding protein (FABP) 4,
promoting fatty acid uptake and leading to accumulation of
intracellular triglycerides in adipocytes (20). In adipocytes, PPARγ elevates
expression of farnesoid X receptor gene, which induces expression
of the stearoyl-CoA desaturase gene in the glucose metabolism
pathway to accelerate fatty acid synthesis from glucose (27). PPARγ activation increases
mitochondrial number and the expression of genes involved in
mitochondrial β-oxidation (28).
Here, LH significantly increased lipid accumulation in
3T3-L1-derived adipocytes and thus may increase the expression of
FATP, FABP4 and mitochondria through its PPARγ agonistic activity
leading to fatty acid accumulation in adipocytes.
Pioglitazone significantly decreased lipid
accumulation in 3T3-L1-derived adipocytes at 0.28 µM. Similarly, in
our previous study, rosiglitazone significantly reduced lipid
accumulation in 3T3-L1-derived adipocytes (18). Rosiglitazone suppresses fibroblast
proliferation via modulation of the p38 MAPK pathway, rather than
by binding to PPARγ (29). This
suggests that TZDs decrease the number of adipocytes
differentiating from 3T3-L1 fibroblasts to decrease lipid
accumulation. LH and LT exhibited an increase in PPARγ binding
activity, promoting lipid accumulation in 3T3-L1-derived
adipocytes, suggesting that they have no suppressive effect on
fibroblast proliferation.
In mitochondria, the β-oxidation system generates
reactive oxygen species (ROS). Hydrogen peroxide
(H2O2), a mitochondrial-derived ROS, causes
chronic inflammation; it is converted to a hydroxy radical via
transition metal catalysis, activating transcription factor NF-κB
expressed in macrophages and promoting gene transcription of IL-1,
IL-2, IL-6, IL-8, TNF-α, intercellular adhesion molecule-1, and
inducible NO synthase, all of which contain NF-κB-binding sequences
(25). In hypertrophied adipose
tissue, chronic inflammation is induced by infiltration of
macrophages that secrete proinflammatory cytokines. The activation
of NF-κB, proinflammatory cytokines and inflammasomes increases
risk of developing tissue inflammation and injury (30). Here, LH and LT exhibited significant
binding activity to PPARγ. LH exhibited stronger DPPH radical
scavenging activity than LT, which may enable it to scavenge excess
superoxide anion and mitochondrial-derived ROS. This may prevent
harmful events such as ferroptosis and pyroptosis in adipose
tissue. Activation of PPARγ by the anti-diabetes compound
rosiglitazone suppresses the production of the inflammatory
cytokine IL-2 from intestinal tissue (31). Additionally, the present study
revealed that LH suppressed IL-2 secretion from cultured
splenocytes. PPARγ, activated by its agonist, promotes small
ubiquitin-related modification (SUMOylation) and forms heterodimers
with a DNA-bound repressor, leading to 19S proteasome-mediated
degradation of the heterodimers, which inhibits expression of
inflammatory cytokines (32). PPARγ
SUMOylation increases insulin sensitivity in murine subcutaneous
white adipose tissue (33).
Therefore, LH is hypothesized to inhibit inflammation of adipose
tissue as a PPARγ agonist and a radical scavenger by preventing
expression of ROS and inflammatory cytokines.
Chronic activation of IL-1 receptors on pancreatic
β-cells by IL-1β secreted from microglia induces pancreas
dysfunction and T2D (34). In
obesity, elevated the expression of IL-1β impairs insulin release,
but IL-1 receptor antagonists treat T2D by increasing insulin
secretion from the pancreas (34).
Troglitazone, a PPARγ agonist, has been reported to suppress IL-1β
synthesis in human monocytes (5),
suggesting that PPARγ agonist may suppress T2D onset. Furthermore,
the proinflammatory cytokine TNF-α is associated with insulin
resistance and disrupts homeostasis of lipid and glucose metabolism
(35). Additionally, PPARγ is
expressed in macrophages and its agonist (an antidiabetic agent,
troglitazone) inhibits the production of the inflammatory cytokine
TNF-α in human monocytes (5). LH
induced decreased TNF-α secretion from murine splenocytes compared
with that of the control, although the difference was not
statistically significant. PPARγ binding and radical scavenging
activities of the present species from the genus Lespedeza
were not strong enough to significantly reduce inflammatory factor
TNF-α secretion from splenocytes.
The anti-inflammatory hormone adiponectin is
synthesized almost exclusively in differentiated adipocytes and is
present at high circulating levels. Adiponectin regulates blood
glucose levels, lipid metabolism, and insulin sensitivity by
binding to its receptors, adiponectin receptors 1 (AdipoR1) and 2
(AdipoR2). PPARγ agonist rosiglitazone promotes adiponectin
transcription, synthesis and secretion of inguinal white adipose
tissue (21). Here, the PPARγ
agonist pioglitazone significantly increased adiponectin secretion
from 3T3-L1-derived adipocytes at 28 and 280 µM (Fig. 2C). LH and LT both exhibited
pronounced PPARγ ligand activity; however, increased adiponectin
secretion from 3T3-L1-derived adipocytes was observed only in
response to LH (Fig. 2D). These
findings suggested that LH exerted a similar binding mechanism to
the PPARγ agonist, thereby increasing adiponectin secretion from
3T3-L1-derived adipocytes.
The adipose tissue serves a crucial role in
regulating insulin sensitivity (36). Macrophages and T cells migrate to
hypertrophied adipose tissue with excess lipid accumulation,
thereby activating inflammatory pathways (37). Chronic inflammation of adipose
tissue caused by the migration of proinflammatory macrophages (M1)
and T cells decreases glucose uptake (38) and impairs insulin signaling, leading
to diabetes mellitus (39). An
increase in number of Tregs prevents adipose tissue inflammation
(40), and accumulation of Tregs in
adipose tissue leads to improved insulin sensitivity in diabetes
mellitus (41). CTLA-4, an immune
checkpoint molecule expressed on Tregs, competes with CD28 on
CD4+ T cells and binds to B7 on APCs, thereby expressing
B7 on their surface; this process is referred to as trogocytosis.
This suppresses APC function and decreases the number of
CD4+ T cells. Treatment with CTLA-4Ig, a fusion protein
that binds to B7, induces anti-inflammatory macrophage 2
polarization in adipose tissue (42). CD8+ T cells in adipose
tissue serve a crucial role in macrophage infiltration and the
initiation and propagation of adipose tissue inflammation (43). Th17 cells, characterized by
production of proinflammatory cytokines such as IL-17A, IL-17F,
IL-21, IL-22 and IL-26, promote adipose tissue inflammation
(44). Th17/Treg imbalance
initiates adipose tissue inflammation, leading to insulin
resistance due to deficiency of Rab4b, an insulin-induced glucose
transporter 4 translocation-controlling factor in adipocytes
(24). The present study aimed to
ascertain the effect of LH on the accumulation of Tregs, Th17
cells, and CD8+ T cells in adipose tissue using flow
cytometric analysis. In the subcutaneous adipose tissue, LH induced
a notable increase in Tregs and CTLA-4+ Tregs, along
with a significant decrease in Th17 cells, the Th17/Treg ratio and
CD8+ T cells. These findings suggested that LH shifted the
Th17/Treg balance towards Treg dominance and decreased levels of
CD4+ and CD8+ cells through trogocytosis by
augmenting CTLA-4 on Tregs, thereby mitigating inflammation in
subcutaneous adipose tissue.
A comparison of gene expression profiles between
mouse visceral adipose tissue and lymph node Treg cells has
revealed an upregulation of transcripts encoding PPARγ in mouse
visceral adipose tissue Tregs (45). In addition, PPARγ activation by its
agonist promotes Foxp3 gene transcription and induces effector Treg
generation (46). Furthermore,
intrinsic metabolic programming in immune cells is associated with
immune cell differentiation and function (47,48).
PPARγ agonist promotes the expression of CD36 and carnitine
palmitoyl transferase I, which imports fatty acids into immune
cells and enhances mitochondrial β-oxidation in the immune cell
(49). Furthermore, in in
vitro assay, PPARγ agonist increases CD4+
Foxp3+ Tregs generation from naïve CD4+ T
cells and boosts transcription of IL-10 and CTLA-4 of Tregs,
indicating that PPARγ agonist promotes function of Tregs (49). These findings suggest that by PPARγ
agonistic activity, LH may promote differentiation and function of
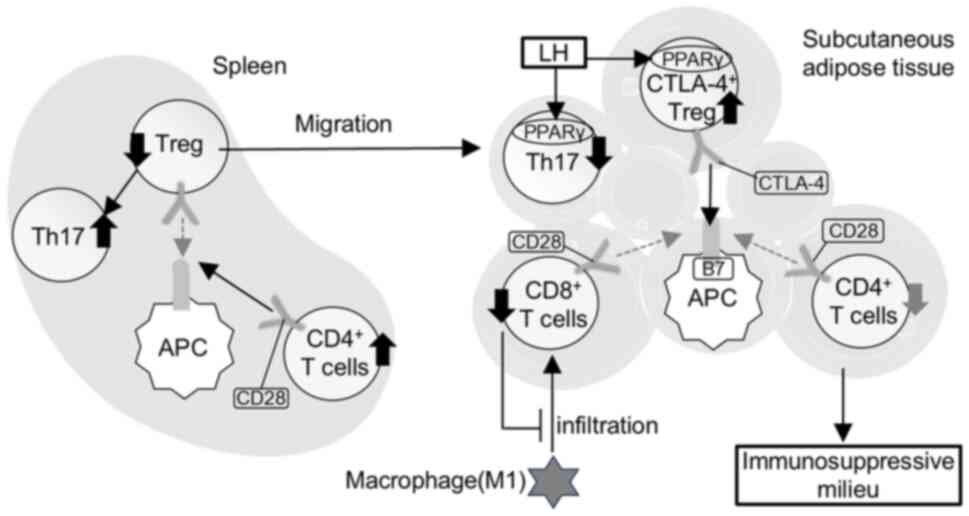
Tregs in adipose tissue. By contrast with the subcutaneous adipose
tissue, LH treatment in the spleen resulted in a substantial
decrease in Tregs with a minimal effect on CTLA-4+
Tregs, a notable increase in Th17 cells and the Th17/Treg ratio and
an increasing trend in CD8+ T cells. A splenic Treg
population expressing low levels of PPARγ contains Treg precursors
migrating to adipose tissue (50).
Therefore, LH may promote Treg migration into subcutaneous adipose
tissue from the spleen, thereby increasing the number of Th17 and
CD8+ T cells due to decreased Tregs in the spleen
(Fig. 9).
Various mechanisms have been proposed for
immunosuppressive mechanism of Foxp3+ Tregs, including
the anergy (immune unresponsiveness) of APC caused by CTLA-4 on
Tregs, immunosuppression by immunosuppressive cytokines IL-10 and
TGF-β secreted from Tregs and the suppression of inflammatory
response via adenosine production by enzymes CD39/CD73 on Tregs.
The adipose tissue of mice administered 0.1% LH showed a decrease
in CD4+ T cells due to an increase in CTLA-4+
Tregs, thus inducing anergy in APCs. By contrast, the adipose
tissue of mice administered 0.2% LH showed an increase in
IL-10+ Tregs. It has been reported that an increase in
IL-10 in adipose tissue suppresses differentiation of naïve T cells
into Th17(51). Therefore, 0.2% LH
was hypothesized to decrease Th17 cells owing to an increase in
IL-10+ Tregs and induce an immunosuppressive milieu in
adipose tissue.
IL-10 serves various roles as an anti-inflammatory
cytokine. Activation of the IL-10 signaling pathway in the
intestine causes phosphorylated STAT3 (pSTAT3)-mediated
anti-inflammatory response to induce epithelial cell proliferation,
leading to healing of inflammatory bowel disease (52-54).
In neurons and astrocytes, IL-10 promotes neurogenesis via the
suppression of NF-κB signaling associated with the pSTAT3-mediated
anti-inflammatory response in healing neuronal damage (55). In adipocytes, activation of the
IL-10/STAT3 signaling cascade suppresses expression of thermogenic
genes; thus, energy expenditure is limited (54). IL-10 ablation in white adipose
tissue improves insulin sensitivity by increasing thermogenesis,
energy expenditure and browning of white adipose tissue in mice
(56). In humans, IL-10 is
primarily produced by inflammatory immune cells in the white
adipose tissue to promote insulin resistance (57). AdipoR1 is expressed on Tregs
(58) and adiponectin stimulation
of AdipoR1 promotes secretion of the anti-inflammatory cytokine
IL-10 from Tregs (59). In
subcutaneous adipose tissue, IL-10+ Tregs showed a
decreasing trend in 0.1% LH-treated mice and an increasing trend in
0.2% LH-treated mice, similar to the trend observed for blood
adiponectin levels. Additionally, blood glucose levels were higher
in 0.2% LH-treated mice than in 0.1% LH-treated mice, suggesting
that insulin sensitivity decreased with increasing
IL-10+ Tregs in adipose tissue. Furthermore,
polyunsaturated fatty acids, endogenous ligands for nuclear
retinoic acid receptor-related orphan receptor γ-expressed in Th17
cells, promote RORγt binding to IL-10 promoter region to increase
IL-10 production from Th17(60).
Although cultured splenocytes of 0.1% LH-treated mice exhibited
increased IL-10 secretion, mice exposed to 0.1 and 0.2% LH
exhibited significantly decreased splenic IL-10+ Tregs
(Fig. 6D) and notably increased
splenic Th17 cells. These findings suggest the possibility that LH
increased IL-10 secretion from splenic Th17.
Elevated fasting glucose levels indicate a high
risk of type 2 diabetes, whereas postprandial hyperglycemia
indicates early-stage diabetes. Here, exposure to 0.1% LH in mice
significantly decreased fasting blood glucose levels, which was
accompanied by a substantial increase in number of
CTLA-4+ Tregs in subcutaneous adipose tissue. In 0.2%
LH-treated mice, a significant decrease in fasting blood glucose
levels was observed due to a notable decrease in Th17 cell number
and Th17/Treg ratio in subcutaneous adipose tissue. Tregs improve
glucose metabolism and insulin sensitivity in female mice by
promoting subcutaneous adipose tissue browning and thermogenesis
(61). PPARγ ligands, including
rosiglitazone and pioglitazone, selectively suppress Th17 cell
differentiation (62) and promote
Treg differentiation (46). IL-17
knockout mice exhibit increased insulin sensitivity, glucose
tolerance and serum adiponectin levels (63). These findings suggest that LH may
prevent type 2 diabetes by increasing Tregs and decreasing Th17
cells in adipose tissue. Furthermore, to investigate whether LH
generates an immunosuppressive milieu in adipose tissue of obese
mice, 0.1% LH solution was administered for 30 days to male BALB/c
mice which showed a significant increase in body weight after
feeding high-fat diet for 8 weeks. The subcutaneous adipose tissue
was analyzed using flow cytometry. Although LH caused a slight
decrease in Th17 cells, it did not affect Tregs (data not shown).
Therefore, it was hypothesized that LH has no therapeutic effect on
insulin resistance in obesity or diabetes.
LH, which is endemic to Japan, has been reported to
exert a scavenging effect on superoxide anion radicals, chelae
Fe2+, which causes lipid peroxidation and exerts an
antiallergic effect (64). Miyase
et al (64,65) revealed that Lespedeza spp.
are rich in isoflavones isoflav-3-en like haginin E and
pterocarp-6a-en, which have hydroxyl groups at the C-3 and C-9
positions. Lespedezols E1, A6 and
E2, which are prenylated at the 8-, 10-, and
5'-positions of isoflavones, respectively, have also been isolated
from LH. Miyase et al (64,65)
reported that lipophilic isoflavone derivatives are active
compounds among Lespedeza spp. because Lespedeza
species can easily hybridize with other species of Lespedeza
and biosynthesize similar constituents (64). Although the LH and LT used here may
contain low-polarity flavonoids, such as isoflavones or prenylated
flavonoids and contain similar constituents as shown in HPLC
profiles, their properties are hypothesized to differ because of
the difference in the content of these components. Prenylflavonoids
are known to accumulate in high concentrations and persist for long
periods in adipose tissue because of their high lipophilic
properties (66).
8-prenylnaringenin, naringenin prenylated at the 8-position, has
been suggested to have greater absorption in the body than
naringenin and efficient accumulation in target tissue (9). Therefore, the prenylflavonoid
constituents of LH may accumulate in adipose tissue and establish
an anti-inflammatory environment, thereby improving insulin
sensitivity and suppressing increased blood glucose levels.
LH activated nuclear receptor PPARγ, suggesting
that components of LH permeated into the nucleus. Therefore, the
adverse effects of long-term LH administration should be
considered. Carnitine shuttle is key for mitochondrial fatty acid
metabolism because the shuttle transport acyl moieties across the
inner mitochondrial membrane for mitochondrial β-oxidation
(67). However, TZDs, PPARγ
agonists, may increase blood lipids levels by inhibiting the
mitochondrial carnitine shuttle to inhibit mitochondrial
β-oxidation, inducing cardiovascular risk (68). Among PPARγ agonists, rosiglitazone
has been removed from the market because of adverse cardiovascular
risk: Rosiglitazone therapy in 4,447 patients with type 2 diabetes
was confirmed to increase risk of heart failure after 5 years of
treatment (69), whereas
pioglitazone significantly delayed the time to heart attack, acute
coronary syndrome and stroke in 4,373 patients with type 2 diabetes
following 4 years of treatment (70). The mechanism underlying the
difference in cardiovascular risk development with PPARγ agonists
remains unclear; however, long-term administration of PPARγ
agonists must be considered for cardiovascular risk. However,
administering 740 mg/day epimedium, which contains 48.2%
prenylflavonoids, for 6 weeks in clinical trials did not show any
adverse symptoms or significant changes in hepatic, hematological,
or renal indices (71). These
findings raise the possibility that LH, which mainly contains
prenylflavonoids has less risk of cardiovascular disease for
long-term administration.
The present study shows that LH exposure suppresses
fasting blood glucose levels and suggests a mechanism that LH
exerts a similar binding mechanism to the PPARγ agonist and
promotes differentiation and function of Tregs by the activation of
PPARγ expressed on the Tregs, which shifts Th17/Treg balance
towards Treg dominance, thereby mitigating inflammation in
subcutaneous adipose tissue. Prenylflavonoid constituents of LH are
known to accumulate in high concentrations and persist for long
periods in adipose tissue because of their high lipophilic
properties and the constituents exhibited a strong binding to
PPARγ. Therefore, LH is thought to establish an anti-inflammatory
environment in adipose tissue to improve the function of adipose
tissue.
Supplementary Material
High performance liquid chromatography
chromatograms of LH and LT. Chromatograms of LH were recorded at
(A) 214 and (B) 280 nm. Chromatograms of LT were recorded at (C)
214 and (D) 280 nm. Chromatograms of (E) formononetin and (F)
lespedezaflavanone H were recorded at 214 nm.
Comparison of peak areas between LH
and LT.
Acknowledgements
The authors would like to thank Ms. Yukie Sato and
Ms. Yu Ting Tang (Tohoku Medical and Pharmaceutical University,
Sendai, Japan) for technical assistance with the experiments.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KK conceived the study, designed and performed
experiments, analyzed data and wrote and reviewed the manuscript.
AT analyzed data and performed experiments. KS designed experiments
and interpretation of data. KK and AT confirm the authenticity of
all the raw data. KS made substantial contributions to supervision
and validation. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by Animal
Experimental Committee of Tohoku Medical and Pharmaceutical
University (approval no. 23032-a).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Surmi BK and Hasty AH: Macrophage
infiltration into adipose tissue: Initiation, propagation and
remodeling. Future Lipidol. 3:545–556. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shimobayashi M, Albert V, Woelnerhanssen
B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S,
Colombi M, Meier JA, et al: Insulin resistance causes inflammation
in adipose tissue. J Clin Invest. 128:1538–1550. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Fooks AN, Beppu LY, Frias AB and D'Cruz
LM: Adipose tissue regulatory T cells: Differentiation and
function. Int Rev Immunol. 42:323–333. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shen N, Wang T, Gan Q, Liu S, Wang L and
Jin B: Plant flavonoids: Classification, distribution,
biosynthesis, and antioxidant activity. Food Chem.
383(132531)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yuan D, Guo Y, Pu F, Yang C, Xiao X, Du H,
He J and Lu S: Opportunities and challenges in enhancing the
bioavailability and bioactivity of dietary flavonoids: A novel
delivery system perspective. Food Chem. 430(137115)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mukai R, Fujikura Y, Murota K, Uehara M,
Minekawa S, Matsui N, Kawamura T, Nemoto H and Terao J: Prenylation
enhances quercetin uptake and reduces efflux in Caco-2 cells and
enhances tissue accumulation in mice fed long-term. J Nutr.
143:1558–1564. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
American Veterinary Medical Association
(AVMA): AVMA guidelines for the euthanasia of animals: 2020
edition. American Veterinary Medical Association, Schaumburg, IL,
2020.
|
|
11
|
Lee SJ, Hossaine MDA and Park SC: A
potential anti-inflammation activity and depigmentation effect of
Lespedeza bicolor extract and its fractions. Saudi J Biol
Sci. 23:9–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mariadoss AVA, Park S, Saravanakumar K,
Sathiyaseelan A and Wang MH: Phytochemical profiling, in vitro
antioxidants, and antidiabetic efficacy of ethyl acetate fraction
of Lespedeza cuneata on streptozotocin-induced diabetic
rats. Environ Sci Pollut Res Int. 30:60976–60993. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim NK, Park HM, Lee J, Ku KM and Lee CH:
Seasonal Variations of metabolome and tyrosinase inhibitory
activity of Lespedeza maximowiczii during growth periods. J
Agric Food Chem. 63:8631–8639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bae J, Lee D, Lee TK, Song JH, Lee JS, Lee
S, Yoo SW, Kang KS, Moon E, Lee S and Kim KH:
(-)-9'-O-(α-l-Rhamnopyranosyl)lyoniresinol from Lespedeza
cuneata suppresses ovarian cancer cell proliferation through
induction of apoptosis. Bioorg Med Chem Lett. 28:122–128.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee JH, Parveen A, Do MH, Lim Y, Shim SH
and Kim SY: Lespedeza cuneata protects the endothelial
dysfunction via eNOS phosphorylation of PI3K/Akt signaling pathway
in HUVECs. Phytomedicine. 48:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Konno T, Sasaki K, Kobayashi K and Murata
T: Indirubin promotes adipocyte differentiation and reduces lipid
accumulation in 3T3-L1 cells via peroxisome proliferator-activated
receptor γ activation. Mol Med Rep. 21:1552–1560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
|
|
18
|
Kobayashi K, Tang YT and Sasaki K:
Paeoniflorin, a constituent of Kami-shoyo-san, suppresses blood
glucose levels in postmenopausal diabetic mice by promoting the
secretion of estradiol from adipocytes. Biochem Biophys Rep.
32(101335)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daynes RA and Jones DC: Emerging roles of
PPARS in inflammation and immunity. Nat Rev Immunol. 2:748–759.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Hardwick JP, Osei-Hyiaman D, Wiland H,
Abdelmegeed MA and Song BJ: PPAR/RXR regulation of fatty acid
metabolism and fatty acid omega-hydroxylase (CYP4) isozymes:
Implications for prevention of lipotoxicity in fatty liver disease.
PPAR Res. 2009(952734)2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Andrade ML, Gilio GR, Perandini LA,
Peixoto AS, Moreno MF, Castro É, Oliveira TE, Vieira TS,
Ortiz-Silva M, Thomazelli CA, et al: PPARγ-induced upregulation of
subcutaneous fat adiponectin secretion, glyceroneogenesis and BCAA
oxidation requires mTORC1 activity. Biochim Biophys Acta Mol Cell
Biol Lipids. 1866(158967)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu Z, Xie Y, Morrison RF, Bucher NL and
Farmer SR: PPARgamma induces the insulin-dependent glucose
transporter GLUT4 in the absence of C/EBPalpha during the
conversion of 3T3 fibroblasts into adipocytes. J Clin Invest.
101:22–32. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Way JM, Harrington WW, Kathleen KB,
Gottschalk WK, Sundseth SS, Mansfield TA, Ramachandran RK, Willson
TM and Kliewer SA: Comprehensive messenger ribonucleic acid
profiling reveals that peroxisome proliferator-activated receptor
gamma activation has coordinate effects on gene expression in
multiple insulin-sensitive tissues. Endocrinology. 142:1269–1277.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gilleron J, Bouget G, Ivanov S, Meziat C,
Ceppo F, Vergoni B, Djedaini M, Soprani A, Dumas K, Jacquel A, et
al: Rab4b deficiency in T cells promotes adipose Treg/Th17
imbalance, adipose tissue dysfunction, and insulin resistance. Cell
Rep. 25:3329–3341.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Oliveira-Marques V, Marinho HS, Cyrne L
and Antunes F: Modulation of NF-kappaB-dependent gene expression by
H2O2: A major role for a simple chemical
process in a complex biological response. Antioxid Redox Signal.
11:2043–2053. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun C, Mao S, Chen S, Zhang W and Liu C:
PPARs-orchestrated metabolic homeostasis in the adipose tissue. Int
J Mol Sci. 22(8974)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shinohara S and Fujimori K: Promotion of
lipogenesis by PPARγ-activated FXR expression in adipocytes.
Biochem Biophys Res Commun. 527:49–55. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bogacka I, Ukropcova B, McNeil M, Gimble
JM and Smith SR: Structural and functional consequences of
mitochondrial biogenesis in human adipocytes in vitro. J Clin
Endocrinol Metab. 90:6650–6656. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Antonelli A, Ferri C, Ferrari SM, Colaci
M, Ruffilli I, Sebastiani M and Fallahi P: Peroxisome
proliferator-activated receptor γ agonists reduce cell
proliferation and viability and increase apoptosis in systemic
sclerosis fibroblasts. Br J Dermatol. 168:129–135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu D, Liu JQ, Mo LH, Luo XQ, Liu ZQ, Wu
GH, Yang LT, Liu DB, Wang S, Liu ZG and Yang PC: Specific
antigen-guiding exosomes inhibit food allergies by inducing
regulatory T cells. Immunol Cell Biol. 98:639–649. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martin H: Role of PPAR-gamma in
inflammation. Prospects for therapeutic intervention by food
components. Mutat Res. 690:57–63. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Katafuchi T, Holland WL, Kollipara RK,
Kittler R, Mangelsdorf DJ and Kliewer SA: PPARγ-K107 SUMOylation
regulates insulin sensitivity but not adiposity in mice. Proc Natl
Acad Sci USA. 115:12102–12111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wiedemann SJ, Trimigliozzi K, Dror E,
Meier DT, Molina-Tijeras JA, Rachid L, Foll CL, Magnan C, Schulze
F, Stawiski M, et al: The cephalic phase of insulin release is
modulated by IL-1β. Cell Metab. 34:991–1003,e6. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cawthorn WP and Sethi JK: TNF-alpha and
adipocyte biology. FEBS Lett. 582:117–131. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Smith U and Kahn BB: Adipose tissue
regulates insulin sensitivity: Role of adipogenesis, de novo
lipogenesis and novel lipids. J Inter Med. 280:465–475.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Invest.
112:1796–1808. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Carey AL, Steinberg GR, Macaulay SL,
Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt
MJ, James DE, et al: Interleukin-6 increases insulin-stimulated
glucose disposal in humans and glucose uptake and fatty acid
oxidation in vitro via AMP-activated protein kinase. Diabetes.
55:2688–2697. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Uysal KT, Wiesbrock SM, Marino MW and
Hotamisligil GS: Protection from obesity-induced insulin resistance
in mice lacking TNF-alpha function. Nature. 389:610–614.
1997.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Lumeng CN, Maillard I and Saltiel AR:
T-ing up inflammation in fat. Nat Med. 15:846–847. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen LW, Chen PH, Tang CH and Yen JH:
Adipose-derived stromal cells reverse insulin resistance through
inhibition of M1 expression in a type 2 diabetes mellitus mouse
model. Stem Cell Re Ther. 13(357)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fujii M, Inoguchi T, Batchuluun B,
Sugiyama N, Kobayashi K, Sonoda N and Takayanagi R: CTLA-4Ig
immunotherapy of obesity-induced insulin resistance by manipulation
of macrophage polarization in adipose tissues. Biochem Biophys Res
Commun. 438:103–109. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nishimura S, Manabe I, Nagasaki M, Eto K,
Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al:
CD8+ effector T cells contribute to macrophage recruitment and
adipose tissue inflammation in obesity. Nature Med. 15:914–920.
2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shin JH, Shin DW and Noh M:
Interleukin-17A inhibits adipocyte differentiation in human
mesenchymal stem cells and regulates pro-inflammatory responses in
adipocytes. Biochem Pharmacol. 77:1835–1844. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cipolletta D, Feuerer M, Li A, Kamei N,
Lee J, Shoelson SE, Benoist C and Mathis D: PPAR-γ is a major
driver of the accumulation and phenotype of adipose tissue Treg
cells. Nature. 486:549–553. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lei J, Hasegawa H, Matsumoto T and
Yasukawa M: Peroxisome proliferator-activated receptor α and γ
agonists together with TGF-β convert human CD4+CD25-T cells into
functional Foxp3+ regulatory T cells. J Immunol. 185:7186–7198.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sun L, Fu J and Zhou Y: Metabolism
controls the balance of Th17/T-regulatory cells. Front Immunol.
8(1632)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Maciolek JA, Pasternak JA and Wilson HL:
Metabolism of activated T lymphocytes. Curr Opin Immunol. 27:60–74.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Miao Y, Zhang C, Yang L, Zeng X, Hu Y, Xue
X, Dai Y and Wei Z: The activation of PPARγ enhances Treg responses
through up-regulating CD36/CPT1-mediated fatty acid oxidation and
subsequent N-glycan branching of TβRII/IL-2Rα. Cell Commun Signal.
20(48)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li C, Muñoz-Rojas AR, Wang G, Mann AO,
Benoist C and Mathis D: PPARγ marks splenic precursors of multiple
nonlymphoid-tissue Treg compartments. Proc Natl Acad Sci USA.
118(e2025197118)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jeong W, Jung JY, Kim SC and Im WT:
Ginsenoside F2 for prophylaxis and treatment of liver disease.
European patent EP2992933A1. Filed September 4, 2015; issued March
9, 2016.
|
|
52
|
Lin Z, Wang Z, Hegarty JP, Lin TR, Wang Y,
Deiling S, Wu R, Thomas NJ and Floros J: Genetic association and
epistatic interaction of the interleukin-10 signaling pathway in
pediatric inflammatory bowel disease. World J Gastroenterol.
23:4897–4909. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fay NC, Muthusamy BP, Nyugen LP, Desai RC,
Taverner A, MacKay J, Seung M, Hunter T, Liu K, Chandalia A, et al:
A novel fusion of IL-10 engineered to traffic across intestinal
epithelium to treat colitis. J Immunol. 205:3191–3204.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Saraiva M, Vieira P and O'Garra A: Biology
and therapeutic potential of interleukin-10. J Exp Med.
217(e20190418)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Vidal PM, Lemmens E, Dooley D and Hendrix
S: The role of ‘anti-inflammatory’ cytokines in axon regeneration.
Cytokine Growth Factor Rev. 24:1–12. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rajbhandari P, Thomas BJ, Feng AC, Hong C,
Wang J, Vergnes L, Sallam T, Wang B, Sandhu J, Seldin MM, et al:
IL-10 signaling remodels adipose chromatin architecture to limit
thermogenesis and energy expenditure. Cell. 172:218–233.e17.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Acosta JR, Tavira B, Douagi I, Kulyté A,
Arner P, Rydén M and Laurencikiene J: Human-specific function of
IL-10 in adipose tissue linked to insulin resistance. J Clin
Endocrinol Metab. 104:4552–4562. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ramos-Ramírez P, Malmhäll C, Johansson K,
Lötvall J and Bossios A: Weight gain alters adiponectin receptor 1
expression on adipose tissue-resident helios+ regulatory T cells.
Scand J Immunol. 83:244–254. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ramos-Ramirez P, Malmhäl C, Tliba O,
Rådinger M and Bossios A: Adiponectin/AdipoR1 axis promotes IL-10
release by human regulatory T cells. Front Immunol.
12(677550)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wu X, Tian J and Wang S: Insight into
non-pathogenic Th17 cells in autoimmune diseases. Front Immunol.
9(1112)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Fang W, Deng Z, Benadjaoud F, Yang D, Yang
C and Shi GP: Regulatory T cells promote adipocyte beiging in
subcutaneous adipose tissue. FASEB J. 34:9755–9770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Klotz L, Burgdorf S, Dani I, Saijo K,
Flossdorf J, Hucke S, Alferink J, Novak N, Beyer M, Mayer G, et al:
The nuclear receptor PPAR gamma selectively inhibits Th17
differentiation in a T cell-intrinsic fashion and suppresses CNS
autoimmunity. J Exp Med. 206:2079–2089. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chang YC, Hee SW and Chuang LM: T helper
17 cells: A new actor on the stage of type 2 diabetes and aging? J
Diabetes Investig. 12:909–913. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Miyase T, Sano M, Nakai H, Muraoka M,
Nakazawa M, Suzuki M, Yoshino K, Nishihara Y and Tanai J:
Antioxidants from Lespedeza homoloba. (I). Phytochemistry.
52:303–310. 1999.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Miyase T, Sano M, Yoshio K and Nonaka K:
Antioxidants from Lespedeza homoloba (II). Phytochemistry.
52:311–319. 1999.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Terao J and Mukai R: Prenylation modulates
the bioavailability and bioaccumulation of dietary flavonoids. Arch
Biochem Biophys. 559:12–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Longo N, Frigeni M and Pasquali M:
Carnitine transport and fatty acid oxidation. Biochem Biophys Acta.
1863:2422–2435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sultan AA, Rattray Z and Rattray NJW:
Toxicometabolomics-based cardiotoxicity evaluation of
Thiazolidinedione exposure in human-derived cardiomyocytes.
Metabolomics. 20(24)2024.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Home PD, Pocock SJ, Beck-Nielsen H, Curtis
PS, Gomis R, Hanefeld M, Jones NP, Komajda M and McMurray JJ:
RECORD Study Team. Rosiglitazone evaluated for cardiovascular
outcomes in oral agent combination therapy for type 2 diabetes
(RECORD): A multicentre, randomised, open-label trial. Lancet.
373:2125–2135. 2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dormandy JA, Charbonnel B, Eckland DJA,
Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre
PJ, Murray GD, et al: Secondary prevention of macrovascular events
in patients with type 2 diabetes in the PROactive study
(PROspective pioglitAzone clinical trial in macroVascular events):
A randomised controlled trial. Lancet. 366:1279–1289.
2005.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yong EL, Cheong WF, Huang Z, Thu WPP,
Cazenave-Gassiot A, Seng KY and Logan S: Randomized, double-blind,
placebo-controlled trial to examine the safety, pharmacokinetics
and effects of epimedium prenylflavonoids, on bone specific
alkaline phosphatase and the osteoclast adaptor protein TRAF6 in
post-menopausal women. Phytomedicine. 91(153680)2021.PubMed/NCBI View Article : Google Scholar
|