Introduction
Paraneoplastic syndrome is used to describe the
clinical manifestations that occur as a result of the secretion of
substances by tumor cells, including hormones, growth factors,
cytokines and tumor antigens (1).
Paraneoplastic glomerulopathy was initially introduced by Galloway
in 1922 in a case of nephrotic syndrome (NS) in connection with
Hodgkin's disease (2). Furthermore,
a study in 1966 revealed that a significant proportion (~11%) of NS
cases observed in adults were linked with the presence of malignant
tumors (3). Data collected from the
Danish Kidney Biopsy Registry between 1985 and 2014 associated 16%
of patients with glomerulopathy with cancer development (4,5). NS is
defined by persistent urine protein excretion, primarily albumin,
>3.5 g/24 h (adults) or 40 mg/h/m2 (children),
indicating ‘nephrotic-range proteinuria’, usually with serum
albumin levels below normal for adults (3.5 g/dl). Although not
necessary for diagnosis, NS is frequently associated with
peripheral edema, ascites, pleural effusion, hyperlipidemia (high
serum cholesterol and triglycerides) or lipiduria (fatty casts or
doubly refractile fat masses) (6).
It encompasses various etiologies, including primary renal disorder
such as minimal change disease, focal segmental glomerulosclerosis
and membranous glomerulonephritis (MG). Moreover, NS can manifest
as a result of systemic disorder that impacts the kidneys and other
organs, such as amyloidosis or lupus erythematosus (6). Membranous nephropathy is the
predominant glomerular disease that leads to NS associated with
solid tumors (7,8). Association between membranous
nephropathy and neoplasia has been reported but there is still
limited understanding of the specific features of this association
(referred to as MN-cancer) (7). The
prevalence of MN-cancer varies between 1 and 22%, in France
(9).
The solid cancers most frequently observed in
association with MN include lung, bronchus and gastric cancer, as
well as renal, prostate and thymus tumors. Colorectal, pancreatic,
esophageal and hepatic carcinomas are among the other types of
cancer associated with MN (10).
Other types of glomerulopathy linked with neoplasia include minimal
change disease, IgA nephropathy, focal segmental glomerulosclerosis
and mesangiocapillary and crescentic glomerulonephritis (11,12).
Minimal change disease is often associated with Hodgkin's lymphoma,
renal cell carcinoma and thymoma (11,13).
IgA nephropathy is associated with cancers of the respiratory
tract, buccal cavity, nasopharynx and melanoma (11,14,15).
Focal segmental glomerulosclerosis is connected to renal cell
carcinoma and gastric and breast cancer (11,16).
Mesangiocapillary glomerulonephritis is observed alongside renal
cell and lung and bronchus carcinoma and thymoma (17). Crescentic glomerulonephritis is
associated with various types of cancer, including renal cell,
esophagus and ovarian carcinoma, as well as gastric cancer
(11). Additionally, amyloidosis is
suspected to be associated with renal cell carcinoma (18), while cancer-associated thrombotic
microangiopathy is often seen in mucin-producing carcinoma,
particularly of the stomach, lung and breast (19).
Paraneoplastic syndromes, which are rare disorders
triggered by an immune response to malignancy, manifest in a
variety of ways depending on the type of cancer and patient
demographic. Paraneoplastic nephropathy, a kidney condition often
seen in neoplasia. Notably, ovarian carcinoma in a 59-year-old
patient from Korea (20) and lung
cancer in an 80-year-old patient from China (21) were both associated with MN and
nephropathy, respectively. Hodgkin's lymphoma, appearing in both a
9-year-old from Romania (22) and a
13-year-old from India (23), is
also a common malignancy, although specific nephropathies were not
detailed for these cases. By contrast, thymoma in a 49-year-old
from China (24) and a 32-year-old
from Korea (25) were linked to
membranous nephropathy and focal segmental glomerulosclerosis,
respectively. Other notable associations include meningioma in a
60- (26) and a 58-year-old
(27) from India, with one case
involving MN. Additionally, gastrointestinal stromal tumor was
noted in a 69-year-old Japanese patient (28), though specific nephropathy details
were not provided. These data highlight the wide age range and
diverse types of cancer involved in paraneoplastic syndrome, with
membranous nephropathy being a recurrent form of nephropathy across
multiple types of cancer (Table
I).
 | Table ITypes of cancer associated with
paraneoplastic nephrotic syndrome. |
Table I
Types of cancer associated with
paraneoplastic nephrotic syndrome.
| First author/s,
year | Country | Age, years | Type of cancer | Kidney biopsy
results | (Refs.) |
|---|
| Kim et al,
2003 | South Korea | 59 | Ovarian
carcinoma | Membranous
glomerulonephritis | (20) |
| Yu et al,
2020 | China | 80 | Lung | Membranous
nephropathy | (21) |
| Sfrijan et
al, 2016 | Romania | 9 | Hodgkin's
lymphoma | N/A | (22) |
| Devi et al,
2022 | India | 13 | Hodgkin's
lymphoma | N/A | (23) |
| Liu et al,
2022 | China | 49 | Thymoma | Membranous
nephropathy | (24) |
| Yoo et al,
2017 | Korea | 32 | Thymoma | Focal segmental
glomerulosclerosis | (25) |
| Sardhara et
al, 2018 | India | 60 | Meningioma | Membranous
glomerulonephritis | (26) |
| Zachariah et
al, 2013 | India | 58 | Meningioma | N/A | (27) |
| Takane et
al, 2014 | Japan | 69 | GIST | N/A | (28) |
The clinical manifestations associated with NS
include pronounced edema, specifically in the periorbital region,
ankles and feet; proteinuria, characterized by the presence of
foamy urine due to excessive protein excretion; increased body
weight due to fluid retention; fatigue and decreased appetite
(29). The diagnostic tests for NS
include urine analysis, which can detect presence of protein, and
blood tests that indicate low levels of albumin, decreased total
protein levels and elevated cholesterol and triglyceride levels
(29). Kidney biopsy is considered
the gold standard in diagnosis of NS (30). The therapeutic approach for managing
NS includes the utilization of angiotensin-converting enzyme (ACE)
inhibitors and angiotensin II receptor blockers. These
pharmacological agents mitigate both blood pressure levels and
urinary protein excretion effectively. An additional therapeutic
approach entails the use of diuretic medication to reduce edema by
stimulating renal fluid excretion. Frequently prescribed diuretic
medications include furosemide, spironolactone and thiazides.
Statins are utilized to reduce cholesterol levels. To decrease risk
of embolic events, it is imperative to administer anticoagulant
medications (29). These include
heparin and vitamin K antagonists, as well as directly acting oral
anticoagulants such as direct factor Xa (such as rivaroxaban,
apixaban, and endoxaban) and thrombin inhibitors (dabigatran)
(31).
Case report
A 44-year-old Caucasian male was admitted to
Emergency County Hospital (Craiova in March 2018, with a history of
smoking (22 pack-years) and chronic ethanol consumption. The
patient presented with abdominal distension and edema in the
genital and leg regions. Medical history did not reveal any
significant health conditions. Upon admission, vital signs were
within normal range: Blood pressure, 130/80 mmHg; heart rate, 80
bpm; respiratory rate, 17 breaths/min and body temperature, 36.7˚C.
The patient was alert with no signs of scleral icterus or anemic
conjunctiva and lymph nodes were non-palpable. The patient did not
report dyspnea and chest auscultation was normal.
The patient had notable edema in the lower
extremities and pronounced scrotal and penile edema. Physical
abdominal examination revealed flank and shifting dullness,
indicating ascites. The patient also reported polyuria and dysuria.
An initial EKG showed sinus rhythm with a heart rate of 87 bpm,
regular QRS complex and no ST-T segment changes. An abdominal
ultrasound in indicated an enlarged liver with a homogeneous
structure and a moderate amount of ascitic fluid, with no pleural
or pericardial effusion.
Initial laboratory investigations were notable for
an inflammatory syndrome [erythrocyte sedimentation rate (ESR), 110
mm/h], normal blood cell count (white blood cells,
8,000/mm3, reference range 4,000-10,000/mm3;
red blood cells=5x106/mm3, reference range
4-5.7x106/mm3;
platelets=300,000/mm3, reference range
150,000-450,000/mm3), normal urea (38 mg/dl, reference
range 20-40 mg/dl) and creatinine (0.9 mg/dl, reference range
0.8-1.2 mg/dl). Serum glucose (90 mg/dl, reference range 70-110
mg/dl), glycosylated hemoglobin (HbA1c) (5%, reference range,
4-6.4%), aspartate aminotransferase (20 U/l, reference range 5-34
U/l), alanine aminotransferase (24 U/l, reference range 3-44 U/l),
prothrombin time (10.5 s, reference range 9.4-13.5 s) and
international normalized ratio (INR) levels (0.9, reference range
0.8-1.16) were within normal ranges. Additionally, N-terminal
pro-b-type natriuretic peptide (64 pg/ml, reference range <121
pg/ml) was tested and found to be within the normal range (Table II).
 | Table IIInitial laboratory findings. |
Table II
Initial laboratory findings.
| Parameter | Value | Reference
range |
|---|
| C reactive protein,
mg/dl | 6.87 | 0.00-5.00 |
| Hemoglobin,
g/dl | 10.50 | 13.50-17.50 |
| Protein, g/l | 3.90-4.20 | 6.00-8.30 |
| Triglyceride,
mg/dl | 213.00 | 50.00-150.00 |
| Cholesterol,
mg/dl | 475.00 | 70.00-220.00 |
| Ureea, mg/dl | 38.00 | 20.00-40.00 |
| Creatinine,
mg/dl | 0.90 | 0.80-1.20 |
| Natrium, mEq/l | 136.00 | 135.00-145.00 |
| Albumin, g/dl | 1.40-2.20 | 3.00-4.50 |
| Proteinuria,
mg/dl | 500.00 | - |
| Total urinary
protein, mg/24 h | 12,500.00 | 0.00-300.00 |
Further laboratory tests the following day confirmed
the inflammatory syndrome, with increased ESR=130 mm/h, reference
range <20 mm/h fibrinogen, 150 mg/dl, reference range 200,400
mg/dl) and C-reactive protein levels (6.87 mg/dl, reference range
0-5 mg/dl). The patient exhibited anemic syndrome (Hb, 10.5 g/dl,
normal range 13.5-17.5 g/dl), hypoproteinemia (3.9 g/l, reference
range 6-8.3 g/l) and hypoalbuminemia (1.4-2.2 g/dl, reference range
3.5-5.2 g/dl), as well as hypertriglyceridemia (213 mg/dl,
reference range 50-150 mg/dl), hypercholesterolemia (475 mg/dl,
reference range 70-220 mg/dl) and hyperuricemia (10.22 mg/dl,
reference range 2.5-7.2 mg/dl). Serum potassium levels were normal
(4 mmol/l, reference range 3.5-5.1 mmol/l). Normal values were also
recorded for bilirubin (0.4 mg/dl, reference range <1.2 mg/dl),
alkaline phosphatase (70 U/l, reference range 50-116 U/l) and
γ-glutamyl transferase (35 U/l, reference range 0-55 U/l). Urine
screening showed proteinuria (500 mg/dl) and total urinary protein
of 12,500 mg/24 h (normal range 0-300 mg/24 h), with no cells or
casts.
Serum protein electrophoresis levels were as
follows: Albumin, 20.40% (normal range 54.30-66.00%), α1-globulin,
4.30% (normal range 1.20-3.30%), α2-globulin, 33.90% (normal range
8.30-15.00%), β1-globulin, 13.30% (normal range 6.50-11.00%),
β2-globulin, 10.60% (normal range 2.50-7.00%) and γ-globulin,
17.50% (normal range 7.00-19.50%). Serum protein electrophoresis
was conducted following the standard protocols established by the
Emergency County Hospital of Craiova. Blood samples were collected
following a 12-h fasting period by an experienced phlebotomist. To
facilitate blood clotting, the samples were left at room
temperature for 20 min. Serum was separated by centrifugation at
3,600 rpm for 10 min at 4˚C. After centrifugation, the clot was
promptly removed, and the serum was stored at -20˚C until analysis.
Serum protein electrophoresis was conducted using Hydragel
30(15) β1-β2 reagents, based on
the principle of agarose gel separation, on a semi-automated
HYDRASYS 2 SCAN system, following the manufacturer's instructions
(32). Carcinoembryonic antigen
(CEA, 9.46 ng/ml, reference range 0.00-4.70 ng/ml) and carbohydrate
antigen (CA 19-9=82.47 U/ml, reference range 0.00-39.00 U/ml)
levels were elevated, while other markers such as CA 72-4 (4.00
U/ml, reference range <6.90 U/ml), α-fetoprotein (2.40 ng/ml,
reference range <7.00 ng.ml) and cytokeratin fragments (1.20
ng/ml, reference range <3.30 ng/ml) were within normal limits
(Table III).
 | Table IIIProtein electrophoresis and positive
tumor markers. |
Table III
Protein electrophoresis and positive
tumor markers.
| Parameter | Value | Reference
range |
|---|
| Albumin, % | 20.40 | 54.30-66.00 |
| α1-globulin, % | 4.30 | 1.20-3.30 |
| α2-globulin, % | 33.90 | 8.30-15.00 |
| β1-globulin, % | 13.30 | 6.50-11.00 |
| β2-globulin, % | 10.60 | 2.50-7.00 |
| γ-globulin, % | 17.50 | 7.00-19.50 |
| CA19-9, U/ml | 82.47 | 0.00-39.00 |
| CEA, ng/ml | 9.46 | 0.00-4.70 |
Further investigation revealed negative results for
viral hepatitis panel, human immunodeficiency virus and
Epstein-Barr virus. Additionally, antinuclear antibody (<1:100),
anti-double-stranded DNA antibody (<25 UI/ml), prostate-specific
antigen (1.3 ng/ml, reference range <2 ng/ml), thyroid function
(triiodothyronine T3=2 nmol/l, reference range 1.3-3.1 nmol/l;
thyroxine T4=75 nmol/l, reference range 66-181 nmol/l), complement
levels (CH50=50 U/ml, reference range, 50-75 U/ml; C3=120 mg/dl
reference range 90-180 mg/dl; C4=22 mg/dl, reference range 10-40
mg/dl) and anti-phospholipase A2 receptor (PLA2R) antibodies
(<1:10, negative <1:10) were within normal ranges.
Additionally, immune complexes were within the normal range (CIC
<20 R.U./ml, negative <20 R.U./ml).
To determine the nature of NS (primary or
secondary), the most frequent secondary etiologies were
investigated. Abdominal paracentesis was performed for both
diagnostic and therapeutic purposes. Laboratory results revealed
serum ascites albumin gradient <1.1 g/dl, along with normal
cytological examination and a negative test for Mycobacterium
tuberculosis. Ascitic fluid samples were collected via
paracentesis and analyzed within 2 h of collection. A volume of 10
ml of the fluid was centrifuged at 1,500 rpm for 5 min. After
discarding the supernatant, the sediment was transferred onto glass
slides to prepare smears. These smears were air-dried at room
temperature and subsequently fixed with methyl alcohol or
alcohol-ether mixture (1:1) for 2-3 min. After fixation, the smears
were stained with Giemsa solution for 20-23 min. Following
staining, the slides were air-dried and examined under a light
microscope. Cytological examination was performed using a
scan-screen method to detect any abnormalities, atypical cells, or
neoplastic changes in cell morphology.
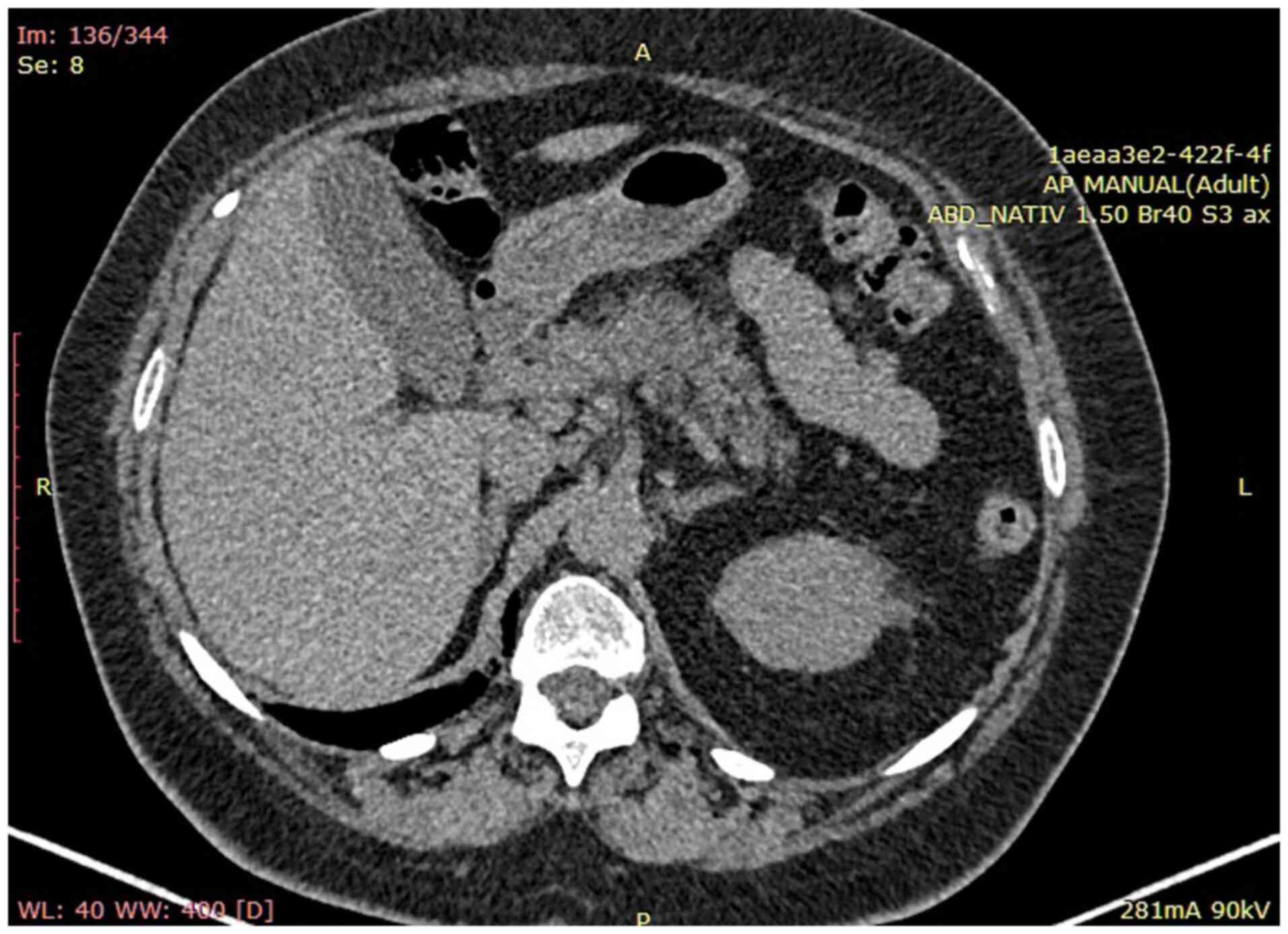
Abdominal computed tomography (CT) scan confirmed
liver enlargement without pathological masses, normal diameter (12
mm) of the portal vein and a notable accumulation of ascitic fluid.
Additionally, CT scan revealed multiple mesenteric lymphadenopathy,
measuring up to 1.3 cm, and increased renal echogenicity. No
pathological alterations were observed in the spleen, prostate, or
pancreas (Fig. 1).
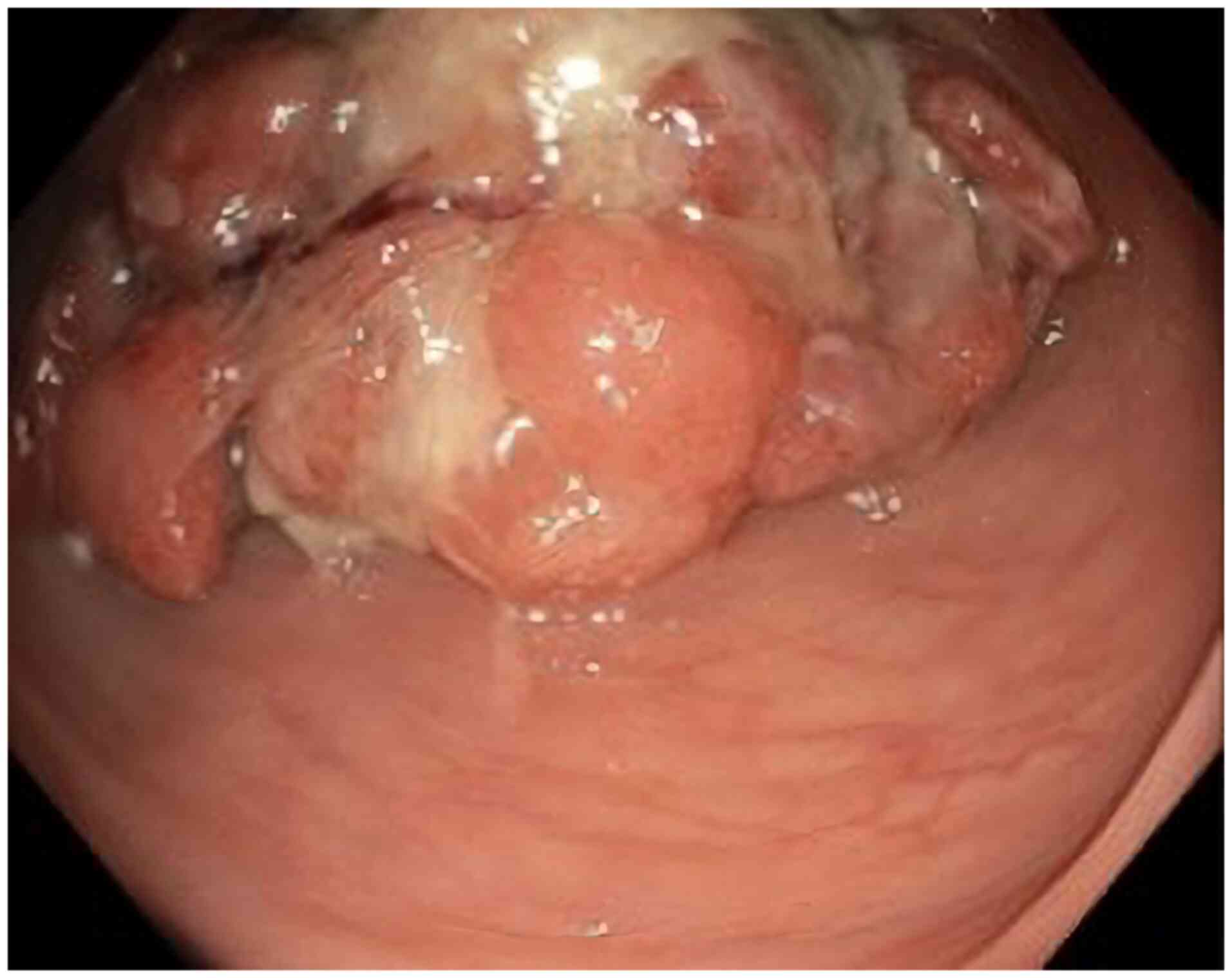
A superior digestive endoscopy did not reveal
notable abnormalities. However, an inferior digestive endoscopy
revealed a pedunculated polyp, which was biopsied for further
examination (Fig. 2). The sample
was first fixed in 10% neutral buffered formaldehyde, at room
temperature for 24-48 h. Following fixation, the sample was
embedded in paraffin and serial sections were cut at a thickness of
4 µm. The sections were stained with hematoxylin at room
temperature for 5 min, followed by eosin for 2 min. The stained
sections were examined under a light microscope at varying
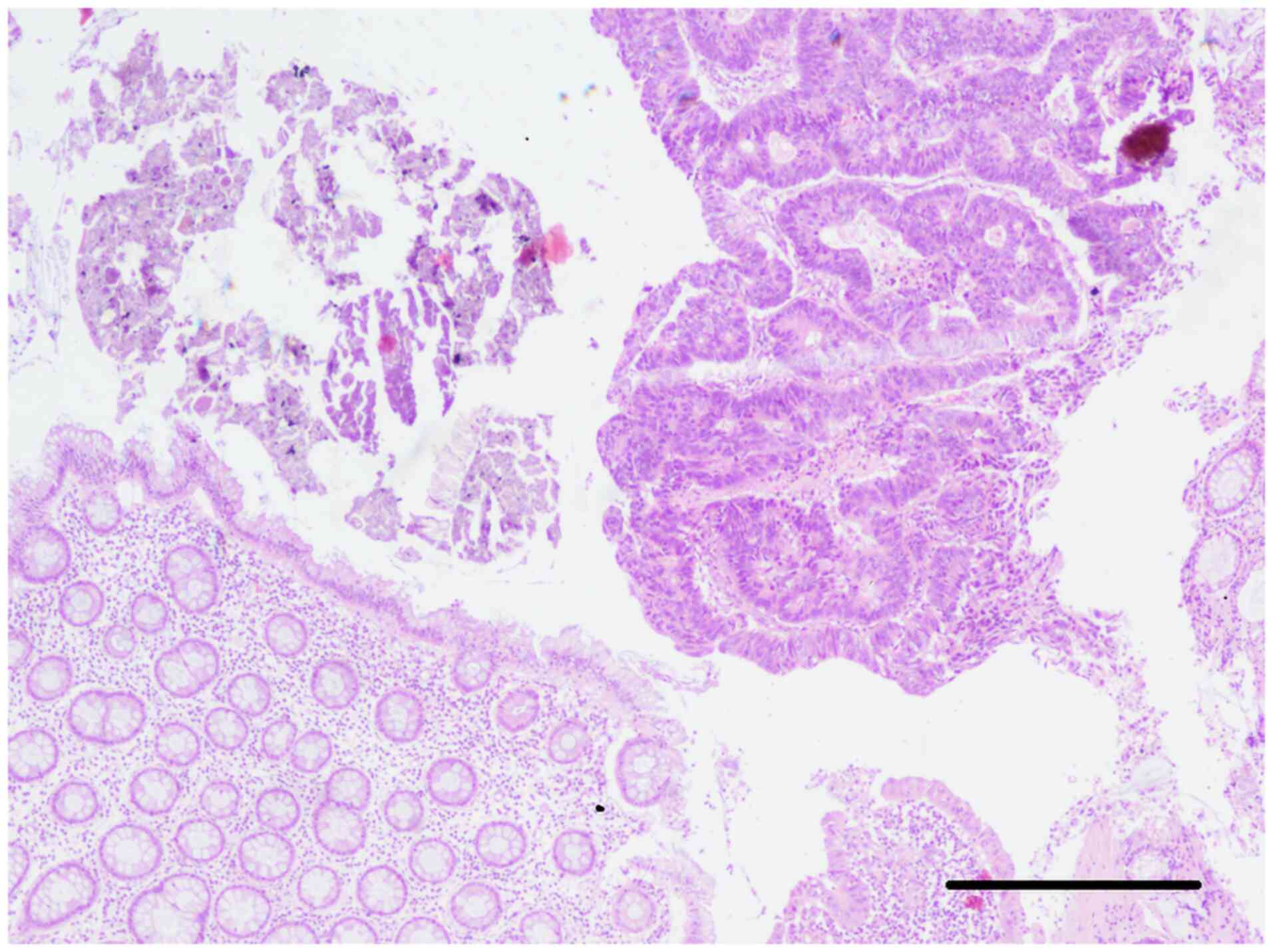
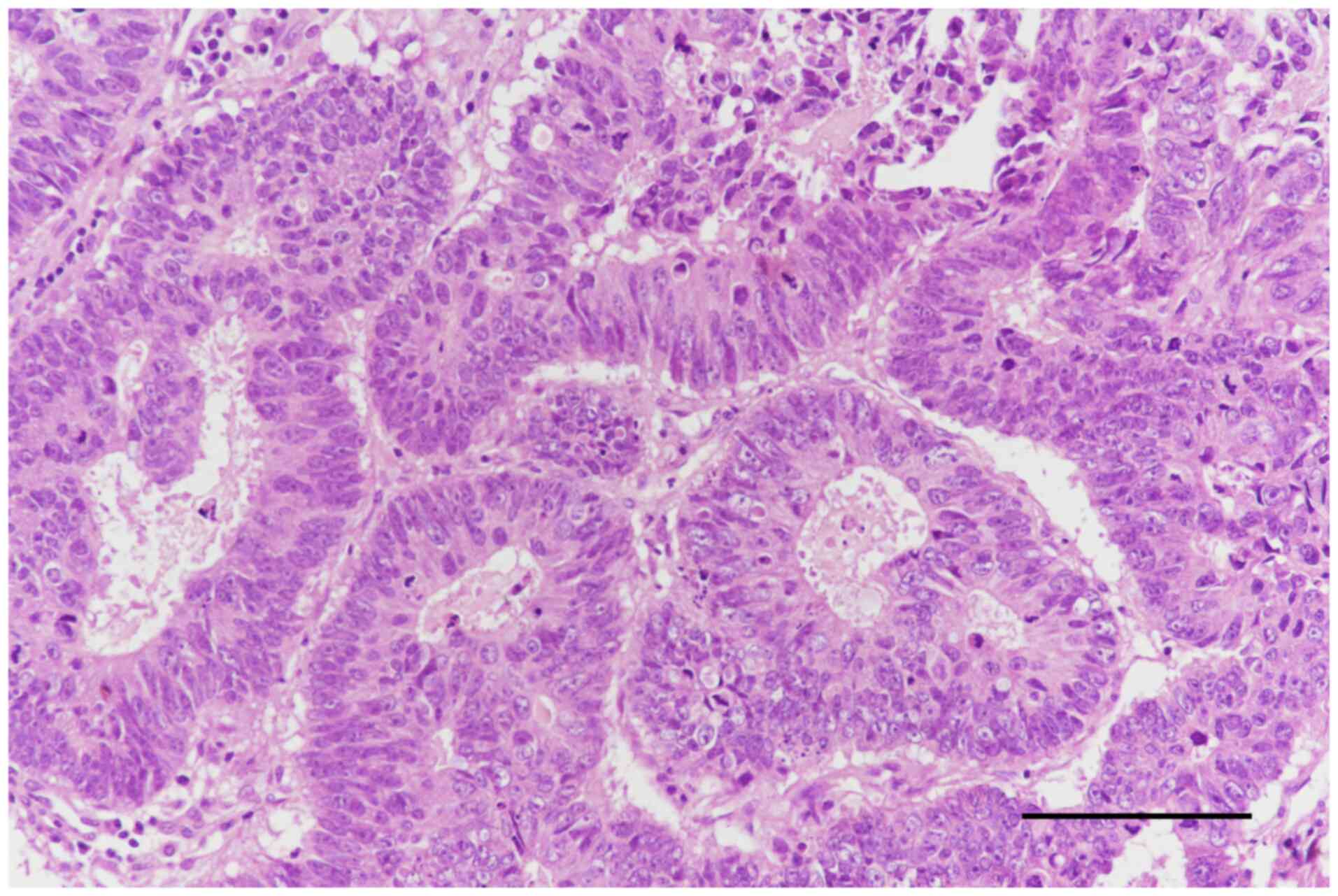
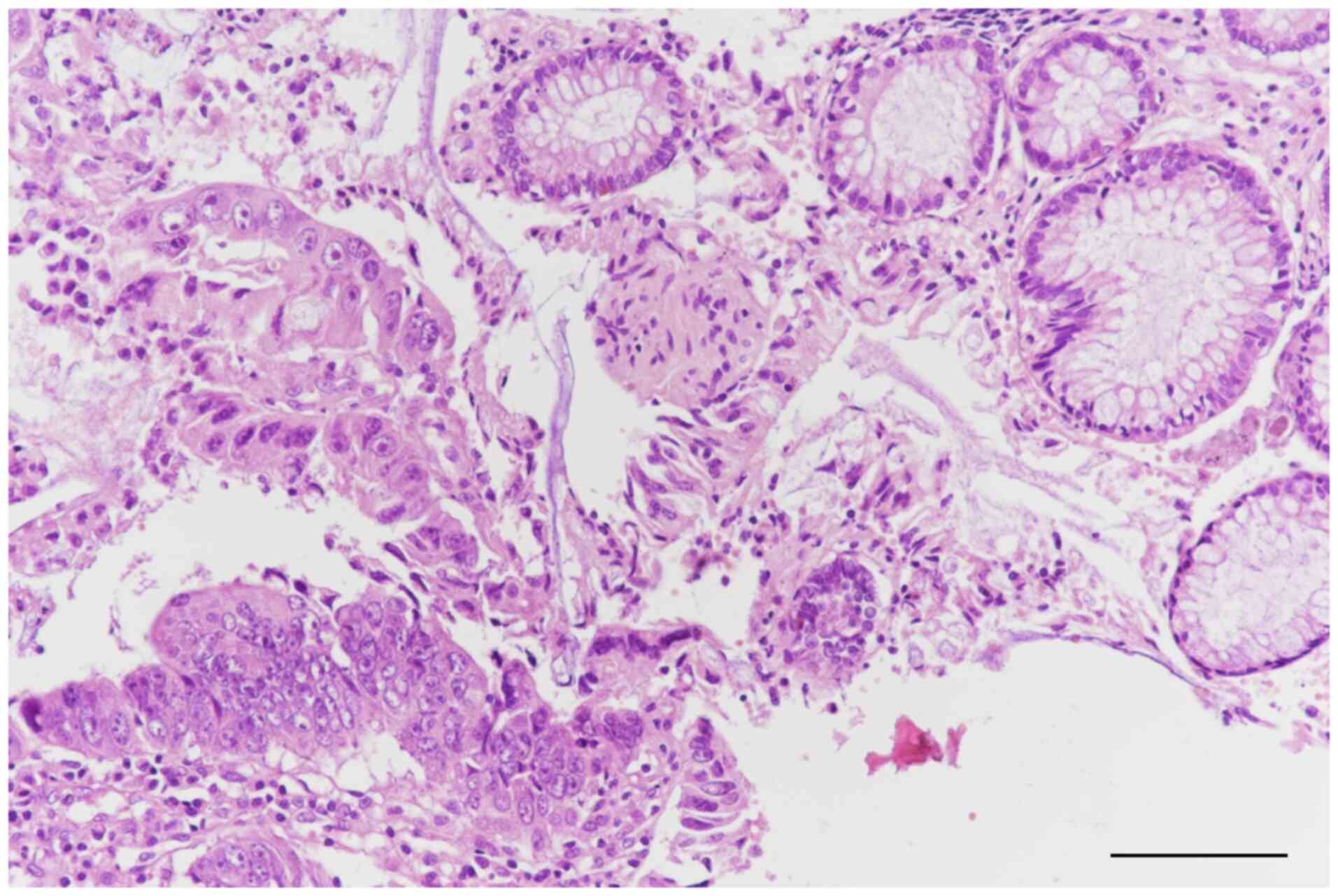
magnifications, in this case 20 and 40x. Histopathological
examination revealed an adenomatous polyp with areas of moderately
differentiated adenocarcinoma that did not involve the muscularis
mucosae layer (Fig. 3, Fig. 4 and Fig.
5).
An inferior digestive endoscopy revealed a
pedunculated polyp in the descending colon and an endoscopic
polypectomy was subsequently performed. Histopathological
examination confirmed the presence of a malignant polyp with areas
of moderately differentiated adenocarcinoma infiltrating the
submucosal layer, as well as regions classified as carcinoma in
situ not extending beyond the muscularis mucosae layer
(Fig. 3).
Given the fragmented state of the specimen, the
multidisciplinary team concluded that the appropriate course of
action was left colectomy with a bloc removal of regional lymph
nodes. This decision was made following normal chest and pelvic CT
and negative ascitic fluid smear cytology for malignancy, which
conclusively excluded the presence of metastases. The pathological
stage was determined to be IIIB, specifically classified as
pT2pN1bM0 using the TNM staging system (33).
The patient underwent eight courses of adjuvant
chemotherapy of oxaliplatin (130 mg/m2 once every three
weeks and oral capecitabine 1,000 mg/m2 twice a day for
14 days, followed by 7 days off. The adjuvant chemotherapy was
administered at the usual dose, since the renal function remained
stable, with no notable adverse effects observed.
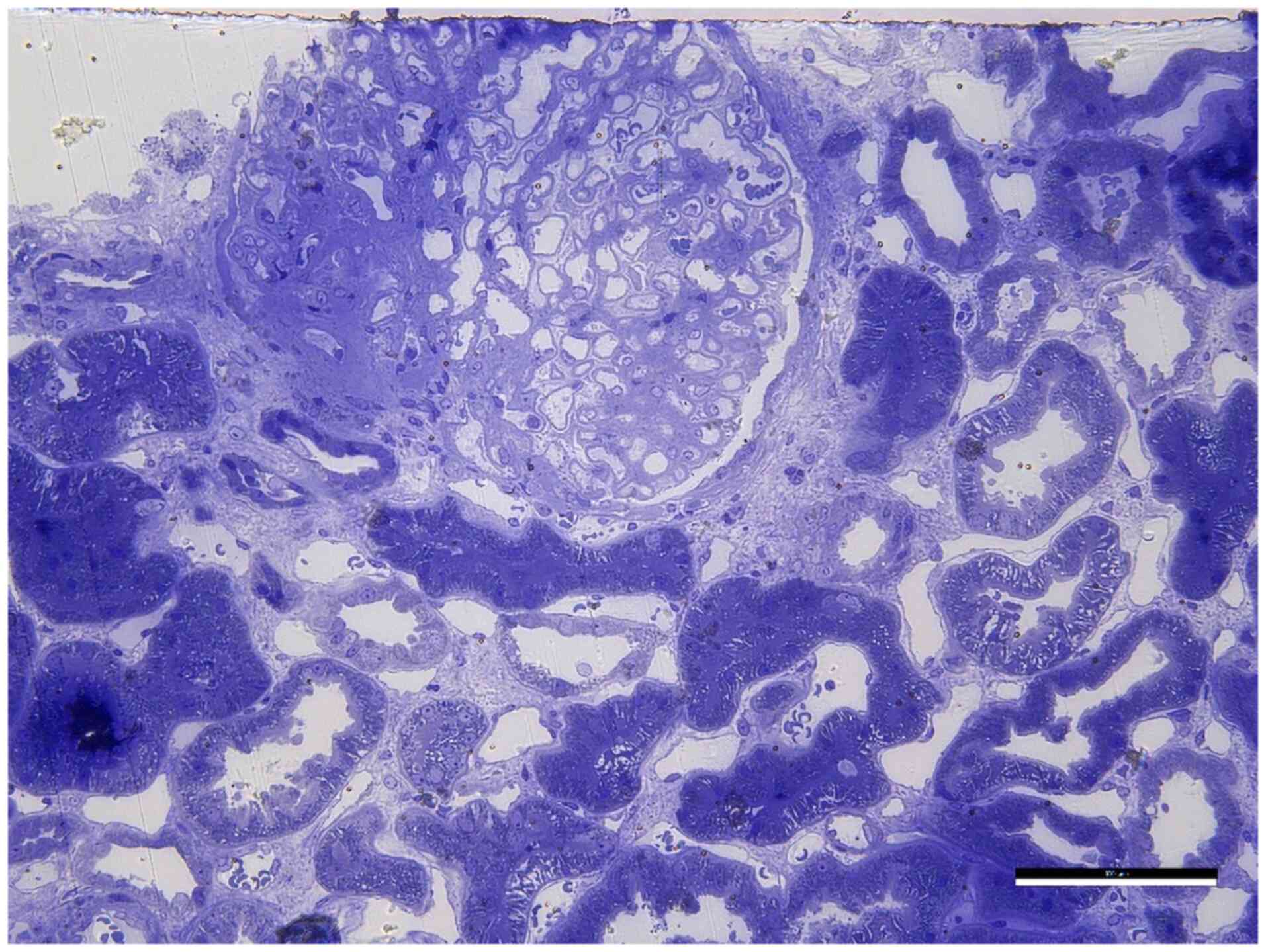
Kidney biopsy (ultrasound-guided) revealed
membranous nephropathy, stage 3 (Fig.
6) with thickening of the glomerular basement membrane (GBM),
indicating moderate progression of the disease, due to the
deposition of immune complexes. Kidney biopsy revealed a fragment
of connective and renal tissue with a single glomerulus. The
glomerulus showed segmental sclerosis affecting ~25% of its
structure, with thickened capillary loop walls. There were rare
interstitial foam cells and discrete lipid load in tubular
epithelia. Arteries and arterioles were normal and there was mild
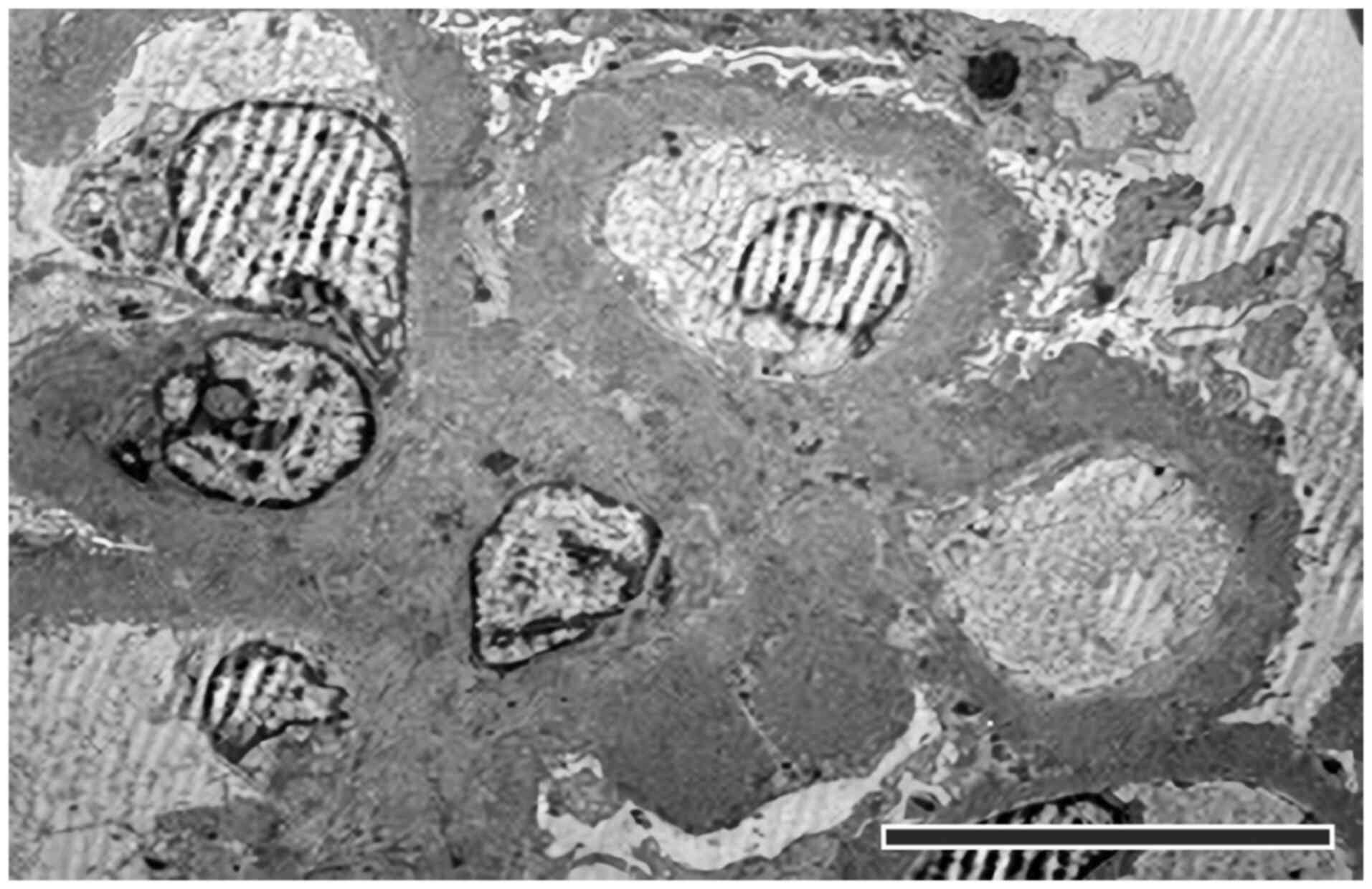
interstitial fibrosis without interstitial inflammation (Fig. 7). Electron microscopy examination
showed a glomerulus with segmental sclerosis and some permeable
capillaries. GBM was thickened, containing numerous dense deposits
on the epithelial and intramembrane surfaces, along with rare large
hump-like deposits. Some capillaries exhibited continuous
endothelium and intraluminal platelets. A circumferential hyaline
deposit was noted at the level of the afferent arteriole, which
narrowed the lumen. Biopsy fragments were fixed in 2% neutral
buffered glutaraldehyde followed by 1% buffered osmium tetraoxide,
embedded in PolyBed 812® resin with polymerization for
72 h at 60˚C, and sectioned as seriate 1 µm semithin and
respectively 0.1 µm thin sections. Semithin sections were stained
with toluidine blue for light microscopy evaluation, and for
ultrastructural microscopy, 0.1 µm-ultrathin sections were
collected on copper grids and examined under an electron
microscope.
The patient was advised to reduce the dietary sodium
(maximum 2 g/day), saturated fat and cholesterol intake. Initial
management focused on diuretic therapy (furosemide, 40 mg, 3
times/day, intravenous; spironolactone, 100 mg/day, orally), with
vigilant monitoring of serum electrolytes. To decrease proteinuria,
angiotensin II enzyme inhibitor was used (perindopril, 5 mg/day,
orally). For the restoration of serum albumin levels, intravenous
administration of human albumin (50 g/l) was implemented once
daily. Thromboembolic events were prevented via subcutaneous
administration of enoxaparin (1 mg/kg) at 12-h intervals, followed
by vitamin K antagonists (acenocumarol 2 mg/day, oral) with
monitoring of INR (maintained between 1.8 and 2.0) and albumin
levels. Notably, prophylactic antibiotic (cefuroxime 500 mg x
2/day, 7 days) therapy was initiated from hospitalization. At last
follow-up (January 2020), clinical condition improved, including a
reduction in peripheral edema.
Discussion
In the present case, typical causes of ascitic
syndrome, including liver cirrhosis, ascites due to heart failure
or peritoneal carcinomatosis, were considered (34). Given the relatively young age,
bacillary peritonitis was initially considered as a potential
cause, but this hypothesis was excluded following cytology and
microbiological (Mycobacterium tuberculosis) analysis of
ascites fluid obtained via paracentesis. The detection of abdominal
adenopathy on CT examination suggested lymphoma; however, blood
tests ruled out this diagnosis.
The diagnosis of NS prompted consideration of
potential underlying primary glomerulonephritis. The conclusive
determination of the patient condition was achieved through a
systematic process of excluding potential causes of secondary NS,
such as drugs, infection or multisystemic etiology (35). The presence of an unexplained
inflammatory syndrome, coupled with perturbations in tumor marker
levels, prompted imaging assessment of the gastrointestinal tract,
leading to the identification of a colonic adenocarcinomatous
polyp. Notably, endoscopic examination play a pivotal role in the
timely detection of digestive malignancies (36) and empirical evidence suggests that
screening strategies contribute to a decrease in both the incidence
and mortality associated with colorectal cancer (37).
The precise mechanisms underlying development of
glomerular disease as a result of malignancy are not yet fully
comprehended (1,17,38,39).
The occurrence of remission in glomerular disease following cancer
removal via surgery or anticancer medications, along with the
subsequent relapse upon return of neoplasia, indicates the presence
of paraneoplastic mechanisms (37).
One potential mechanism is the production of autoantibodies that
specifically target a tumor antigen that shares immunological
characteristics with a component of the glomerulus, resulting in
formation of immune complexes in the local area. Trapping of these
immune complexes in glomerular capillaries or deposition of
antibodies bound to the tumor antigen within the glomerular
membrane may be involved. External factors, such as oncogenic
viruses, also need to be considered (40).
Identification of IgG subtypes within immune
deposits in membranous nephropathy has the potential to assist in
differentiation of various diagnoses. The IgG4 subtype is prevalent
in cases of idiopathic and recurrent membranous nephropathy,
whereas IgG1, IgG2 and IgG3 subtypes are more frequently observed
in secondary membranous nephropathy and allograft cases (41). These findings highlight the role of
immunological mechanisms in pathogenesis of renal involvement in
systemic malignancy, which is relevant in the present case of
paraneoplastic NS (42,43).
Paraneoplastic nephropathy represents a key
intersection between kidney disease and systemic malignancy,
illustrating how renal manifestations can facilitate diagnosis of
unsuspected cancers. NS arises via various mechanisms influenced by
the presence of malignancies. Membranous nephropathy is a
predominant form of glomerular disease associated with a range of
solid tumors, underscoring the need for clinicians to remain
vigilant when encountering unexplained NS. The present case
highlighted that paraneoplastic syndromes, while rare, can present
uniquely and lead to early detection of underlying malignancies.
Further research is essential in elucidating these associations,
developing targeted therapeutic strategies and enhancing
understanding of the pathophysiological underpinnings of
paraneoplastic syndrome to improve both diagnosis and management of
affected patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RS and ȘBV conceived the study. CDN, DM, DeP, MA,
AVGD, GGD, RES, MDS and DaP performed experiments and analyzed
data. MA, RS and AVGD wrote the manuscript. GGD, RES, MDS and AVGD
edited and reviewed the manuscript. GGD, AVGD and MDS supervised
the study. All authors have read and approved the final manuscript.
RS and AVGD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient.
Patient consent for publication
Written informed consent has been obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ronco PM: Paraneoplastic glomerulopathies:
New insights into an old entity. Kidney Int. 56:355–377.
1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galloway J: Remarks on Hodgkin's disease.
Br Med J. 2:1201–1208. 1922.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee JC, Yamauchi H and Hopper J JR: The
association of cancer and the nephrotic syndrome. Ann Intern Med.
64:41–51. 1966.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Birkeland SA and Storm HH:
Glomerulonephritis and malignancy: A population-based analysis.
Kidney Int. 63:716–721. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heaf JG, Hansen A and Laier GH:
Quantification of cancer risk in glomerulonephritis. BMC Nephrol.
19(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Glassock RJ, Fervenza FC, Hebert L and
Cameron JS: Nephrotic syndrome redux. Nephrol Dial Transplant.
30:12–17. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lefaucheur C, Stengel B, Nochy D, Martel
P, Hill G and Rossert J: GN-PROGRESS Study Group. Membranous
nephropathy and cancer: Epidemiologic evidence and determinants of
high-risk cancer association. Kidney Int. 70:1510–1517.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Davison A: Renal diseases associated with
malignancies. Nephrol Dial Transplant. 16:13–14. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bacchetta J, Juillard L, Cochat P and Droz
JP: Paraneoplastic glomerular diseases and malignancies. Crit Rev
Oncol Hematol. 70:39–58. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Monga D: Chapter 6: Glomerular Diseases
and Cancer, 2016. Available from: https://www.asn-online.org/education/distancelearning/curricula/onco/Chapter6.pdf.
Accessed January 4, 2019.
|
|
11
|
Jhaveri KD, Shah HH, Calderon K, Campenot
ES and Radhakrishnan J: Glomerular diseases seen with cancer and
chemotherapy: A narrative review. Kidney Int. 84:34–44.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lionaki S, Marinaki S, Panagiotellis K,
Tsoumbou I, Liapis G, Vlahadami I, Tzioufas A, Sfikakis P and
Boletis I: Glomerular diseases associated with malignancies:
Histopathological pattern and association with circulating
autoantibodies. Antibodies (Basel). 9(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
González-Fontal GR, Restrepo JG and
Henao-Martínez AF: Minimal-change disease as a paraneoplastic
syndrome in a patient with ovarian carcinoma. NDT Plus. 4:427–429.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sanathkumar HT, Thirumalvalavan K, Raj TY,
Srinivasaprasad ND, Sujith S and Fernando EM: Association of IgA
nephropathy with squamous cell carcinoma of the tongue: -Case
report and review of literature. Indian J Nephrol. 31:290–292.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rehnberg J, Ludvigsson JF, Carrero JJ and
Emilsson L: Cancer risk in patients with immunoglobulin A
nephropathy: A Swedish population-based cohort study. Nephrol Dial
Transplant. 37:749–759. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dabrowski D, Ozluk E, Barbeito S and Wei
EX: Focal segmental glomerulosclerosis preceding type 2 papillary
renal cell carcinoma. Case Rep Pathol. 15(8811905)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lien YH and Lai LW: Pathogenesis,
diagnosis and management of paraneoplastic glomerulonephritis. Nat
Rev Nephrol. 7:85–95. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vanatta PR, Silva FG, Taylor WE and Costa
JC: Renal cell carcinoma and systemic amyloidosis: Demonstration of
AA protein and review of the literature. Hum Pathol. 14:195–201.
1983.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Babu KG and Bhat GR: Cancer-associated
thrombotic microangiopathy. Ecancermedicalscience.
28(649)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim YT, Rha SY, Shim CY, Sohn JH, Kim C,
Yu NC, Chung HC, Kim JH, Han DS, Kim BS and Roh JK: A case of
paraneoplastic nephrotic syndrome in a patient with ovarian
carcinoma. Yonsei Med J. 44:539–543. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu X, Fan Z, Chen W and Wang Z: Lung
cancer with nephrotic syndrome as a paraneoplastic syndrome: A case
report. Mol Clin Oncol. 13(86)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sfrijan D, Tieranu I, Necula I, Popa L and
Balgradean M: Nephrotic syndrome, paraneoplastic syndrome
associated to hodgkin lymphoma. Maedica (Bucur). 11:64–67.
2016.PubMed/NCBI
|
|
23
|
Padmanaban PD, Jayaraman D, Shanmugam SG
and Geminiganesan S: Nephrotic syndrome and hodgkins lymphoma-an
unusual association. EJIFCC. 33:262–267. 2022.PubMed/NCBI
|
|
24
|
Liu H, Dong Z, Zhang M, Pang R, Xu J, He
P, Mei W, Zhang S, You G and Li W: Case report: Complex
paraneoplastic syndromes in thymoma with nephrotic syndrome,
cutaneous amyloidosis, myasthenia gravis, and Morvan's syndrome.
Front Oncol. 12(1002808)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yoo SH, Kim HJ, Kim JH, Lee GW, Lee JH,
Kim SH, Kim JW, Kim JW, Lee JO, Kim YJ, et al: Nephrotic syndrome
associated with metastatic thymoma treated with chemotherapy.
Medicine (Baltimore). 96(e5408)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sardhara J, Shukla M, Jamdar J, Jaiswal
AK, Jaiswal S, Kaul A, Bhaisora KS, Das KK, Mehrotra A and Behari
S: Paraneoplastic nephrotic syndrome in a patient with planum
sphenoidale meningioma. Asian J Neurosurg. 13:864–866.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zachariah PP, Mathew A, Rajesh R, Kurien G
and Unni VN: Nephrotic syndrome associated with meningioma. Indian
J Nephrol. 23:63–66. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takane K, Midorikawa Y, Yamazaki S,
Kajiwara T, Yoshida N, Kusumi Y and Takayama T: Gastrointestinal
stromal tumor with nephrotic syndrome as a paraneoplastic syndrome:
A case report. J Med Case Rep. 8(108)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moța E, Văduva C and Zaharie S: Compendium
of nephrology 1st Edition, Editura Medicală Universitară, Craiova,
2010.
|
|
30
|
Farahani E, Nili F, Moghimian M, Jahanzad
I, Minoo FS, Abdollahi A and Salarvand S: Analysis of prevalence
and trends in the biopsy-proven native kidney diseases in Iranian
population: A 12-year survey from a referral center. Iran J Pathol.
18:202–209. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Longo D, Fauci A, Kasper D, Hauser S,
Jameson J and Loscalzo J: Harrison's Manual of Medicine. 18th
edition. ALL, București, 2013.
|
|
32
|
Amin Nordin FD, Mohd Khalid MKN, Abdul
Aziz SM, Mohamad Bakri NA, Ahmad Ridzuan SN, Abdul Jalil J, Habib A
and Yakob Y: Performance comparison of EasyFix G26 and HYDRASYS 2
SCAN for the detection of serum monoclonal proteins. J Clin Lab
Anal. 34(e23254)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vogel JD, Felder SI, Bhama AR, Hawkins AT,
Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ,
Lightner AL, et al: The American Society of Colon and Rectal
Surgeons Clinical Practice Guidelines for the Management of Colon
Cancer. Dis Colon Rectum. 65:148–177. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Raza A and Aggarwal S: Membranous
Glomerulonephritis. StatPearls Publishing, Treasure Island, FL,
2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499865/.
|
|
35
|
Ho W and Sanyal A: Ascites: Diagnosis and
management. Med Clin North Am. 93:801–817. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nishi S, Ubara Y, Utsunomiya Y, Okada K,
Obata Y, Kai H, Kiyomoto H, Goto S, Konta T, Sasatomi Y, et al:
Evidence-based clinical practice guidelines for nephrotic syndrome
2014. Clin Exp Nephrol. 20:342–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mitruţ P, Enescu A, Streba LA, Burada F,
Cojocaru G, Simionescu C, Mărgăritescu C and Genunche-Dumitrescu A:
The endoscopic and morphological forms of early gastric cancer. Rom
J Morphol Embryol. 48:373–379. 2007.PubMed/NCBI
|
|
38
|
Buskermolen M, Cenin DR, Helsingen LM,
Guyatt G, Vandvik PO, Haug U, Bretthauer M and Lansdorp-Vogelaar I:
Colorectal cancer screening with faecal immunochemical testing,
sigmoidoscopy or colonoscopy: A microsimulation modelling study.
BMJ. 367(l5383)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Faria TV, Baptista MA, Burdmann EA and
Cury P: Glomerular deposition of immune complexes as a first
manifestation of malignant melanoma-a case report. Ren Fail.
32:1223–1225. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Takeda S, Chinda J, Murakami T, Numata A,
Iwazu Y, Akimoto T, Hamano Y, Muto S, Takahashi M and Kusano E:
Development of features of glomerulopathy in tumor-bearing rats: A
potential model for paraneoplastic glomerulopathy. Nephrol Dial
Transplant. 27:1786–1792. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ohtani H, Wakui H, Komatsuda A, Okuyama S,
Masai R, Maki N, Kigawa A, Sawada K and Imai H: Distribution of
glomerular IgG subclass deposits in malignancy-associated
membranous nephropathy. Nephrol Dial Transplant. 19:574–579.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Stepan AE, Mărgăritescu C, Stoica LE,
Stepan MD and Simionescu CE: Clear cell renal cell
carcinomas-epithelial and mesenchymal immunophenotype. Rom J
Morphol Embryol. 59:1189–1194. 2018.PubMed/NCBI
|
|
43
|
Stepan AE, Ciurea RN, Drăgoescu PO,
Florescu MM and Stepan MD: Immunoexpression of transcription
factors in urothelial bladder carcinomas. Rom J Morphol Embryol.
58:863–869. 2017.PubMed/NCBI
|