Introduction
Alzheimer's disease (AD) is a neurodegenerative
disorder and the most common type of dementia, accounting for 60 to
80% of cases. Dementia encompasses a group of neurodegenerative
disorders characterized by chronic and progressive cognitive
deterioration in various higher cortical functions, including
memory, thinking and decision-making abilities, which significantly
impair independent living. The global prevalence of AD has
substantial public health implications. In 2018, it was estimated
that ~50 million individuals worldwide suffered from dementia,
rendering AD a critical global public health concern. According to
recent data, the prevalence of dementia in Europe is projected to
double by the year 2050(1).
Aging is a key risk factor for the development of
AD. Of note, <5% of AD cases occur prior to the age of 65 years
and are classified as early-onset AD, while the majority of cases
occur after 65 years of age and are characterized as sporadic or
late-onset AD (2). In line with the
hypothesis that AD is age-related, normal aging is associated with
a decline in cognitive abilities. However, in AD, these
pathophysiological changes are exacerbated by neuroinflammation,
cellular biological alterations and injuries (3).
The Alzheimer's Disease Assessment Scale-Cognitive
(ADAS-Cog) Subscale test is widely used in research and clinical
trials to measure cognitive function, primarily assessing language,
memory and praxis. This score is considered the gold standard for
evaluating treatments against dementia (4).
Recently, there has been growing interest in the
role of omega-3 fatty acids (FAs) in promoting health and reducing
morbidity. Omega-3 FAs are polyunsaturated FAs with long
hydrocarbon chains, including α-linolenic acid (ALA, 18:3 ω-3),
stearidonic acid (SDA, 18:4 ω-3), eicosapentaenoic acid (EPA, 20:5
ω-3), docosapentaenoic acid (22:5 ω-3) and docosahexaenoic acid
(DHA, 22:6 ω-3). EPA and DHA are synthesized from the essential
omega-3 FA ALA through elongation and desaturation, catalyzed by
delta-15 desaturase. ALA is found in plants, particularly in
vegetable oils, seeds and nuts. These FAs play a critical role in
the healthy development and functioning of the human body. Various
potential health benefits have been investigated (5).
Dietary supplementation with omega-3 FAs may
influence brain function by altering membrane fluidity, the
activity of membrane-bound enzymes, ion channel function, receptor
affinity and number, and signal transduction pathways that regulate
neurotransmitter and neuronal growth factor activity (6). Epidemiological research has suggested
an association between a low omega-3 FA intake and an increased
risk of cognitive decline or dementia, particularly AD (7). In 2004, MacLean et al (8) provided clinical evidence linking
omega-3 FAs to the prevention of AD. Omega-3 FAs are essential for
the assembly, maturation and physiological function of neuronal
structures. Additionally, omega-3 FAs are crucial for higher
cognitive functions, such as cognition and memory (9).
The association between omega-3 FAs and cognition
remains under investigation, with accumulating evidence and
inconsistent results regarding the efficacy of dietary
supplementation on the cognitive functions of patients with AD.
While recent clinical and epidemiological research has indicated
that specific dietary components may influence the risk of
developing AD, the cognitive health benefits of fish and omega-3
FAs in older healthy individuals appear to be more consistent than
in patients diagnosed with AD (5).
The critical question of whether dietary
supplementation with omega-3 FAs, such as DHA, can alter the risk
of developing AD or the progression of the condition remains
unanswered. Randomized controlled trials (RCTs) are essential for
evaluating the efficacy of various treatment options. Therefore,
the present study aimed to identify all RCTs examining the
association between omega-3 FA supplementation and cognitive
function in patients with AD, as measured by the ADAS-Cog test, and
to conduct a meta-analysis and determine whether these nutrients
improve cognition.
Data and methods
Search strategy
A comprehensive literature search was conducted in
two electronic databases, PubMed and Cochrane Library, for all
eligible published RCTs assessing cognition following omega-3 FA
supplementation compared to a placebo, from inception up to
December 31, 2023. Key words, synonyms and Boolean operators were
utilized to create the search strategy. The search query on the
Medline database was as follows: (Alzheimer Disease) AND [(Omega3
Fatty) OR (Omega-3) OR (Fatty acids) OR (Omega-3 fatty acids) OR
(Omega 3 fatty acids)] AND [(RCT) OR (randomized controlled trial)
OR (clinical trial)]. Similar queries were employed for controlled
vocabulary searches. Filters applied included article type
(Clinical trial, RCT) and species (humans). There were no
restrictions on language and time. Unpublished manuscripts were not
considered.
Study selection
Studies were included based on the following
Population, Intervention, Comparator, Outcome, Study type (PICOS)
criteria: i) Participants: Adults with AD; ii) intervention:
Omega-3 FA supplementation; iii) comparator: Placebo; outcome:
Cognition, assessed using the ADAS-Cog test; and iv) study type:
RCTs.
Statistical analysis
Data analysis was conducted using RevMan 5.3
software (https://revman.cochrane.org/info). The outcomes were
treated as continuous variables and analyzed using the random
effects model. The point estimate for continuous variables was the
mean difference (MD) along with the corresponding 95% confidence
intervals (CIs). To impute a standard deviation of the change from
baseline for the experimental intervention, an appropriate formula
was used according to the Cochrane Handbook for Systematic Reviews
of Interventions (10). Statistical
homogeneity was evaluated with the Chi-squared test and the
I2 test. A value of P<0.05 was considered to indicate
a statistically significant difference. Sensitivity analysis was
also performed by successively deleting each study and reanalyzing
the data set for all remaining studies.
Quality assessment and data
extraction
The Revised Cochrane risk of bias tool for
randomized trials (RoB 2.0) (10)
was used to assess the methodological quality of the included
studies. Two researchers (TVK and KD) independently used a
standardized form to collect data on the RCTs. The form included
data on the names of authors, year of publication, study design,
study characteristics (sample size, and age and sex of the study
participants, duration of the intervention), intervention (type of
omega-3 FA and dosage), outcome assessment and results.
Discrepancies between the two reviewers were resolved through
discussion and consensus with a third senior reviewer (DK). The
grading of retrieved evidence for the efficacy outcome (improved
cognition) was performed in accordance with the GRADE framework
(11).
Results
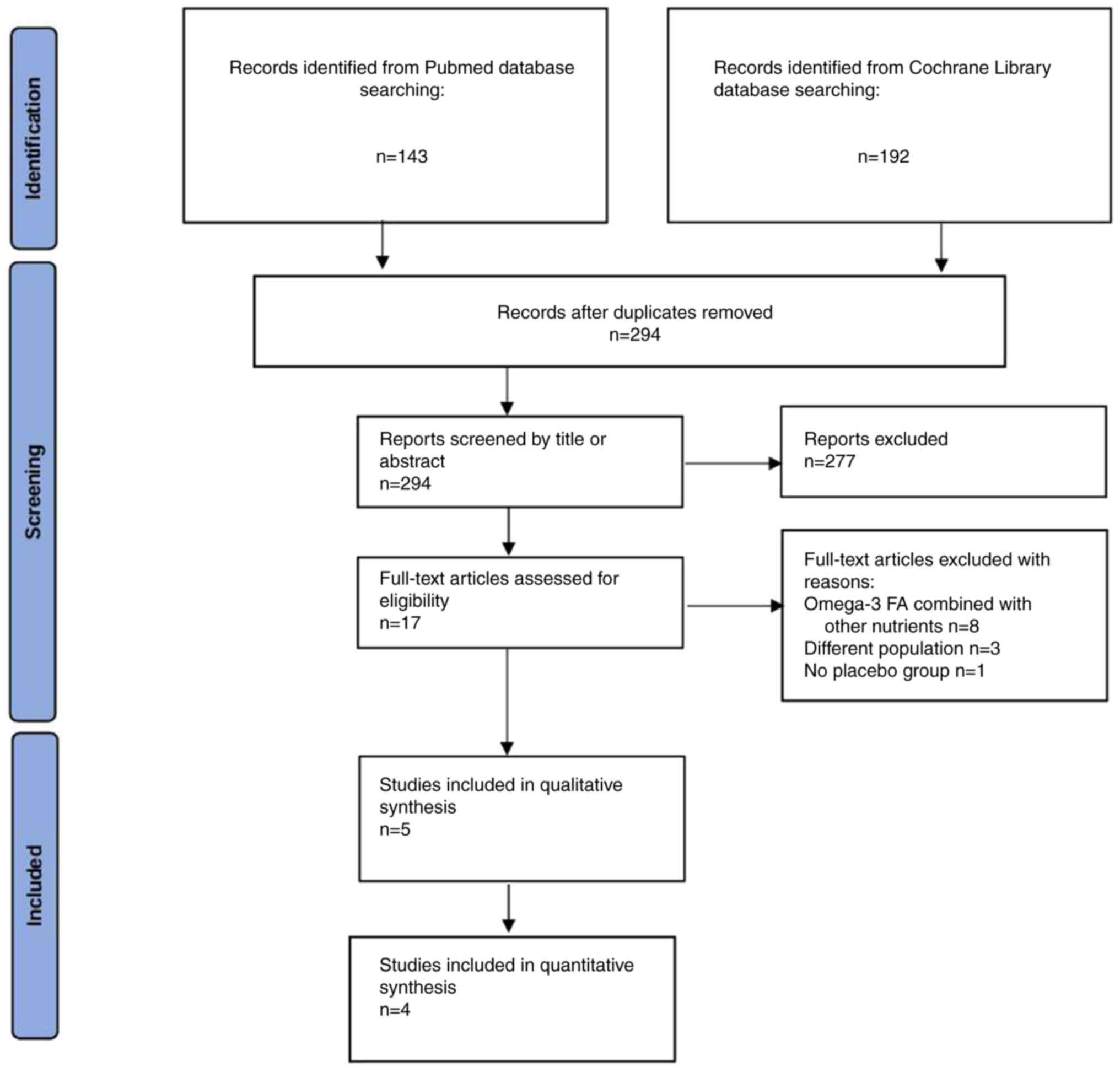
Selected studies
Initially, 143 results were retrieved from the
PubMed database and 192 results from the Cochrane Library database.
After removing duplicate records and evaluating the titles and
abstracts to identify relevant articles, five studies (12-16)
met the eligibility criteria and were included in the qualitative
synthesis, with four of these studies (12-15)
being included in the present meta-analysis. The study by Shinto
et al (16) was excluded
from the quantitative synthesis, as the results were not presented
in an appropriate format. Data were collected from a total of 702
patients with AD, with 376 participants assigned to omega-3 FA
supplementation and 326 participants assigned to a placebo. A flow
diagram illustrating the study selection process is presented in
Fig. 1.
Characteristics of selected
studies
The five clinical studies were conducted between
2006 and 2022. The age of the study participants ranged from 64 to
85 years. The clinical trials varied in duration from 24 weeks to
24 months. Omega-3 FAs (DHA, EPA, or combinations thereof) were
administered orally in the form of capsules. Both females and males
were included in the studies. The ADAS-Cog test was performed at
the beginning of the studies and at the final time endpoint in all
studies. All the studies included were RCTs. The characteristics of
the included studies are presented in Table I.
 | Table ISummary of the baseline
characteristics of the participants, which are of interest, across
the selected trials. |
Table I
Summary of the baseline
characteristics of the participants, which are of interest, across
the selected trials.
| | Authors (Refs.) |
|---|
| Characteristic | Freund-Levi et
al (12) | Chiu et al
(15) | Quinn et al
(13) | Shinto et al
(16) | Lin et al
(14) |
|---|
| No. of participants
on omega-3 FAs | 89 | 20 | 238 | 13 | 123 |
| No. of participants
on the placebo | 85 | 15 | 134 | 13 | 40 |
| Age of the
participants (years) on omega-3 FAs | 72.6±9 | 74.0±3.9 | 76±9.3 | 75.9±8.1 | EPA group,
77.80±8.49; DHA group, 78.95±7.89; EPA + DHA group, 76.73±9.15 |
| Age of the
participants (years) on the placebo | 72.9±8.6 | 76.5±4.7 | 76±7.8 | 75.2±10.8 | 78.10±8.59 |
| Females on omega-3
FAs (n, %) | 51 (57%) | 65% | 47.1% | 39% | EPA group, 32.5%; DHA
group, 29.27%; EPA + DHA group, 35.71% |
| Females on the
placebo (n, %) | 39 (46%) | 46.7% | 59.8% | 54% | 37.50% |
| Omega-3 FA Route of
administration | DHA + EPA Oral
(capsules) | EPA + DHA Oral
(capsules) | DHA Oral
(capsules) | DHA + EPA Oral
(capsules) | EPA, DHA and EPA +
DHA Oral (capsules) |
| Dosage | 430 mg DHA+150
EPA | 1,080 mg EPA + 720 mg
DHA | 2 g DHA | 675 mg DHA + 975 mg
EPA | EPA group, 1.6 g; DHA
group, 0.7 g; EPA + DHA group, 0.8 g EPA + 0.35 g DHA |
| Duration | 6 months | 24 weeks | 18 months | 12 months | 24 months |
| ADAS-Cog baseline for
omega-3 FA supplementation | 25.7±10.1 | 9.17±7.19 | 23.77 (8.9) | 31.8 (9.4) | EPA group,
15.29±8.48; DHA group, 14.60±6.38; EPA + DHA group, 16.33±8.46 |
| ADAS-Cog baseline for
placebo supplementation | 27.2±10.1 | 7.99±7.13 | 23.96 (9.2) | 32.2 (9.5) | 14.76±6.98 |
| ADAS-Cog final for
omega-3 FA supplementation | 27.7±11.1 | 5.90±5.63 | 31.75±11.8 | - | EPA group,
17.93±6.43; DHA group, 17.53±7.17; EPA + DHA group, 18.7±3.68 |
| ADAS-Cog final for
placebo supplementation | 28.3±10.8 | 5.57±4.76 | 32.23±10.32 | - | 15.61±4.03 |
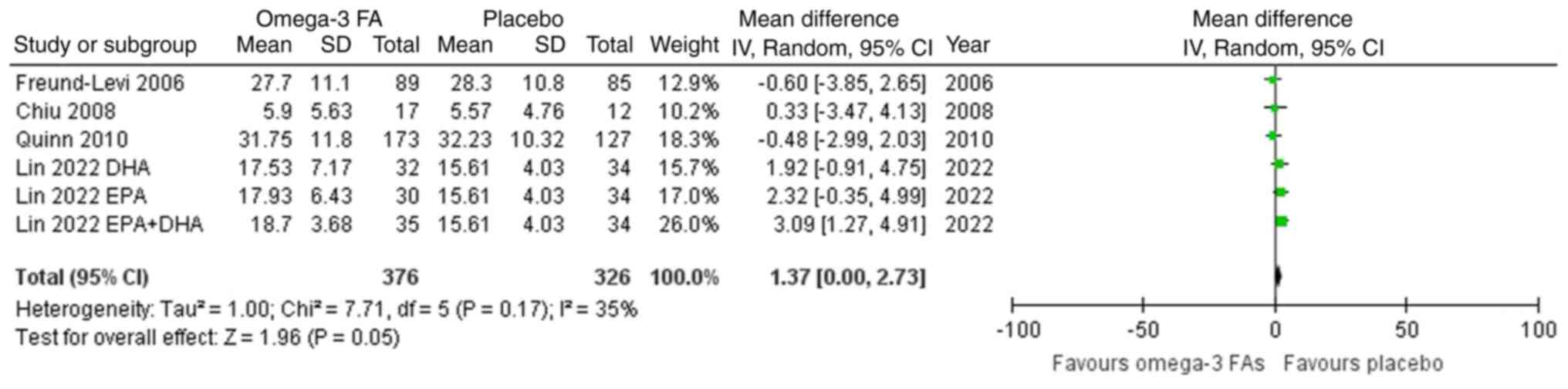
Meta-analysis
As demonstrated in Fig.
2, omega-3 FA supplementation compared to a placebo resulted in
a non-significant impact on the ADAS-Cog score (MD=1.37; 95% CI,
0.00-2.73). The included studies were similar (I2=35%;
P=0.17). The test for overall effect (Z=1.96; P=0.05) confirmed no
statistically significant difference. No heterogeneity was
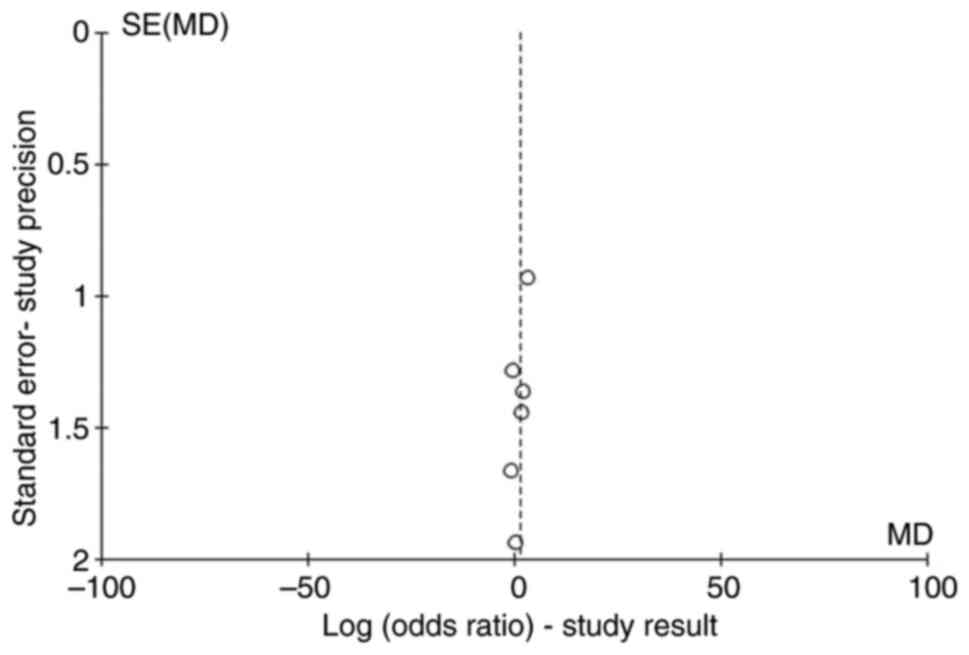
demonstrated for the comparison. Publication bias was assessed with
funnel plots and the risk was low (Fig.
3). Egger's test was also performed. It yielded a value of 0.07
(>0.05) with a regression coefficient of -1.5; the results
demonstrated no major publication bias, but a possible sign against
publication of smaller size studies.
Quality assessment
The risk of bias assessment of the included trials
according to the revised Cochrane risk of bias tool for randomized
trials (RoB 2.0) is presented in Table
II. The study by Freund-Levi et al (12) was reported as being randomized
without describing the exact method of randomization; thus, the
risk of bias regarding the randomization process could not be
adequately assessed. Both the intervention and placebo groups
received the intended interventions, and the effect of assignment
to the intervention was estimated using both intention-to-treat and
per-protocol analyses. As regards outcome measurement, the assessor
was not blinded (the study was not triple-blinded). The overall
risk was stated as ‘some concerns.’ The study by Chiu et al
(15) did not elaborate on the
process used to achieve randomization, so the randomization bias
risk could not be measured. The assessor of the outcome was not
blinded in this study either. The overall risk was characterized as
some concerns.’ Shinto et al (16) did not describe the randomization
process, but provided a detailed table with the number of dropouts
in each group without mentioning an intention-to-treat analysis.
The overall risk of bias of the study was stated as some concerns.’
Quinn et al (13) adequately
described the randomization process, but the outcome assessor was
not blinded. The overall risk was stated as some concerns.’ Lastly,
the study conducted by Lin et al (14) did not provide a detailed description
of the randomization process and did not include a blinded outcome
assessor. Overall, the risk of bias was stated as some
concerns.’
 | Table IIQuality assessment of the included
interventional trials. |
Table II
Quality assessment of the included
interventional trials.
| Authors | Randomization
process | Deviation of
intended intervention | Missing outcome
data | Measurement of the
outcome | Selection of the
reported result | Overall | (Refs.) |
|---|
| Freund-Levi et
al | Unclear | Low risk | Low risk | Some concerns | Low risk | Some concerns | (12) |
| Chiu et
al | Unclear | Low risk | Low risk | Some concerns | Low risk | Some concerns | (15) |
| Shinto et
al | Unclear | Low risk | Low risk | Some concerns | Low risk | Some concerns | (16) |
| Quinn et
al | Low risk | Low risk | Low risk | Some concerns | Low risk | Some concerns | (13) |
| Lin et
al | Unclear | Low risk | Low risk | Some concerns | Low risk | Some concerns | (14) |
Discussion
AD is the leading cause of dementia and a
significant public health concern globally, particularly as the
population ages. It is a multifactorial neurodegenerative disease
characterized by a continuous and slow progression that affects the
brain and interferes with independent living. Currently, no
curative treatment is available, and the pathophysiological
cellular and molecular mechanisms responsible for the development
of AD remain unclear (1).
Cyclooxygenase and lipooxygenase pathways, along
with cytochrome P450 enzymes, are critical for the metabolism of
omega-3 dietary long-chain polyunsaturated FAs. These nutrients may
have beneficial properties, such as anti-inflammatory,
anti-apoptotic, antioxidant and neurotrophic effects. Specifically,
omega-3 FAs inhibit the activities of cyclooxygenase-2 and nitric
oxide synthase-2, and suppress nuclear factor-κB, leading to
decreased levels of cytokines and monocytic chemotactic protein-1,
thus enhancing anti-inflammatory activity. Omega-3 FAs also prevent
neuronal apoptosis by lowering caspase levels, exert antioxidant
effects by inducing transcription factors, and increase nerve
growth factor levels (17). Despite
the growing interest and extensive research in nutrition over
recent years, with common assumptions that diet and nutrients may
affect the progression of AD, available data from studies
evaluating omega-3 FA supplementation remain controversial and
limited. Several research groups worldwide have used different
animal models over the last two decades to examine the potential
association between omega-3 FA supplementation and Aβ pathology and
cognitive decline. Despite notable heterogeneity, evidence suggests
that these dietary components can protect against cognitive
deterioration in AD and associated neuropathology.
The systematic review and meta-analysis conducted by
Hooijmans et al (18) in
2012 revealed that long-term omega-3 FA supplementation (≥10% of
the expected lifespan in rats and mice) reduced brain Aβ levels,
neuronal loss, and improved cognitive functions in animal models of
AD. Subgroup analysis indicated potential differences between
species (mice and rats) and sex (18). In humans, a key risk factor for AD
development is the loss of sex steroid hormones, specifically
androgens and estrogens, with age. Cognitive decline is slower, and
females exhibit improved verbal abilities than males (19).
Freund-Levi et al (12) conducted a randomized, double-blind,
placebo-controlled study with 6 months of follow-up. The treatment
group received 1,720 mg DHA plus 600 mg EPA daily, while the
placebo group received 4,000 mg isocaloric corn oil daily for 6
months. ADAS-Cog was assessed at baseline and at 6 months. No
statistically significant difference was observed between the
groups on the ADAS-Cog (P>0.05) at 6 months. However, a
significant reduction in decline rate was noted in a subgroup of
patients with very mild cognitive dysfunction (n=32) in the omega-3
FA group compared to placebo (P=0.01) (12).
Chiu et al (15) conducted a randomized, double-blind,
placebo-controlled study with 24 weeks of treatment. Patients
received 1,080 mg EPA plus 720 mg DHA daily, while the placebo
group received olive oil esters. Cognitive evaluation was performed
at baseline and weeks 6, 12, 18 and 24 using ADAS-Cog. No
significant cognitive improvement was observed in the treated group
(15).
Quinn et al (13) conducted an 18-month randomized,
double-blind, placebo-controlled trial. The intervention group
received 2 g DHA daily, while the placebo group received corn or
soy oil. ADAS-Cog assessment was conducted at baseline, six months,
and 12 months. No statistically significant difference was found
between the groups regarding ADAS-Cog (P=0.41) after 18 months
(13).
Shinto et al (16) conducted a 12-month, three-arm,
parallel-group, randomized, double-blind, placebo-controlled pilot
clinical trial. Participants received omega-3 FA fish oil
concentrate containing 675 mg DHA and 975 mg EPA daily, while the
placebo group received soy oil. Cognitive subscale evaluation was
conducted at baseline and 12 months. No significant difference in
cognitive performance was observed between the omega-3 FA and
placebo groups (P=0.86) (16). Lin
et al (14) conducted a
randomized, double-blind, placebo-controlled trial with four
groups: The placebo, DHA 0.7 g daily, EPA 1.6 g daily, or EPA 0.8 g
plus DHA 0.35 g daily for 24 months. Overall, omega-3 FA
supplementation did not improve cognitive outcomes (14).
Phillips et al (20) conducted a 4-month randomized,
double-blind, placebo-controlled study. The intervention group
received 625 mg DHA and 600 mg EPA daily, while the placebo group
received olive oil. Cognitive assessment was conducted using the
MMSE at 1 and 4 months. No significant difference in cognition was
observed between the groups (P<0.05) over the 4-month period
(20).
The fact that through time several studies have
presented conflicting results regarding the efficacy of omega-3 FA
in the management of AD may be attributed to different study
designs, different population characteristics, different types of
interventions, differences in duration of intervention,
methodological differences, such as the lack of blinding and
randomization, discrepancies in statistical analysis and various
other environmental and contextual factors.
Previous studies have indicated uncertain
associations between omega-3 FA supplementation and cognitive
improvement, with no significant outcomes reported (12,13,15,16,20).
AD is a high-burden disease with an increasing prevalence as the
population ages. It is a clinically complex, age-related
neurodegenerative disorder with significant pathological brain
changes and no curative treatment. Current therapeutic approaches
primarily alleviate symptoms rather than halt disease progression.
Nutritional interventions may enhance pharmaceutical treatment
outcomes. The present systematic review and meta-analysis aimed to
determine whether dietary omega-3 FA administration could improve
cognitive parameters, as indicated by the ADAS-Cog test, in
patients with AD.
The authors aimed to address the need for a clearer
discussion of the clinical impact of the findings and the potential
benefits of omega-3 fatty acids in AD. While the findings of the
present meta-analysis do not demonstrate significant cognitive
benefits of omega-3 FA supplementation for patients with AD, the
clinical implications remain noteworthy. Omega-3 FAs, particularly
DHA and EPA, are recognized for their anti-inflammatory,
neuroprotective and antioxidant properties, which may provide
neurobiological benefits beyond measurable cognitive outcomes.
Although the evidence does not support their routine use in AD
treatment, omega-3 FAs could potentially play a preventative role
by mitigating early pathological processes such as
neuroinflammation and oxidative stress. Furthermore, the observed
subgroup effects, such as reduced cognitive decline in patients
with very mild cognitive dysfunction, highlight the need for
targeted interventions at earlier disease stages. Future studies
are required to investigate the timing, dosage and
population-specific factors that may optimize the therapeutic or
preventative utility of omega-3 FAs in patients with AD.
The limited number of RCTs included small sample
sizes, limited statistical power, and varying omega-3 FA types,
dosages and ratios used restricted subgroup analysis. Future
studies are thus warranted to address these limitations. The
heterogeneity observed (I2 35%) may be attributed to
differences in treatment duration (6 months to 24 months), the type
of FAs used (DHA + EPA or DHA alone), differences in demographics
(male-female composition of each intervention and comparator group,
and mean age), small sample sizes [Shinto et al (16) and Chiu et al (15)].
In conclusion, omega-3 FA supplementation does not
appear to significantly benefit cognition in patients with AD.
Therefore, omega-3 FA supplementation should not be recommended for
the treatment of AD, although the results should be cautiously
interpreted due to the limited number of included studies. Larger
RCTs and further investigation are required to explore the
potential beneficial role of omega-3 FAs in preventing cognitive
deterioration in individuals at risk of developing AD. Developing
nutrition strategies to maintain cognitive function and improve the
quality of life for AD patients should be a research priority.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KD and DK conceptualized the study. TVK KD, DK, GF,
VEG and DAS made a substantial contribution to data interpretation
and analysis and wrote and prepared the draft of the manuscript. DK
and KD analyzed the data and provided critical revisions. All
authors contributed to manuscript revision and have read and
approved the final version of the manuscript. VEG and TVK confirm
the authenticity of all raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Scheltens P, De Strooper B, Kivipelto M,
Holstege H, Chételat G, Teunissen CE, Cummings J and van der Flier
WM: Alzheimer's disease. Lancet. 397:1577–1590. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Long JM and Holtzman DM: Alzheimer
disease: An update on pathobiology and treatment strategies. Cell.
179:312–339. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Herrup K: Reimagining Alzheimer's
disease-an age-based hypothesis. J Neurosci. 30:16755–16762.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kueper JK, Speechley M and Montero-Odasso
M: The Alzheimer's disease assessment scale-cognitive subscale
(ADAS-Cog): Modifications and responsiveness in pre-dementia
populations. A narrative review. J Alzheimers Dis. 63:423–444.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shahidi F and Ambigaipalan P: omega-3
polyunsaturated fatty acids and their health benefits. Annu Rev
Food Sci Technol. 9:345–381. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yehuda S, Rabinovitz S and Mostofsky DI:
Essential fatty acids and the brain: From infancy to aging.
Neurobiol Aging. 26 (Suppl 1):S98–S102. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cole GM, Ma QL and Frautschy SA: Omega-3
fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids.
81:213–221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
MacLean CH, Mojica WA, Morton SC, Pencharz
J, Hasenfeld Garland R, Tu W, Newberry SJ, Jungvig LK, Grossman J,
Khanna P, et al: Effects of omega-3 fatty acids on lipids and
glycemic control in type II diabetes and the metabolic syndrome and
on inflammatory bowel disease, rheumatoid arthritis, renal disease,
systemic lupus erythematosus, and osteoporosis. Evid Rep Technol
Assess (Summ). (89):1–4. 2004.PubMed/NCBI
|
|
9
|
Smollich M: Special | Omega-3-Fatty Acids
Omega-3 fatty acids and brain function. Ernahrungs Umschau.
62:170–177. 2015.https://www.ernaehrungs-umschau.de/fileadmin/Ernaehrungs-Umschau/pdfs/pdf_2015/10_15/EU10_2015_WuF_Smollich_Eng.pdf.
|
|
10
|
Higgins JPT, Sterne JAC, Savović J, Page
MJ, Hróbjartsson A, Boutron I, Reeves B and Eldridge S: A revised
tool for assessing risk of bias in randomized trials. Cochrane
Database Syst Rev. 10:29–31. 2016.
|
|
11
|
Brozek JL, Akl EA, Alonso-Coello P, Lang
D, Jaeschke R, Williams JW, Phillips B, Lelgemann M, Lethaby A,
Bousquet J, et al: Grading quality of evidence and strength of
recommendations in clinical practice guidelines. Part 1 of 3. An
overview of the GRADE approach and grading quality of evidence
about interventions. Allergy. 64:669–677. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Freund-Levi Y, Eriksdotter-Jönhagen M,
Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B,
Wahlund LO and Palmblad J: Omega-3 fatty acid treatment in 174
patients with mild to moderate Alzheimer disease: OmegAD study: A
randomized double-blind trial. Arch Neurol. 63:1402–1408.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Quinn JF, Raman R, Thomas RG, Yurko-Mauro
K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR Jr, Weiner M,
et al: Docosahexaenoic acid supplementation and cognitive decline
in Alzheimer disease: A randomized trial. JAMA. 304:1903–1911.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin PY, Cheng C, Satyanarayanan SK, Chiu
LT, Chien YC, Chuu CP, Lan TH and Su KP: Omega-3 fatty acids and
blood-based biomarkers in Alzheimer's disease and mild cognitive
impairment: A randomized placebo-controlled trial. Brain Behav.
Immun. 99:289–298. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chiu CC, Su KP, Cheng TC, Liu HC, Chang
CJ, Dewey ME, Stewart R and Huang SY: The effects of omega-3 fatty
acids monotherapy in Alzheimer's disease and mild cognitive
impairment: A preliminary randomized double-blind
placebo-controlled study. Prog Neuropsychopharmacol Biol
Psychiatry. 32:1538–1544. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shinto L, Quinn J, Montine T, Dodge HH,
Woodward W, Baldauf-Wagner S, Waichunas D, Bumgarner L, Bourdette
D, Silbert L and Kaye J: A randomized placebo-controlled pilot
trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer's
disease. J Alzheimers Dis. 38:111–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ajith TA: A recent update on the effects
of omega-3 fatty acids in Alzheimer's disease. Curr Clin Pharmacol.
13:252–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hooijmans CR, Pasker-de Jong PCM, de Vries
RB and Ritskes-Hoitinga M: The effects of long-term omega-3 fatty
acid supplementation on cognition and Alzheimer's pathology in
animal models of Alzheimer's disease: A systematic review and
meta-analysis. J Alzheimers Dis. 28:191–209. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ferretti MT, Iulita MF, Cavedo E, Chiesa
PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard
H, Misoch S, Giacobini E, et al: Sex differences in Alzheimer
disease - the gateway to precision medicine. Nat Rev Neurol.
14:457–469. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Phillips MA, Childs CE, Calder PC and
Rogers PJ: No effect of omega-3 fatty acid supplementation on
cognition and mood in individuals with cognitive impairment and
probable Alzheimer's disease: A randomised controlled trial. Int J
Mol Sci. 16:24600–24613. 2015.PubMed/NCBI View Article : Google Scholar
|