Introduction
Liver fibrosis is caused by several types of liver injuries, including viral hepatitis, alcoholic hepatitis and non-alcoholic steatohepatitis. A meta-analysis showed that the global prevalence rate of advanced liver fibrosis in the general population is 3.3% (95% CI, 2.4-4.2%), with an increasing trend observed in recent years (1). During liver injury, activated Kupffer cells serve as the primary producers of pro-inflammatory cytokines, including interleukin-6, tumor necrosis factor-α and transforming growth factor-beta (TGF-β). These cytokines activate hepatic stellate cells (HSCs), which, upon activation, produce excess extracellular matrix molecules such as collagen types I and III, as well as other proteins that constitute pathological fibrous tissues. This process ultimately leads to scar tissue formation and liver fibrosis (2,3).
The Sestrin (SESN) family consists of three highly evolutionarily conserved, stress-inducible proteins: SESN1, SESN2 and SESN3. Among them, SESN2 has been the most extensively studied SESN. SESN2 expression is upregulated in cells in response to oxidative stress (4), hypoxia (5,6) and nutritional stress (7,8). Under these conditions, activating transcription factor 4 (ATF4) expression is induced to stimulate SESN2 expression. SESN2 exerts cytoprotective effects by activating AMP-activated protein kinase and inhibiting the mechanistic target of rapamycin complex 1, thereby attenuating anabolic activities and maintaining cellular homeostasis (9). In liver disease, SESN2 is abnormally expressed and is correlated with disease progression. This increased SESN2 production suppresses HSCs' activation and intrahepatic inflammation, thereby inhibiting the occurrence and progression of fibrogenesis. Furthermore, artificial induction of SESN2 via the ATF4 pathway ameliorates hepatic steatosis in mice (10). Overexpression of SESN2 in the mouse liver reduces collagen type Iα 1 (COL1A1) expression (11).
HSC activation and the progression of fibrosis are influenced by various amino acids. Branched-chain amino acids have been shown to suppress liver fibrosis in rats treated with carbon tetrachloride (12,13) and in diethyl nitrosamine-treated cirrhotic rats (14). Glutamine also inhibits carbon tetrachloride-induced liver fibrosis in mice (15). Branched-chain amino acids reduce COL1A1 expression, the primary component of collagen (16,17). Additionally, a combination of five amino acids and an amino acid derivative-leucine (Leu), isoleucine, valine, arginine, glutamine, and N-acetylcysteine-reduces the secretion of procollagen 1 and 3 in TGF-β-treated HSCs (18). Conversely, homocysteine promotes HSC proliferation (19), while Leu enhances procollagen alpha1 translation in HSCs (20). However, the effects of amino acids on quiescent HSCs remains unclear.
Previously, it was reported that specific amino acids differentially regulate SESN2 expression, which is upregulated by amino acid deprivation in C2C12 cells (21). Based on these findings, it was hypothesized that specific amino acids regulate SESN2 expression and influence COL1A1 production and cell proliferation, even in quiescent HSCs. It has been reported that RI-T cells, a rat HSC cell line, can be cultured in both Roswell Park Memorial Institute (RPMI)-1640 medium and Dulbecco's modified Eagle's medium (DMEM) (22,23). Unlike RPMI-1640, DMEM does not contain Asp, Asn, Glu and Pro. In the present study, the effect of these two media was first compared on SESN2 expression as an initial screening. The effect of amino acid composition on SESN2 and COL1A1 expression, as well as cell proliferation, was then investigated in RI-T cells.
Materials and methods
Cell culture and in vitro study
Fetal bovine serum (FBS) was purchased from ICN Biomedicals. DMEM and RPMI-1640 media were obtained from Nacalai Tesque, Inc. Aspartic acid (Asp), asparagine (Asn), glutamic acid (Glu), proline (Pro), sodium pyruvate and actinomycin were also sourced from Nacalai Tesque, Inc. The C2C12 myocytes (cat. no. RCB0987) were obtained from the Riken Cell Bank. RI-T rat HSC lines (cat. no. JCRB1088) were purchased from the JCRB cell bank. C2C12, HepG2 (a liver cancer cell line; cat. no. RCB1648; Riken Cell Bank) and GH3 (a pituitary cell line producing growth hormone and prolactin; cat. no. CCL-82.1; American Type Culture Collection) cells were cultured in DMEM supplemented with 15.5 µg/ml kanamycin, 100 µg/ml penicillin G and 10% FBS. RI-T cells were maintained in RPMI-1640 medium with same supplements. Cells were plated in 6-well plates for western blot experiments or in 12-well plates for reverse transcription-quantitative PCR (RT-qPCR) experiments and cultured until 80% confluence was reached.
After reaching the desired confluence, cells were washed with phosphate buffered saline and treated with media containing specific amino acids for 5, 24, or 48 h to examine the effects of specific amino acids on SESN2, ATF4, COL1A1 and asparagine synthetase (ASNS) mRNA levels as well as COL1A1 protein levels. The final concentrations of Asp, Asn, Glu and Pro added to DMEM matched those found in RPMI-1640 medium. Additionally, sodium pyruvate was added to the RPMI-1640 medium to adjust its concentration to match that of DMEM. For experiments evaluating SESN2 transcriptional activation, 1 mM actinomycin was added to the medium, as previously described (24). Unless otherwise specified, serum-free medium was used in all experiments. Preliminary time-course experiments showed detectable changes in SESN2 mRNA expression within 5 h, thus guiding the incubation time for these studies.
RT-qPCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. RNA concentration was determined by measuring absorbance at 260 nm using a spectrophotometer. Reverse transcription was performed using 2 µg of total RNA in a 25 µl reaction volume at 42˚C for 60 min. The synthesized complementary DNA solution (0.8 µl) was used as the template for RT-qPCR, which was conducted using the Thunderbird SYBR PCR mix (Toyobo Life Science) on a LightCycler 96 System (Roche Diagnostics). The thermocycling conditions were as follows: Initial denaturation at 95˚C for 60 sec, followed by 45 cycles of 95˚C for 15 sec, 60˚C for 15 sec, and 72˚C for 45 sec. All gene expression data were normalized to β-actin mRNA levels and relative quantification was performed using the 2-ΔΔCq method as previously described (25). Primer sequences used in the present study are listed in Table I.
 |
Table I
Primer sequences used in reverse transcription-quantitative PCR.
|
Table I
Primer sequences used in reverse transcription-quantitative PCR.
| Gene name |
Primer sequence (5'-3') |
Species |
| β-actin |
F: AGCCATGTACGTAGCCATCC |
Rattus norvegicus |
| |
R: CTCTCAGCTGTGGTGGTGAA |
|
| SESN2 |
F: ATCGCCAGTTCTCCTCGTTC |
|
| |
R: TCGGCTACGATCATGGTGTG |
|
| ATF4 |
F: CGGCAAGGAGGATGCCTTTT |
|
| |
R: TGTCTGAGGGGGCTCCTTAT |
|
| COL1A1 |
F: AAAGGCTGGAGAACGAGGTG |
|
| |
R: CAAGGTCTCCAGGAACACCC |
|
| ASNS |
F: CGATGAAGATTGCGCACAGG |
|
| |
R: GATGGTTTTCTCGATGCCGC |
|
| β-actin |
F: AGCCATGTACGTAGCCATCC |
Mus musculus |
| |
R: CTCTCAGCTGTGGTGGTGAA |
|
| SESN2 |
F: ACTGCGTCTTTGGCATCA |
|
| |
R: CATCCTACGGGTCGTCTTCT |
|
| ATF4 |
F: GAATGGCCGGCTATGGATGA |
|
| |
R: TCTGGCATGGTTTCCAGGTC |
|
| ASNS |
F: CCTGGACTCGAGCTTGGTTG |
|
| |
R: GATCACCACGCTGTCTGTGTT |
|
| β-actin |
F: GTCACCAACTGGGACGACAT |
Homo sapiens |
| |
R: GAGGCGTACAGGGATAGCAC |
|
| SESN2 |
F: GCTGTTGCCCGAATCCTAGT |
|
| |
R: ATGTGACCAGCAAAGGCTCA |
|
| ATF4 |
F: CTTGATGTCCCCCTTCGACC |
|
| |
R: CTTGTCGCTGGAGAACCCAT |
|
| ASNS |
F: GCTGCTAGAAAGGTGGCAGA |
|
| |
R: ACCATGGGCAGCAGTAGTTC |
|
Protein extraction and western blotting
RI-T cells were washed twice with ice-cold phosphate buffered saline, followed by treatment with ice-cold lysis buffer [50 mM Tris-HCl, pH 8.0; 120 mM NaCl; 20 mM NaF; 1% Nonidet™ P-40; 1 mM ethylene glycol tetra-acetic acid; 1 mM ethylenediaminetetraacetic acid; 15 mM sodium dihydrogen pyrophosphate; 2 mM sodium orthovanadate; 1 mM β-glycerophosphate; and protease inhibitor cocktail (Nacalai Tesque, Inc.)]. The lysates were centrifuged at 13,000 x g for 12 min at 4˚C, and the supernatant was transferred to new tubes. The protein concentration in each lysate was determined using the Bradford protein assay (Bio-Rad Laboratories, Inc.). Lysates containing 30 µg of total protein were boiled in 2X sample buffer (125 mM Tris-HCl, pH 6.8; 4% sodium dodecyl sulfate; 100 mM dithiothreitol; 20% glycerol and 1% bromophenol blue) for 5 min. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5% acrylamide) and transferred to polyvinylidene fluoride membranes. After blocking with Blocking One (Nacalai Tesque, Inc.) for 30 min at room temperature, the membranes were incubated overnight at 4˚C with primary antibodies, followed by washing with Tris-buffered saline containing 0.1% Tween-20. Secondary antibodies were then applied for 1 h at 22˚C. The following primary antibodies were used: α-tubulin (cat. no. T6074; MilliporeSigma) and COL1A1 (cat. no. bs-10423R; BIOSS). Secondary antibodies included horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG (cat. nos. NA934 and NA931, respectively; GE Healthcare). Protein bands were detected using Amersham ECL Prime chemiluminescent Reagent (GE Healthcare) and quantified using an ImageQuant LAS 500 system with the ImageQuant TL software (v.8-2; Cytiva). The band intensities are expressed in arbitrary units.
Small interfering RNA (siRNA)
For siRNA experiments, rat ATF4 siRNA targeting the sequence TGGATAAGAAGCTGAAAAAGATG and rat SESN2 siRNA targeting the sequence TGCGTCTTTGGTATCAGATATGA were obtained from Eurofins Genomics. The negative control siRNA targeting the sequence TTCTCCGAACGTGTCACGT was purchased from Shanghai GenePharma Co., Ltd. The sense and antisense sequences for all siRNAs used are listed in Table II. RI-T cells were seeded in 12-well plates and cultured in RPMI-1640 medium until they reached 50% confluency. A total of 50 pmol of siRNA or control siRNA was transfected into the cells using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. After 24 h, the medium was replaced with DMEM, with or without Asn and Pro (AP). Total RNA was extracted after 5 h of incubation.
 |
Table II
Sense and antisense sequences for small interfering RNAs.
|
Table II
Sense and antisense sequences for small interfering RNAs.
| Target gene |
Sequence (5'-3') |
Species |
| ATF4 |
Sense: UCUUUUUCAGCUUCUUAUCCA |
Rattus norvegicus |
| |
Antisense: GAUAAGAAGCUGAAAAAGAUG |
|
| SESN2 |
Sense: AUAUCUGAUACCAAAGACGCA |
|
| |
Antisense: CGUCUUUGGUAUCAGAUAUGA |
|
| Control |
Sense: UUCUCCGAACGUGUCACGUDTDT |
|
| |
Antisense: ACGUGACACGUUCGGAGAADTDT |
|
Cell proliferation assay
RI-T cells were seeded in 96-well plates at a density of 7,000 cells per well. After 6 h of incubation at 37˚C, AP was added to DMEM, and the cells were incubated for an additional 24 h. Proliferation was assessed using a Cell Counting Kit-8 (Dojindo Laboratories, Inc.), according to the manufacturer's instructions. Briefly, 20 µl of water-soluble tetrazolium salt-8 reagent was added to each well. After a 2-h incubation at 37˚C, the absorbance was measured at 450 nm using a microplate reader.
Statistical analyses
Data are expressed as the mean ± standard error of the mean (SEM). Statistical differences were determined using one-way analysis of variance (ANOVA), followed by Tukey-Kramer test (SAS Institute, Inc.). For comparisons between two groups, unpaired Student's t-test was used. P<0.05 was considered to indicate a statistically significant difference. Sample sizes were calculated based on preliminary data (mean ± SEM) using G*Power software (version 3.1.9.6; www.gpower.hhu.de,) as α=0.05 and 1-β=0.8.
Results
The addition of Asp, Asn, Glu and Pro (4AAs), which are deficient in DMEM, reduce the SESN2 and ATF4 mRNA levels in RI-T cells to levels comparable with those in RI-T cells cultured in RPMI medium
To compare the effects of DMEM and RPMI on SESN2 and ATF4 mRNA levels in RI-T cells, SESN2 mRNA levels were measured. SESN2 mRNA levels were significantly higher in RI-T cells cultured in DMEM compared with those in RPMI-1640 (DMEM, 1±0.07; RPMI-1640, 0.1±0.01) (Fig. 1A). Similarly, ATF4 mRNA levels were also elevated in DMEM-treated RI-T cells (DMEM, 1.00±0.06; RPMI-1640, 0.23±0) (Fig. 1B). When 4AAs were added to DMEM, SESN2 mRNA levels decreased significantly (DMEM, 1±0.04; DMEM + 4AAs, 0.12±0) (Fig. 1C), as did ATF4 mRNA levels (DMEM, 1±0.04; DMEM + 4AAs, 0.35±0.01) (Fig. 1D) These results indicated that 4AAs contribute to the reduction of SESN2 and ATF4 mRNA levels.
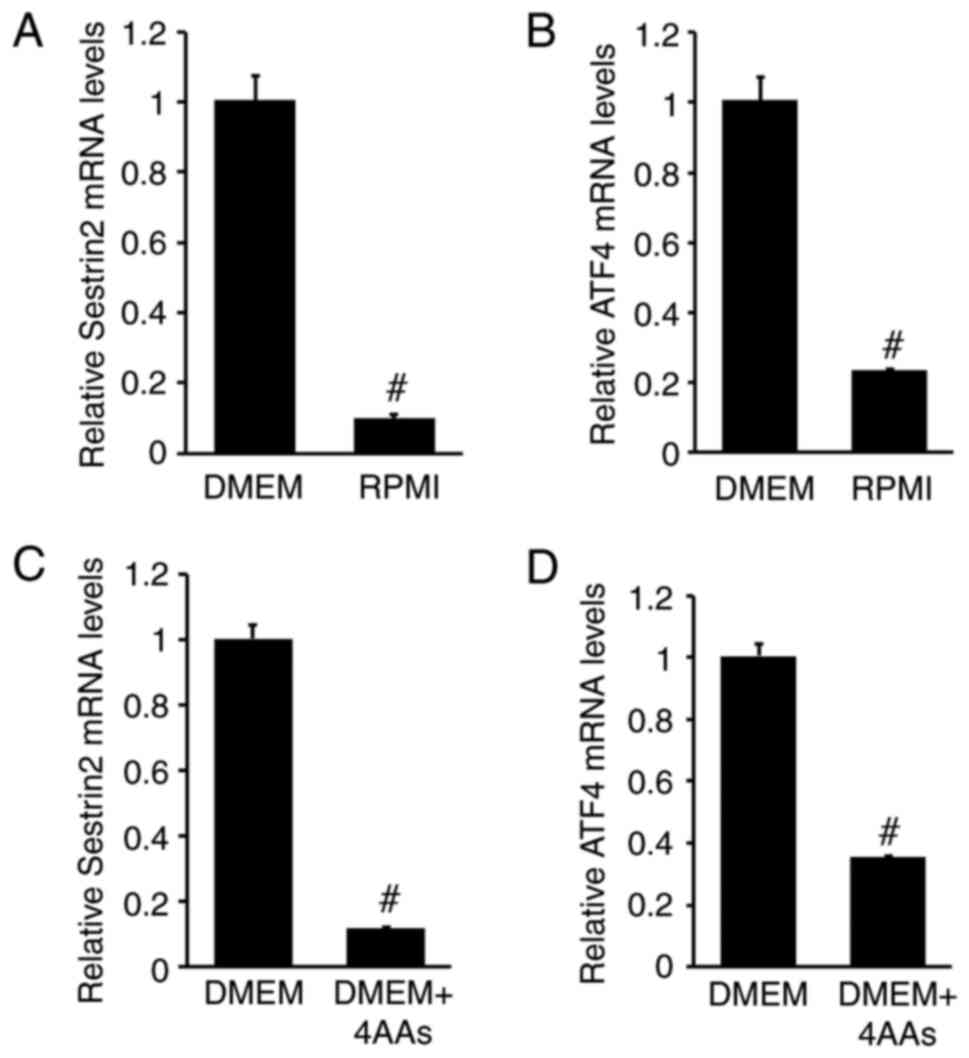 |
Figure 1
Addition of Asp, Asn, Glu and Pro (4AAs), which are deficient in DMEM, reduces SESN2 and ATF4 mRNA levels in RI-T cells to levels comparable with those in RPMI-1640 medium-cultured RI-T cells. RI-T cells were cultured in DMEM or RPMI medium for 5 h, after which total RNA was isolated. (A) SESN2 and (B) ATF4 mRNA levels were analyzed using RT-qPCR. To examine the effect of 4AAs (Asp, Asn, Glu and Pro) on SESN2 and ATF4 mRNA levels, RI-T cells cultured in DMEM were treated with 4AAs for 5 h and total RNA was isolated from the cells. (C) SESN2 and (D) ATF4 mRNA levels were analyzed using RT-qPCR (n=6 per treatment group). Statistical differences were determined using Student's t-test. #P<0.05 vs. DMEM. Asp, aspartic acid; Asn, asparagine; Glu, glutamic acid; Pro, proline; DMEM, Dulbecco's modified Eagle's medium; SESN2, sestrin 2; ATF4, activating transcription factor 4; SESN2, sestrin 2; RT-qPCR, reverse transcription-quantitative PCR.
|
Asn and Pro downregulate SESN2 mRNA levels in RI-T cells cultured in DMEM
To identify the specific amino acids responsible for the reducing SESN2 mRNA levels, individual amino acids were removed from the 4AA mixture. The addition of 4AAs significantly decreased the SESN2 mRNA levels. When Asn was removed, SESN2 mRNA levels were restored to their original levels, while Pro removal partially reversed the reduction. By contrast, the removal of Asp or Glu had no significant effect on SESN2 mRNA suppression [DMEM, 1±0.02; 4AAs, 0.07±0.01, Asp (-), 0.07±0; Asn (-), 1.01±0.09; Glu (-), 0.08±0; Pro (-), 0.42±0.01] (Fig. 2A). Furthermore, the addition of both Asn and Pro to DMEM completely suppressed SESN2 mRNA levels, similar to the effect of f 4AAs. However, when added individually, Asn and Pro reduced SESN2 mRNA levels by 58 and 22%, respectively (DMEM, 1.01±0; 4AAs, 0.1±0.02; Asn + Pro, 0.06±0; Asn, 0.42±0.01; Pro, 0.78±0.04) (Fig. 2B).
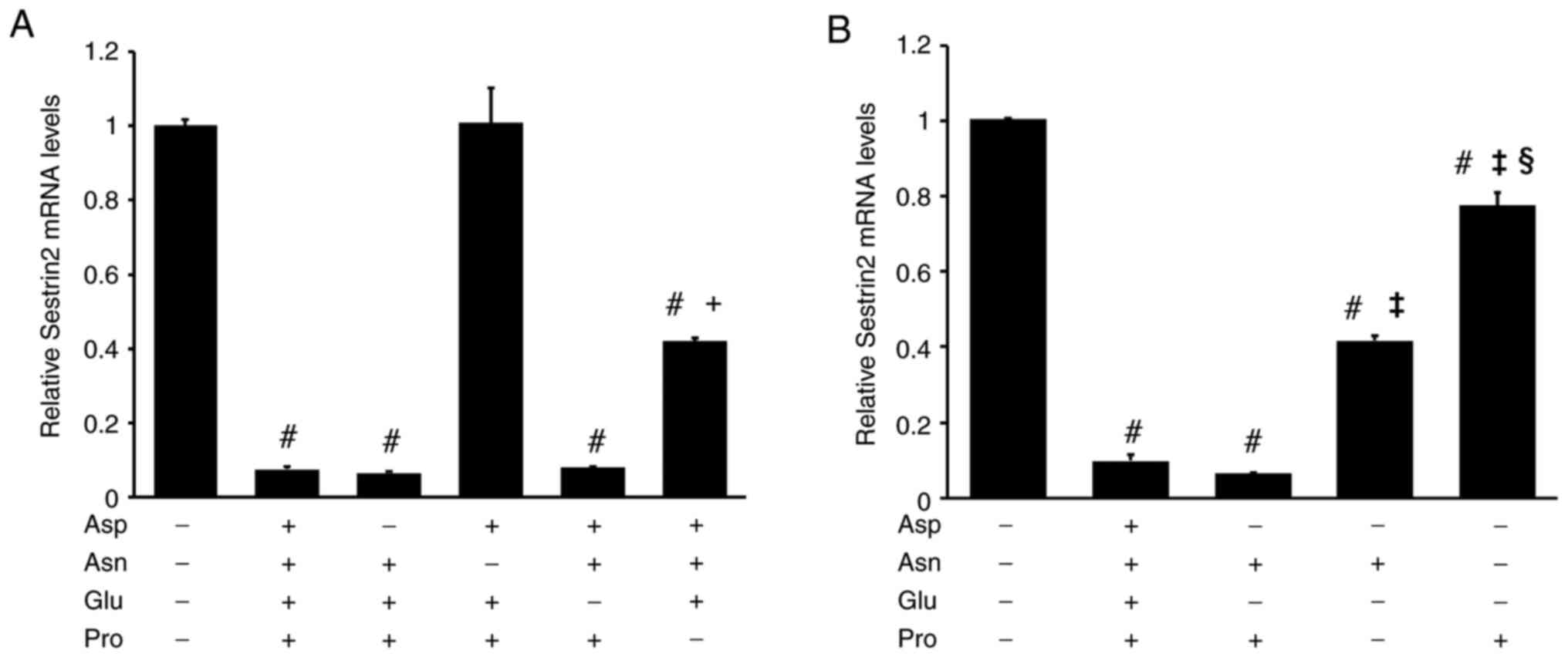 |
Figure 2
Both Asn and Pro are required to fully reduce SESN2 mRNA levels in RI-T cells. (A) RI-T cells cultured in Dulbecco's modified Eagle's medium were treated with either a combination of Asp, Asn, Glu and Pro (4AAs) or with 4AAs lacking one specific amino acid for 5 h, followed by total RNA isolation. SESN2 mRNA levels were analyzed by RT-qPCR. The removal of Asp or Glu from 4AAs did not alter the suppression of SESN2 expression, indicating that these amino acids are not essential for this effect (n=6 per treatment group). Statistical differences were determined using ANOVA followed by the Tukey-Kramer test. #P<0.05 vs. 4AAs (-); +P<0.05 vs. Asn (-). (B) RI-T cells were treated with Asn, Pro, or a combination of both for 5 h, and total RNA was isolated from the cells. SESN2 mRNA levels were determined by RT-qPCR. Neither Asn nor Pro alone was sufficient to suppress SESN2 mRNA levels. Both Asn and Pro were required (n=6 per treatment group). Statistical differences were determined using ANOVA followed by the Tukey-Kramer test. #P<0.05 vs. 4AAs (-); ‡P<0.05 vs. Asn + Pro; §P<0.05 vs. Asn. Asn, asparagine; Pro, proline; SESN2, sestrin 2; RT-qPCR, reverse transcription-quantitative PCR.
|
Asn and Pro downregulate ATF4 and ASNS mRNA levels in RI-T cells
To confirm the involvement of ASNS in SESN2 mRNA reduction following Asn and/or Pro addition, ASNS mRNA levels were examined. The addition of 4AAs or a combination of Asn and Pro to DMEM almost completely decreased ASNS mRNA levels. Asn alone reduced ASNS mRNA levels by 63%, whereas Pro had no significant effect (DMEM, 1±0.04; 4AAs, 0.03±0, Asn + Pro, 0.02±0; Asn, 0.37±0.01; Pro, 1.06±0.02) (Fig. 3A). The inhibitory effects of Asn and Pro on ATF4 mRNA levels were similar to their effects on ASNS mRNA levels. ATF4 and ASNS mRNA levels were significantly suppressed only when both Asn and Pro were present (DMEM, 1±0.02; 4AAs, 0.24±0, Asn + Pro, 0.21±0.01; Asn, 0.65±0.01; Pro, 0.92±0.01) (Fig. 3B).
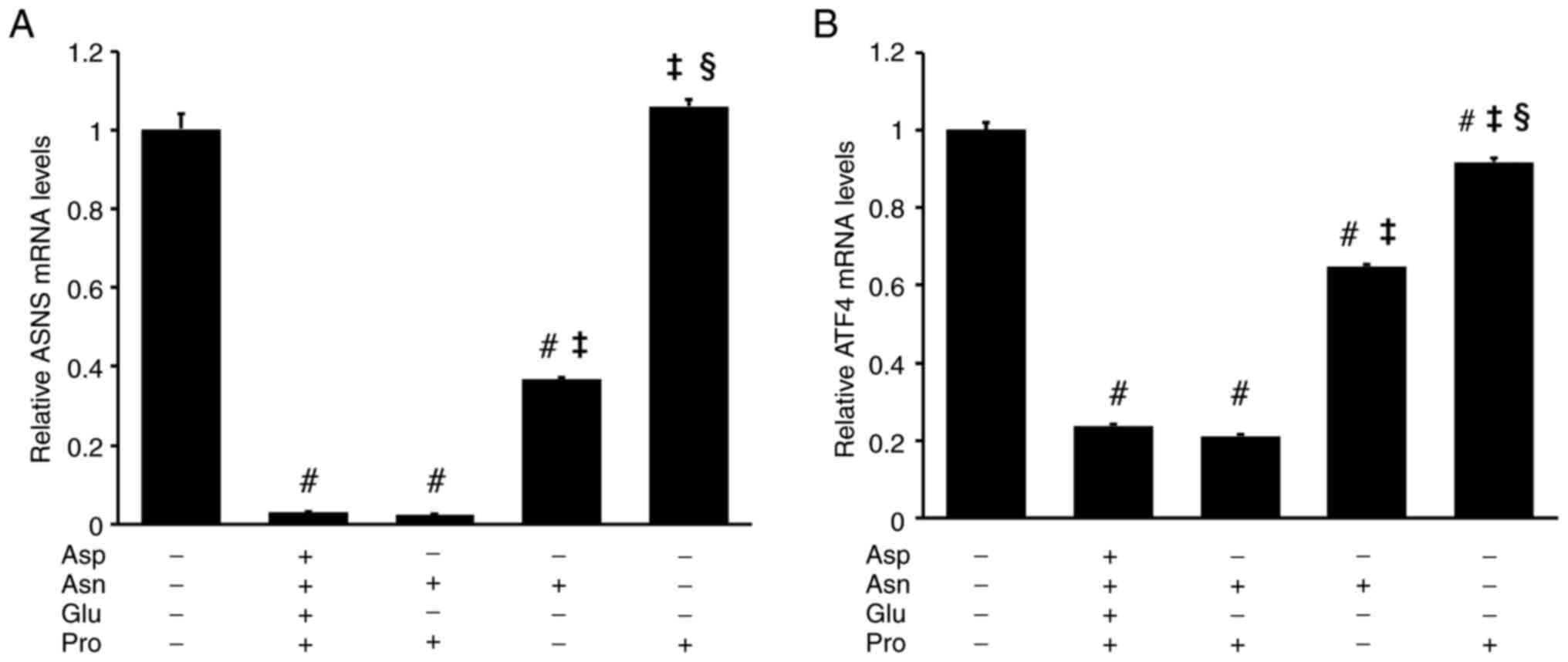 |
Figure 3
Both Asn and Pro are required to reduce ASNS and ATF4 mRNA levels in RI-T cells. RI-T cells cultured in Dulbecco's modified Eagle's medium were treated with Asp, Asn, Glu, Pro, or a combination of these amino acids for 5 h, and total RNA was isolated from the cells. (A) ASNS and (B) ATF4 mRNA levels were analyzed using reverse transcription-quantitative PCR (n=6 per treatment group). Statistical differences were determined using ANOVA followed by the Tukey-Kramer test. #P<0.05 vs. 4AAs (-); ‡P<0.05 vs. Asn + Pro; §P<0.05 vs. Asn. Asn, asparagine; Pro, proline; ASNS, asparagine synthetase; ATF4, activating transcription factor 4; Asp, aspartic acid; Glu, glutamic acid.
|
Effects of 4AAs on SESN2, ATF4 and ASNS mRNA levels in other cell lines
To determine whether the effect of 4AAs on SESN2 and ATF4 mRNA levels was specific to RI-T cells, similar experiments were conducted on C2C12, GH3 and HepG2 cells. In C2C12 cells, the addition of 4AAs to DMEM had no significant effect on SESN2 or ATF4 mRNA levels (SESN2: DMEM, 1±0.03; DMEM + 4AAs, 0.94±0.02; ATF4: DMEM, 1±0.02; DMEM + 4AAs, 0.97±0.03) (Fig. 4A). Similarly, in GH3 cells, SESN2 and ATF4 mRNA levels remained unchanged with 4AA supplementation (SESN2: DMEM, 1±0.04; DMEM + 4AAs, 1.06±0.03, ATF4: DMEM, 1±0.01; DMEM + 4AAs, 0.98±0.01) (Fig. 4B). The same trend was observed in HepG2 cells (SESN2: DMEM, 1±0.01; DMEM + 4AAs, 0.97±0.03; ATF4: DMEM, 1±0.04; DMEM + 4AAs, 0.95±0.03) (Fig. 4C). Additionally, ASNS mRNA levels in RI-T cells were significantly lower than those in GH3, HepG2 and C2C12 cells (RI-T, 1±0.04; GH3, 52.8±1.64; HepG2, 8.64±0.48; C2C12, 2.88±0.11) (Fig. 4D).
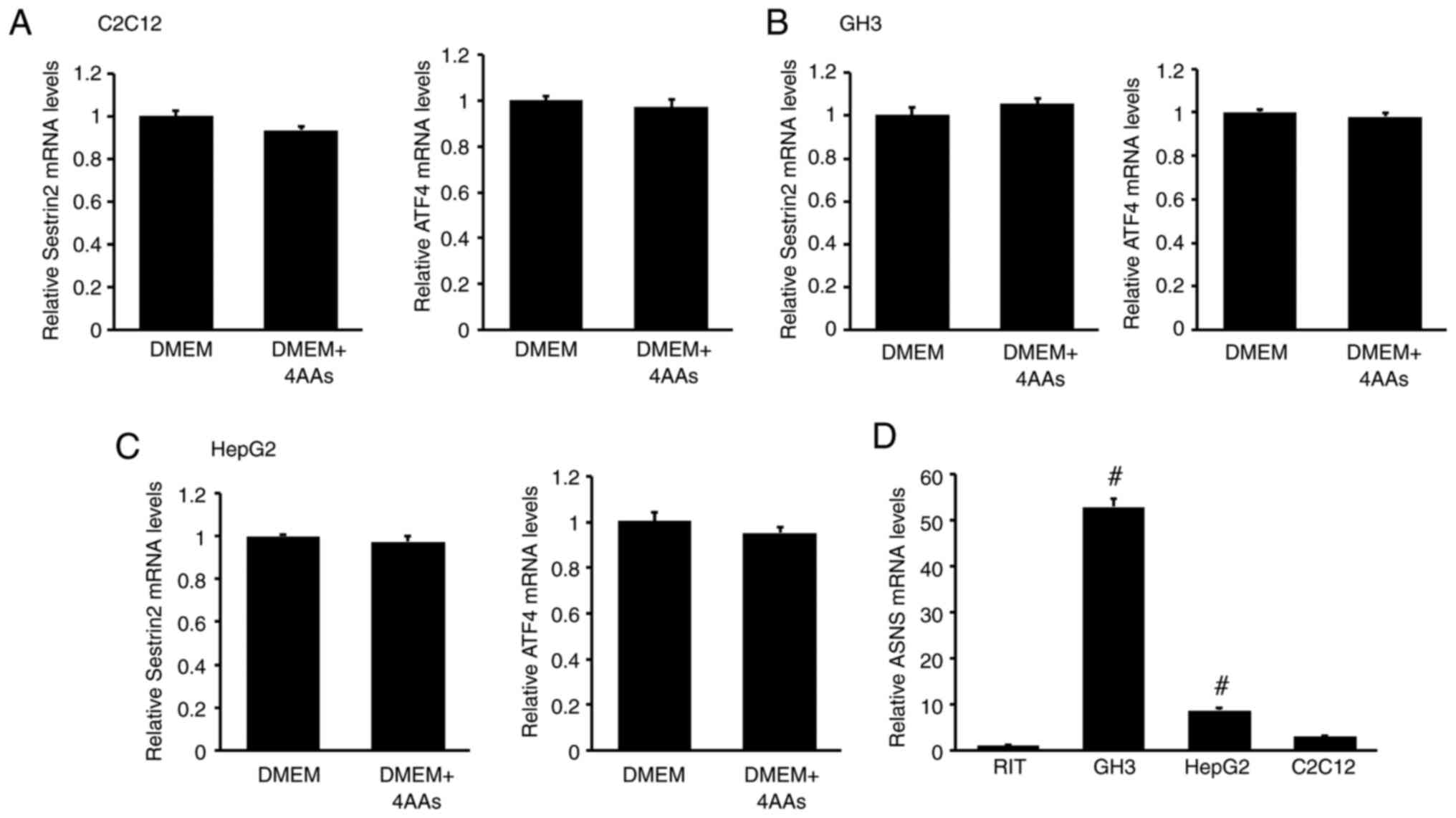 |
Figure 4
Addition of aspartic acid, asparagine, glutamic acid and proline (4AAs) does not influence SESN2 and ATF4 mRNA levels in C2C12, GH3 and HepG2 cells, which exhibit higher ASNS mRNA levels than RI-T cells. (A) C2C12, (B) GH3 and (C) HepG2 cells were treated with 4AAs for 5 h, and total RNA was isolated from the cells. SESN2 and ATF4 mRNA levels were analyzed by RT-qPCR (n=6 per treatment group). Statistical differences were determined using Student's t-test. The addition of 4AAs had no significant effect on SESN2 or ATF4 mRNA levels. (D) ASNS mRNA levels in RI-T, C2C12, HepG2 and GH3 cells were determined by RT-qPCR (n=6 per treatment group). Statistical differences were determined using ANOVA followed by Tukey-Kramer test. #P<0.05 vs. RI-T. ASNS, asparagine synthetase; SESN2, sestrin 2; ATF4, activating transcription factor 4; RT-qPCR, reverse transcription-quantitative PCR.
|
Effects of ATF4 siRNA on ATF4, SESN2 and COL1A1 mRNA levels in RI-T cells
To investigate the role of ATF4 in regulating SESN2 mRNA levels in RI-T cells, ATF4 siRNA was used. ATF4 siRNA significantly reduced SESN2 mRNA levels in RI-T cells cultured in DMEM without AP (control: DMEM, 1.01±0.08; DMEM + AP, 0.06±0; ATF4 siRNA: DMEM, 0.33±0.02; DMEM + AP, 0.04±0.01) (Fig. 5A). Similarly, ATF4 mRNA levels were also reduced by ATF4 siRNA (control: DMEM, 1.01±0.06; DMEM + AP, 0.18±0; ATF4 siRNA: DMEM, 0.31±0.01; DMEM + AP, 0.10±0) (Fig. 5B). ATF4 siRNA also lowered COL1A1 mRNA levels (control: DMEM, 1±0.02; DMEM + AP, 0.89±0.04; ATF4 siRNA: DMEM, 0.69±0.03; DMEM + AP, 0.63±0.02). However, the effect of ATF4 siRNA on COL1A1 mRNA levels was less pronounced than its effect on SESN2 mRNA levels, and AP supplementation reduced COL1A1 mRNA levels by only 11% (Fig. 5C).
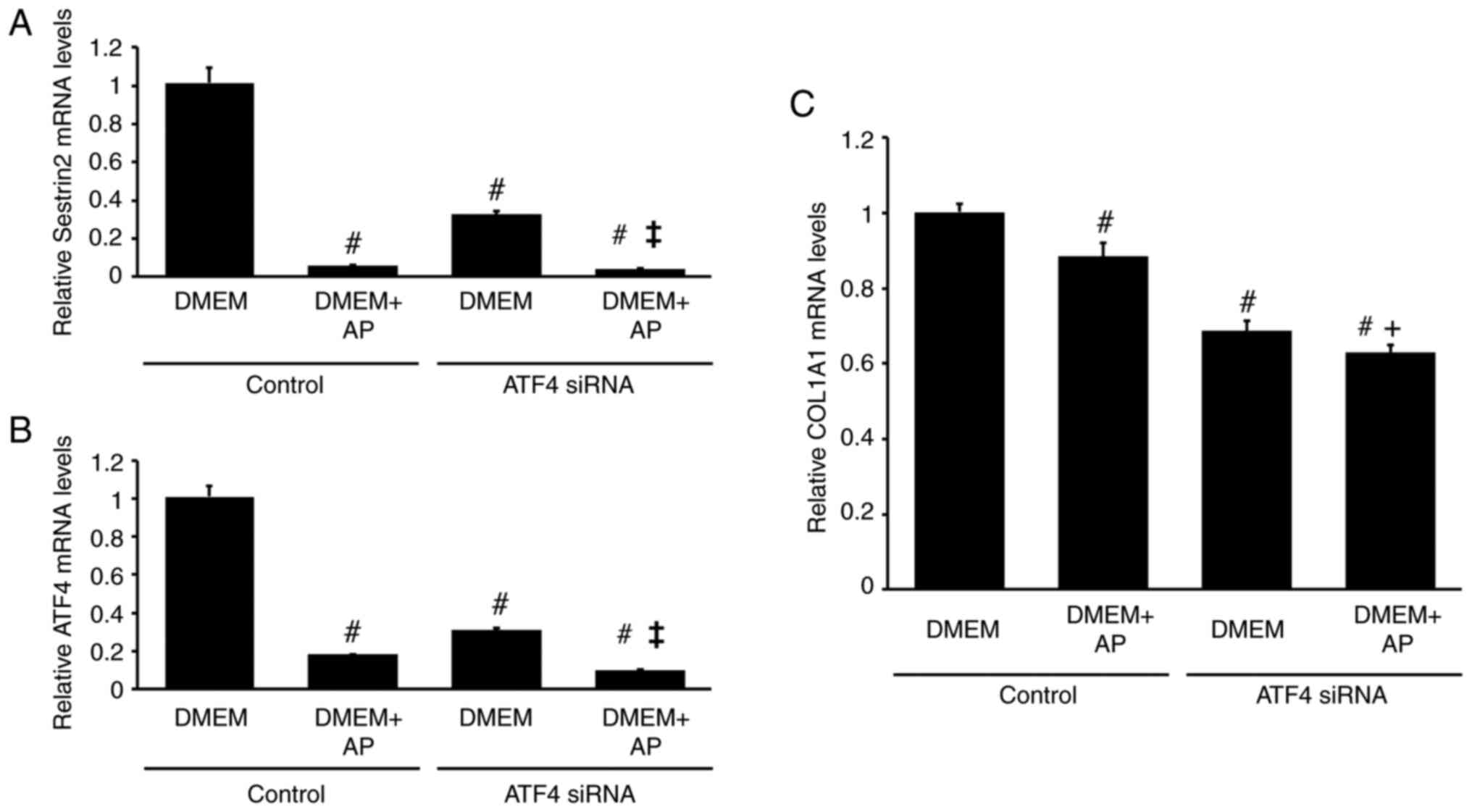 |
Figure 5
Knockdown of ATF4 by siRNA reduces SESN2 and COL1A1 mRNA levels in RI-T cells. RI-T cells were treated with ATF4-targeting siRNA or negative-control siRNA for 24 h. The medium was then changed to DMEM with or without asparagine + proline (AP), and the cells were incubated for an additional 5 h before RNA isolation. (A) ATF4, (B) SESN2 and (C) COL1A1 mRNA levels were analyzed by reverse transcription-quantitative PCR (n=6 per treatment group). Statistical differences were determined using ANOVA followed by Tukey-Kramer test. #P<0.05 vs. DMEM in control; ‡P<0.05 vs. DMEM in ATF4 siRNA; +P<0.05 vs. DMEM + AP in control. ATF4, activating transcription factor; siRNA, small interfering RNA; SESN2, sestrin 2; COL1A1, collagen type I α1 chain; DMEM, Dulbecco's modified Eagle's medium.
|
Effects of SESN2 siRNA on SESN2 and COL1A1 mRNA levels in RI-T cells
To examine the role of SESN2 in regulating of COL1A1 mRNA levels in RI-T cells, SESN2 siRNA was used. SESN2 siRNA significantly decreased SESN2 mRNA levels in RI-T cells cultured in DMEM without AP (control: DMEM, 1±0.04; DMEM + AP, 0.08±0; SESN2 siRNA: DMEM, 0.48±0.01; DMEM + AP, 0.03±0) (Fig. 6A). Furthermore, SESN2 siRNA lowered COL1A1 mRNA levels (control: DMEM, 1±0.03; DMEM + AP, 1±0.05; SESN2 siRNA: DMEM, 0.63±0.01; DMEM + AP, 0.61±0.01) (Fig. 6B).
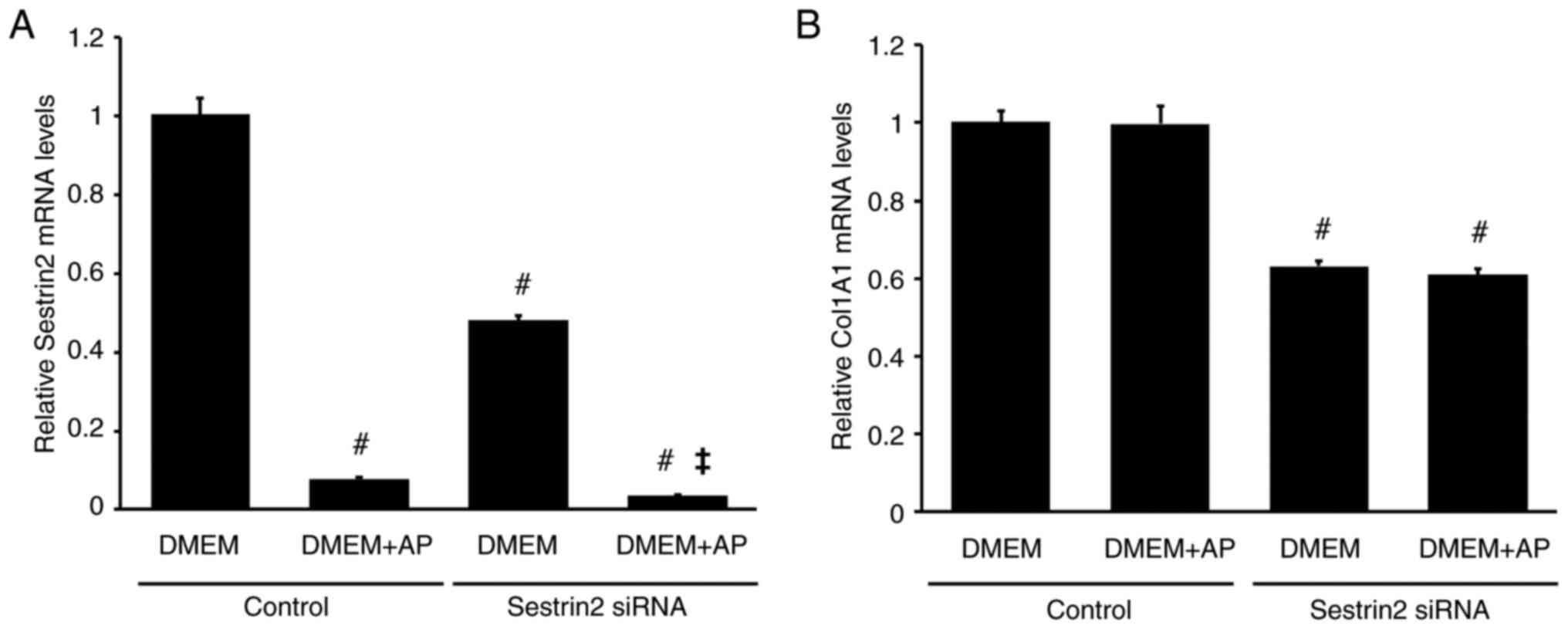 |
Figure 6
SESN2 knockdown by siRNA partially decreases COL1A1 mRNA levels in RI-T cells. RI-T cells were treated with SESN2-targeting siRNA or negative-control siRNA for 24 h. The medium was then changed to DMEM with or without asparagine + proline (AP), followed by an additional 5 h of incubation before RNA isolation. (A) SESN2 and (B) COL1A1 mRNA levels were analyzed by reverse transcription-quantitative PCR (n=6 per treatment group). Statistical differences were determined using ANOVA followed by Tukey-Kramer test. #P<0.05 vs. DMEM in control; ‡P<0.05 vs. DMEM in SESN2 siRNA. SESN2, sestrin 2; siRNA, small interfering RNA; COL1A1, collagen type I α1 chain; DMEM, Dulbecco's modified Eagle's medium.
|
Effects of actinomycin on SESN2 and ATF4 mRNA levels in RI-T cells
To confirm whether ATF4 and SESN2 transcription is involved in amino acid-mediated changes in mRNA levels, actinomycin was used. Actinomycin almost completely suppressed SESN2 mRNA expression in RI-T cells (control: DMEM, 1±0.04, DMEM + AP, 0.07±0.03; actinomycin: DMEM, 0.01±0; DMEM + AP, 0.01±0), indicating that SESN2 mRNA in RI-T cells was newly synthesized (Fig. 7A). Actinomycin also reduced ATF4 mRNA levels in RI-T cells cultured in DMEM. However, in RI-T cells cultured in DMEM + AP, actinomycin had no significant effect on ATF4 mRNA levels (control: DMEM, 1.01±0.08; DMEM + AP, 0.24±0.02; actinomycin: DMEM, 0.23±0.01; DMEM + AP, 0.2±0.01) (Fig. 7B).
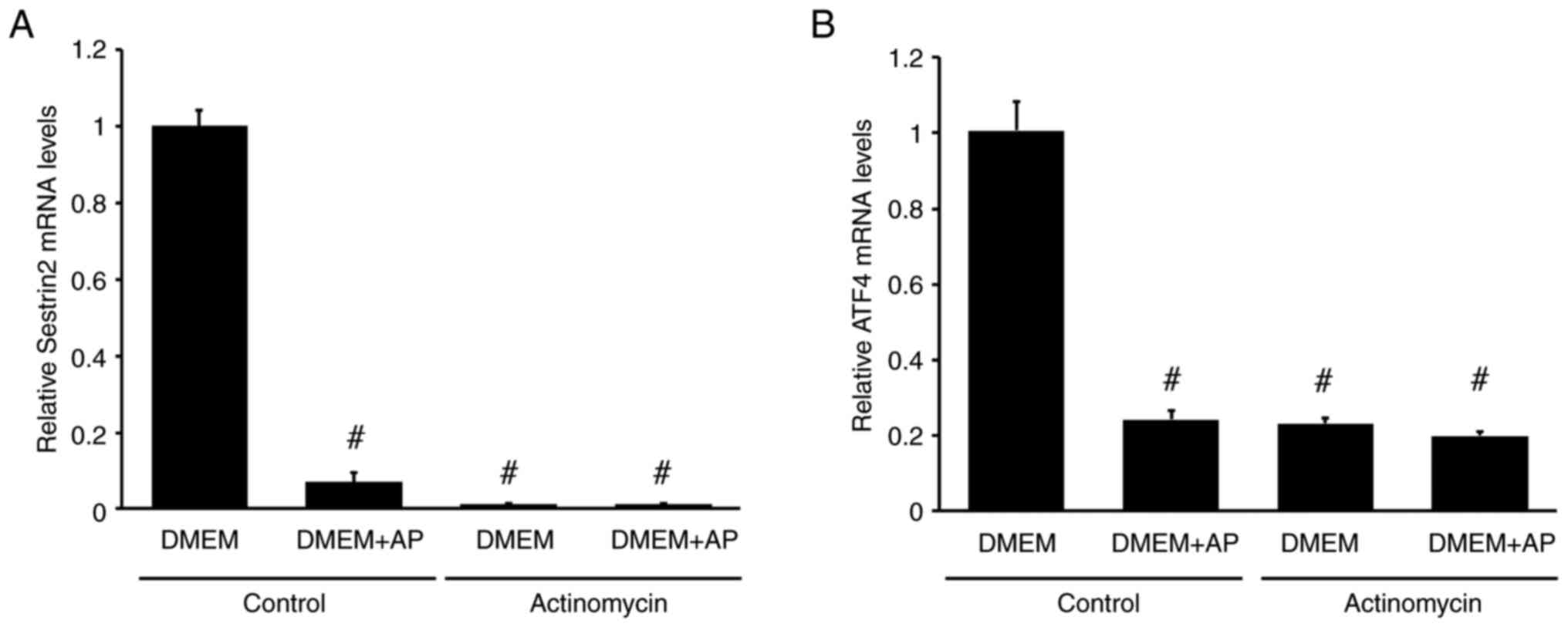 |
Figure 7
Actinomycin decreases SESN2 and ATF4 mRNA levels in RI-T cells. RI-T cells were treated with asparagine + proline (AP) in the presence or absence of actinomycin for 5 h, and total RNA was isolated from the cells. (A) SESN2 and (B) ATF4 mRNA levels were analyzed by reverse transcription-quantitative PCR (n=6 per treatment group). Statistical differences were determined using ANOVA, followed by Tukey-Kramer test. #P<0.05 vs. DMEM in control. SESN2, sestrin 2; ATF4, activating transcription factor 4; DMEM, Dulbecco's modified Eagle's medium.
|
Effects of AP on COL1A1, SESN2 and ATF4 mRNA levels in RI-T cells cultured in DMEM for 5 h or 48 h with FBS
The addition of AP significantly decreased SESN2 mRNA levels in RI-T cells (5 h: DMEM, 1±0.05; DMEM + AP, 0.05±0; 48 h: DMEM, 0.4±0.04; DMEM + AP, 0.09±0.01) (Fig. 8A). The response of ATF4 mRNA to AP was similar to that of SESN2 mRNA (5 h: DMEM, 1±0.04; DMEM + AP, 0.17±0.02; 48 h: DMEM, 0.65±0.09; DMEM + AP, 0.15±0.01) (Fig. 8B). The experiment was conducted in the presence of FBS, and the inhibitory effects of AP on SESN2 and ATF4 mRNA levels were observed irrespective of FBS. Although AP supplementation did not affect COL1A1 mRNA levels in RI-T cells cultured in DMEM for 5 h, it significantly increased COL1A1 mRNA levels after 48 h of culture (5 h: DMEM, 1±0.04; DMEM + AP, 1.02±0.06; 48 h: DMEM, 0.71±0.05; DMEM + AP, 1.36±0.1) (Fig. 8C).
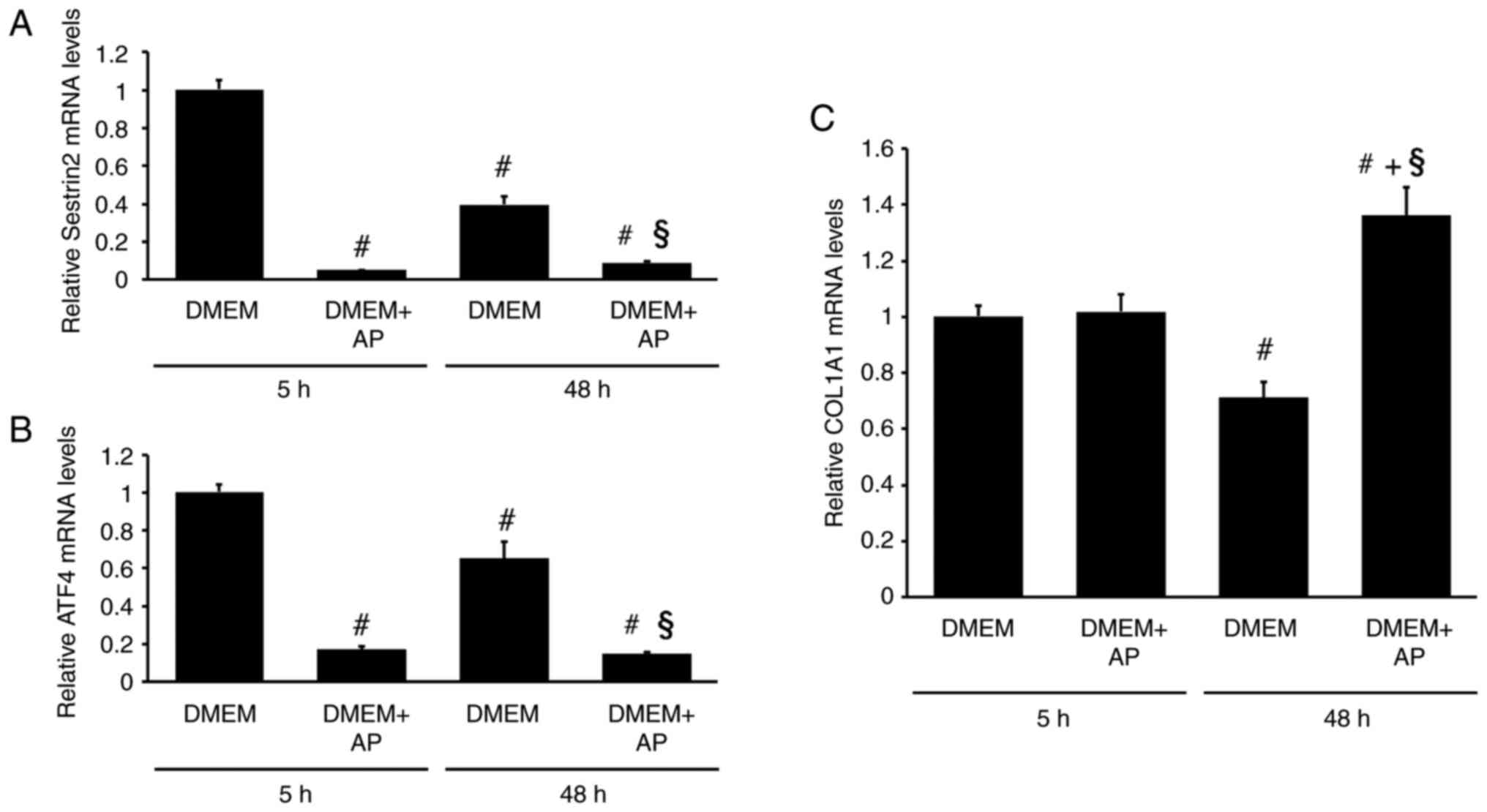 |
Figure 8
Addition of asparagine and proline (AP) increases COL1A1 mRNA levels while reducing SESN2 and ATF4 mRNA levels in RI-T cells cultured in DMEM containing FBS for 48 h. RI-T cells were treated with AP in the presence of FBS for 5 or 48 h, and total RNA was isolated from the cells. (A) SESN2, (B) ATF4 and (C) COL1A1 mRNA levels were analyzed using reverse transcription-quantitative PCR (n=6 per treatment group). Statistical differences were determined using ANOVA followed by Tukey-Kramer test. #P<0.05 vs. DMEM, 5 h; §P<0.05 vs. DMEM, 48 h; +P<0.05 vs. DMEM + AP, 5 h. COL1A1, collagen type I α1 chain; SESN2, sestrin 2; ATF4, activating transcription factor 4; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
|
Effects of AP on COL1A1 protein levels in RI-T cells and their proliferation
The addition of AP increased the COL1A1 and COL1A2 protein levels in RI-T cells cultured in DMEM with FBS for 24 h (COL1A1: DMEM, 0.05±0.01; DMEM + AP, 0.09±0.01. COL1A2: DMEM, 0.8±0.07; DMEM + AP, 1.06±0.04) (Fig. 9A). Moreover, in the presence of FBS, AP supplementation promoted RI-T cell proliferation after 24 h of incubation (control, 0.07±0; AP, 0.24±0.01) (Fig. 9B).
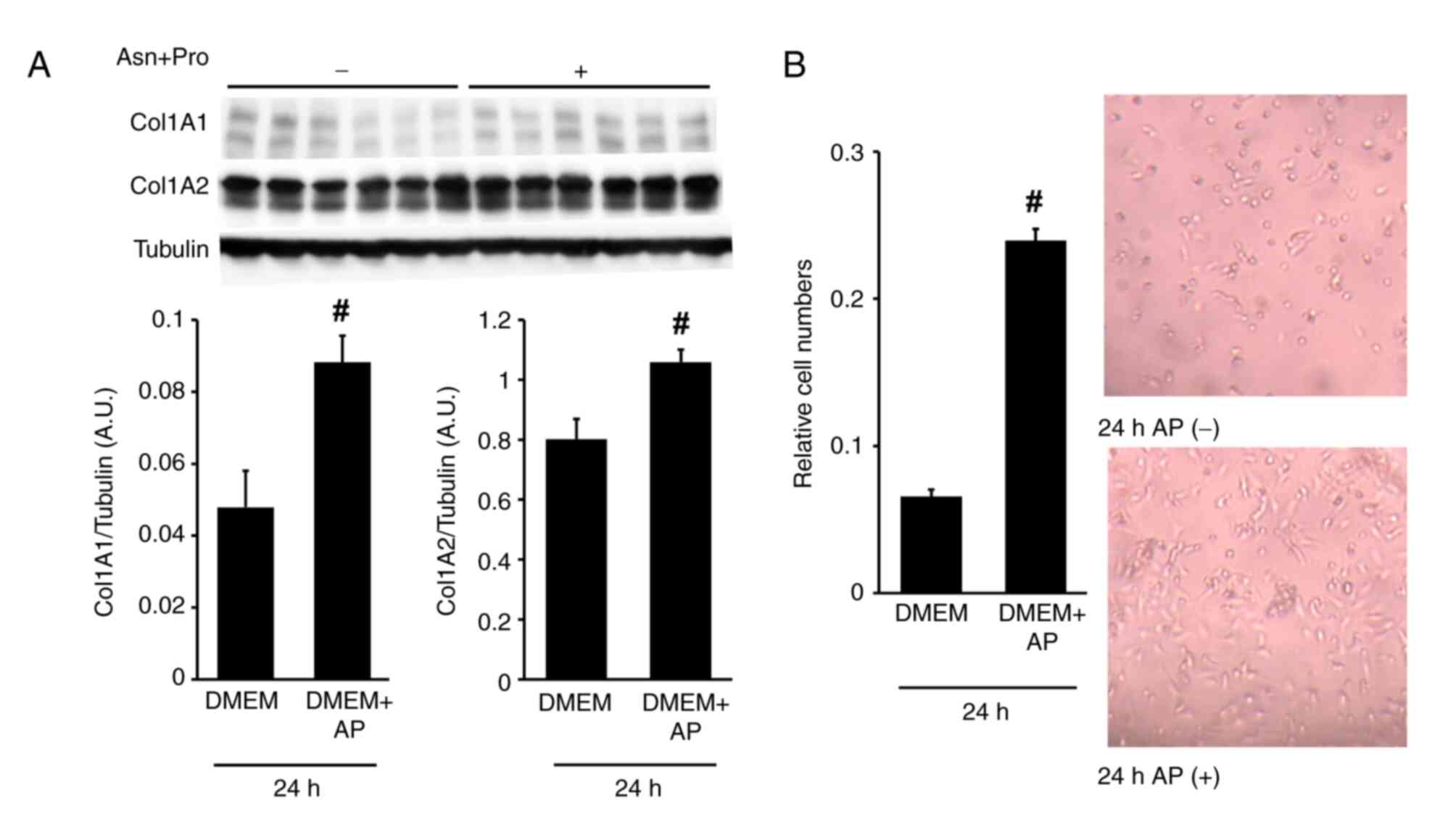 |
Figure 9
Addition of Asn and Pro (AP) promotes RI-T cell proliferation and increases collagen type I protein levels cultured in DMEM with FBS. RI-T cells were treated with AP in the presence of FBS for 24 h. (A) After incubation, COL1A1 and COL1A2 protein levels were analyzed using western blotting; and (B) cell proliferation was assessed using the Cell Counting Kit-8 assay. Representative images of cells treated with or without AP for 24 h are shown. n=6 per treatment group. Statistical differences were determined using Student's t-test. #P<0.05 vs. DMEM. Asn, asparagine; Pro, proline; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; COL1A1, collagen type I α1 chain; COL1A2, collagen type Iα 2.
|
Discussion
The present study revealed that supplementation of AP to DMEM resulted in a reduction in the mRNA levels of ATF4 and SESN2 in RI-T cells after 5 h of culture. These findings suggest that although AP is a non-essential amino acid, its levels in RI-T cells may be insufficient. A deficiency in extracellular AP induces a state of relative amino acid deprivation, which can be mitigated by AP supplementation. Additionally, AP supplementation slightly decreased COL1A1 mRNA levels. In long-term cultures, AP supplementation similarly reduced ATF4 and SESN2 mRNA levels, consistent with the short-term results. However, unlike in short-term cultures, both mRNA and protein levels of COL1A1 increased over time.
SESN2 typically increases in response to various stress factors and plays a protective role against cell damage (26). Amino acid deprivation increases ATF4 expression, which in turn, leads to increased SESN2 expression as part of a cellular stress response (27). RI-T cells were cultured in RPMI-1640 medium (22) or DMEM (23). Compared with RPMI-1640 medium, DMEM lacks 4AAs, and RI-T cells cultured in DMEM exhibited significantly higher ATF4 and SESN2 mRNA levels than those in RPMI medium (Fig. 1). Therefore, the study focused on 4AAs and it was found that AP supplementation reduced SESN2 and ATF4 mRNA levels in RI-T cells within 5 h of culture. Although AP is a non-essential amino acid, its addition to DMEM may alleviate amino acid deprivation stress in RI-T cells. Knockdown of ATF4 using siRNA reduced SESN2 and COL1A1 mRNA levels (Fig. 5), while knockdown of SESN2 also led to a decrease in COL1A1 mRNA levels (Fig. 6). These findings suggested that the absence of AP is a type of amino acid deprivation in RI-T cells. Furthermore, AP supplementation reduced ATF4 mRNA levels to a similar extent as Actinomycin, an RNA synthesis inhibitor, suggesting that AP affects transcription. Additionally, these results indicate that ATF4 and SESN2 contribute to COL1A1 expression. However, while AP markedly reduced SESN2 mRNA levels, its effect on COL1A1 mRNA levels was modest. Specifically, ATF4 and SESN2 knockdown decreased COL1A1 mRNA levels by 31 and 37%, respectively, whereas AP supplementation reduced COL1A1 mRNA levels by at most 11% (Figs. 5 and 6). These results suggested that AP may regulate COL1A1 expression through both SESN2-dependent and SESN2-independent pathways.
In RI-T cells, both Asn and Pro were required to reduce SESN2 mRNA levels. When Asn or Pro was individually removed from 4AA, the ability of 4AA to suppress SESN2 mRNA levels was either completely or partially lost, respectively. This indicates that Asn is essential for the suppression of SESN2 mRNA levels (Fig. 2A). However, Asn alone reduced SESN2 mRNA levels by only 58%, and Pro alone by only 22%, whereas supplementation with both Asn and Pro completely suppressed SESN2 mRNA levels (Fig. 2B). These results suggest that Asn and Pro act synergistically to downregulate SESN2 expression. Asn is a non-essential amino acid synthesized intracellularly by ASNS but can also be transported from the extracellular environment. Some cell types with low extracellular Asn levels depend heavily on ASNS (28). To investigate this further, ASNS mRNA levels were examined in RI-T cells and were compared to those in C2C12, GH3 and HepG2 cells. ASNS mRNA levels in RI-T cells were lower than those in C2C12, GH3 and HepG2 cells (Fig. 4). Consistently, supplementation with 4AAs reduced ATF4 mRNA levels in RI-T cells but had no effect on C2C12, GH3, or HepG2 cells. These findings suggest that RI-T cells may rely on extracellular Asn for maintaining cellular function. The precise role of Pro is unclear; however, our data showed that Pro decreased ATF4 mRNA levels (Fig. 3B), consistent with previous findings that Pro downregulates ATF4 expression in stem cells (29). Since ATF4 stimulates SESN2 expression, Pro may indirectly reduce SESN2 mRNA levels in RI-T cells by decreasing ATF4 expression.
Amino acids influence HSC function, including collagen production and cell proliferation (27,30,31). It was investigated whether AP affects COL1A1 mRNA and protein levels as well as the proliferation of RI-T cells. Since long-term cultures without serum may not sustain adequate cell function, 10% FBS was added to the medium in these experiments (Figs. 8 and 9). Consistent with the short-term results, AP supplementation in the presence of FBS decreased SESN2 and ATF4 mRNA levels after both 5 and 48-h treatments. These findings suggest that DMEM, which lacks AP, induces amino acid deprivation stress in RI-T cells, even in the presence of FBS, leading to the induction of ATF4 and SESN2. By contrast, AP supplementation increased COL1A1 mRNA levels after 48 h of treatment and COL1A1 protein levels after 24 h with the cell proliferation. Since activation of the ATF4/SESN2 pathway inhibits anabolic reactions and cell proliferation (9), our results suggest that AP alleviated this stress response, thereby promoting RI-T cell proliferation. This favorable environment may have enhanced COL1A1 synthesis, a characteristic function of HSCs.
SESN2 is abnormally expressed in liver diseases including fibrosis, and its expression level is associated with disease progression. This increase in SESN2 expression is considered an adaptive mechanism that reduces liver fibrosis. SESN2 overexpression reduces COL1A1 expression in HSC-T6 cells and mitigates carbon tetrachloride-induced liver fibrosis in mice (11,26,32). However, our findings contrast with these observations, as SESN2 siRNA reduced COL1A1 mRNA levels in short-term cultures. To the best of the authors' knowledge, this is the first study to demonstrate that SESN2 enhances COL1A1 expression. This discrepancy may stem from differences between activated HSCs, which are influenced by excess cytokines in liver diseases, and quiescent (inactivated) HSCs.
In summary, Asn was essential for suppressing SESN2 mRNA levels in RI-T cells cultured in DMEM. While Pro alone had a weaker suppressive effect, it enhanced the effect of Asn when combined. In short-term cultures, supplementation with both Asn and Pro completely suppressed SESN2 and ATF4 mRNA levels while having a minimal effect on COL1A1 mRNA levels. However, knockdown of SESN2 or ATF4 resulted in a more substantial reduction in COL1A1 mRNA levels than the supplementation with both Asn and Pro. These results suggest that SESN2, which is induced by amino acid insufficiency, contributes to the upregulation of COL1A1 mRNA levels. This COL1A1-inducing effect of SESN2 was in contrast to the inhibitory effect of SESN2 on activated HSCs. Additionally, Asn and Pro increased COL1A1 mRNA levels through pathways independent of SESN2. A limitation of the present study is that the effects of Asn and Pro were observed only in HSC lines. It remains unknown whether HSCs in vivo have low ASNS activity. The authors plan to confirm the AP effect in normal rat HSCs, which are collected from normal rats. Further studies are needed to elucidate the amino acid-mediated regulatory mechanisms of COL1A1 production in vivo.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by the Yukiyoshi Gakuen Research Grants (grant nos. K2021-11 and K2022-2).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
RS conceptualized the study, conducted investigation, curated data, performed formal analysis, wrote the original draft and visualized data. MO and HS conducted investigation. YO conceptualized the study, visualized and validated data, wrote, reviewed and edited the manuscript conducted project administration, supervised the study, and acquired funding. RS and YO confirmed the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Zamani M, Alizadeh-Tabari S, Ajmera V, Singh S, Murad MH and Loomba R: Global prevalence of advanced liver fibrosis and cirrhosis in the general population: A systematic review and meta-analysis. Clin Gastroenterol Hepatol: S1542-3565(24)00790-0, 2024 (Epub ahead of print).
|
|
2
|
Friedman SL: Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 88:125–172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Higashi T, Friedman SL and Hoshida Y: Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 121:27–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Budanov AV and Karin M: p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 134:451–460. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al: Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 21:6017–6031. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Essler S, Dehne N and Brune B: Role of sestrin2 in peroxide signaling in macrophages. FEBS Lett. 583:3531–3535. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Seo K, Ki SH and Shin SM: Sestrin2-AMPK activation protects mitochondrial function against glucose deprivation-induced cytotoxicity. Cell Signal. 27:1533–1543. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rajanala SH, Ringquist R and Cryns VL: Methionine restriction activates the integrated stress response in triple-negative breast cancer cells by a GCN2- and PERK-independent mechanism. Am J Cancer Res. 9:1766–1775. 2019.PubMed/NCBI
|
|
9
|
Sun W, Wang Y, Zheng Y and Quan N: The emerging role of sestrin2 in cell metabolism, and cardiovascular and age-related diseases. Aging Dis. 11:154–163. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim HJ, Joe Y, Kim SK, Park SU, Park J, Chen Y, Kim J, Ryu J, Cho GJ, Surh YJ, et al: Carbon monoxide protects against hepatic steatosis in mice by inducing sestrin-2 via the PERK-eIF2α-ATF4 pathway. Free Radic Biol Med. 110:81–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang JH, Kim KM, Cho SS, Shin SM, Ka SO, Na CS, Park BH, Jegal KH, Kim JK, Ku SK, et al: Inhibitory effect of sestrin 2 on hepatic stellate cell activation and liver fibrosis. Antioxid Redox Signal. 31:243–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y and Kido Y: Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res. 32:522–529. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khedr NF and Khedr EG: Branched chain amino acids supplementation modulates TGF-β1/Smad signaling pathway and interleukins in CCl4-induced liver fibrosis. Fundam Clin Pharmacol. 31:534–545. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, Choi JY and Yoon SK: Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 8(e77899)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shrestha N, Chand L, Han MK, Lee SO, Kim CY and Jeong YJ: Glutamine inhibits CCl4 induced liver fibrosis in mice and TGF-β1 mediated epithelial-mesenchymal transition in mouse hepatocytes. Food Chem Toxicol. 93:129–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee HL, Lee J, Cha JH, Cho S, Sung PS, Hur W, Yoon SK and Bae SH: Anti-fibrotic effects of branched-chain amino acids on hepatic stellate cells. Korean J Intern Med. 37:53–62. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Blackstock CD, Higashi Y, Sukhanov S, Shai SY, Stefanovic B, Tabony AM, Yoshida T and Delafontaine P: Insulin-like growth factor-1 increases synthesis of collagen type I via induction of the mRNA-binding protein LARP6 expression and binding to the 5' stem-loop of COL1a1 and COL1a2 mRNA. J Biol Chem. 289:7264–7274. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Daou N, Viader A, Cokol M, Nitzel A, Chakravarthy MV, Afeyan R, Tramontin T, Marukian S and Hamill MJ: A novel, multitargeted endogenous metabolic modulator composition impacts metabolism, inflammation, and fibrosis in nonalcoholic steatohepatitis-relevant primary human cell models. Sci Rep. 11(11861)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zou CG, Gao SY, Zhao YS, Li SD, Cao XZ, Zhang Y and Zhang KQ: Homocysteine enhances cell proliferation in hepatic myofibroblastic stellate cells. J Mol Med (Berl). 87:75–84. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pérez de Obanos MP, López-Zabalza MJ, Arriazu E, Modol T, Prieto J, Herraiz MT and Iraburu MJ: Reactive oxygen species (ROS) mediate the effects of leucine on translation regulation and type I collagen production in hepatic stellate cells. Biochim Biophys Acta. 1773:1681–1688. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sawa R, Ohnishi A, Ohno M, Nagata M, Wake I and Okimura Y: Specific amino acids regulate sestrin2 mRNA and protein levels in an ATF4-dependent manner in C2C12 myocytes. Biochim Biophys Acta Gen Subj. 1866(130174)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sekiguchi H, Hemmi N, Maki T, Ozawa A, Kadowaki E, Kamiie J, Yamamoto M, Arishima K and Sakaue M: Culture on a fragmin/protamine-coated plate suppresses the collagen type IαI and TGF-β1 mRNA expression of rat hepatic stellate RI-T cells. J Vet Med Sci. 75:553–559. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Inami M, Fukushima A, Ueno T, Yamada T, Tsunemi A, Matsumoto Y, Fukuda N, Soma M and Moriyama M: Reduction of dimethylnitrosamine-induced liver fibrosis by the novel gene regulator pi polyamide targeting transforming growth factor β1 gene. Biol Pharm Bull. 38:1836–1842. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Théret N, Lehti K, Musso O and Clément B: MMP2 activation by collagen I and concanavalin A in cultured human hepatic stellate cells. Hepatology. 30:462–468. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Herningtyas EH, Okimura Y, Handayaningsih AE, Yamamoto D, Maki T, Iida K, Takahashi Y, Kaji H and Chihara K: Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochim Biophys Acta. 1780:1115–1120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu C, Jiang Y, Xu W and Bao X: Sestrin2: Multifaceted functions, molecular basis, and its implications in liver diseases. Cell Death Dis. 14(160)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deng Y, Hu M, Huang S and Fu N: Molecular mechanism and therapeutic significance of essential amino acids in metabolically associated fatty liver disease. J Nutr Biochem. 126(109581)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li H, Ning S, Ghandi M, Kryukov GV, Gopal S, Deik A, Souza A, Pierce K, Keskula P, Hernandez D, et al: The landscape of cancer cell line metabolism. Nat Med. 25:850–860. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
D'Aniello C, Fico A, Casalino L, Guardiola O, Di Napoli G, Cermola F, De Cesare D, Tatè R, Cobellis G, Patriarca EJ and Minchiotti G: A novel autoregulatory loop between the Gcn2-Atf4 pathway and l-Proline metabolism controls stem cell identity. Cell Death Differ. 22:1094–1105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Izaki S, Goto H and Yokota S: Increased chemosensitivity and elevated reactive oxygen species are mediated by glutathione reduction in glutamine deprived neuroblastoma cells. J Cancer Res Clin Oncol. 134:761–768. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chisari AN, Sancho P, Caja L, Bertran E and Fabregat I: Lack of amino acids in mouse hepatocytes in culture induces the selection of preneoplastic cells. Cell Signal. 24:325–332. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hu YB, Ye XT, Zhou QQ and Fu RQ: Sestrin 2 attenuates rat hepatic stellate cell (HSC) activation and liver fibrosis via an mTOR/AMPK-dependent mechanism. Cell Physiol Biochem. 51:2111–2122. 2018.PubMed/NCBI View Article : Google Scholar
|























