Introduction
The treatment of disseminated prostate cancer
remains a great challenge in current oncology practice (1). The proliferation of prostate cancer
is testosterone-driven, and androgen deprivation by surgical or
chemical castration causes a remission lasting 1.5–3 years
(2). However, a clonal selection
during androgen deprivation therapy promotes the development of
androgen-independent (hormone-refractory) cells, which become
phenotypically dominant. The median survival of patients with
androgen-independent prostate cancer is 12–24 months, depending on
the treatment (3,4). Possible molecular pathways of
androgen independence include a hypersensitive pathway, when more
androgen receptors are produced to compensate for the low level of
androgens, a promiscuous pathway, when androgen receptors are
activated by non-androgenic steroids, and an outlaw pathway, when
the androgen receptors are phosphorylated by either the
mitogen-activated receptor tyrosine kinases (RTKs) or their
downstream signaling kinases (5).
The detection of phenotypic changes associated with
the development of androgen independence may influence patient
management, suggesting the initiation of a second-line therapy.
Moreover, accurate molecular phenotyping may suggest the most
suitable molecular targets for a second-line therapy, making
androgen-independent prostate cancer treatment more personalized.
One challenge includes the heterogeneity of gene expression in
metastases. In principle, biopsy-based methods enable the detection
of a multitude of genes simultaneously. However, repeated sampling
of several metastases is more than questionable in the clinical
setting. The use of radionuclide molecular imaging would enable
repeated imaging of the aberrant expression of different gene
products in all metastases simultaneously. Moreover, the use of
contemporary combined imaging devices (PET/CT or SPECT/CT) would
provide anatomical landmarks for biochemical changes.
One candidate for imaging in disseminated prostate
cancer is human epidermal growth factor receptor type 2 (HER2), a
receptor tyrosine kinase that is involved in the outlaw pathway.
HER2 is one of four members of the human epidermal growth factor
receptor (EGFR or HER) family, which also includes EGFR (ErbB1),
HER3 (ErbB3) and HER4 (ErbB4). While HER2 is weakly expressed in
normal adult tissue, several types of cancer overexpress HER2 in
both primary tumors and their metastases. In prostate cancer, the
progression towards androgen independence is characterized by a
gradual increase in HER2 expression by tumor cells. The initial
function of HER2 may be to permit prostate cancer cell survival in
an androgen-depleted environment (6). Changes in the hormonal environment
precipitate a cascade of events in gene expression and in the
signaling network of the cell. This in turn provides a selective
survival and growth advantage for HER-2-expressing subpopulations
of cells, and accelerates the progression of the tumor towards
androgen independence. This process also renders the cells more
resistant to therapy (7,8). In the absence of androgen, the
overexpression of HER2 activates the transcription of PSA. Unlike
other kinases, HER2 is capable of activating the androgen receptor
(AR) pathway, even in the absence of its ligand (9–11).
This suggests that increased expression of HER2 may be a
prostate-specific, rather than tumor-specific, mechanism of
survival in an androgen-depleted environment (12).
HER2 is a molecular target for several anti-cancer
drugs. Extensive studies have been dedicated to the development of
radiolabeled imaging probes for the visualization of HER2, with the
aim of identifying patients who may benefit from HER2-targeting
therapy (13). One obvious
potential targeting probe for the imaging of HER2-overexpression is
trastuzumab, a humanized anti-HER2 monoclonal antibody. Trastuzumab
is currently approved for the treatment of HER2-expressing breast
cancer. The antibody is commercially available and clinical trials
have demonstrated its safety. 111In-DTPA-trastuzumab has
been used to identify breast cancer patients responding to
trastuzumab treatment (alone or in combination with chemotherapy)
(14).
A promising alternative to radiolabeled anti-HER2
monoclonal antibodies may be Affibody molecules (15). Affibody molecules are proteins
composed of a three-helix bundle based on the scaffold of one of
the IgG-binding domains of Protein A. By randomizing thirteen of
the amino acid residues in the helices 1 and 2, combinatorial
Affibody libraries have been created for the selection of binders
for a multitude of proteins (16).
In contrast to the 150 kDa weight of an antibody, the molecular
weight of Affibody scaffold is only 7 kDa, which provides rapid
extravasation and penetration in the extracellular space of tumors.
An Affibody molecule, ZHER2:342, with an affinity
(dissociation constant, KD) to HER2 of 22 pM, has been developed
(17). Several radiolabeled
derivatives of ZHER2:342 have demonstrated excellent
targeting of HER2-expressing xenografts in murine models (15). A pilot clinical study confirmed
that [111In]- and [68Ga]-labeled
ZHER2:342 can be used to successfully visualize
HER2-expressing metastases (18).
Recently, the Affibody scaffold was re-engineered in order to
improve its properties and to provide a surface distinctly
different from that of the bacterial parental scaffold (19). A DOTA-conjugated anti-HER2 Affibody
molecule, ABY-025, which is based on a new scaffold, has
demonstrated highly specific targeting of HER2-expressing ovarian
carcinoma xenografts in mice (20). This makes ABY-025 a promising
candidate for the imaging of HER2 expression in prostate cancer
metastases.
This study aimed to establish the level of HER2
expression in a number of prostate cancer cell lines in order that
they be used as models in further studies, and to evaluate the
binding and cellular possessing of [111In]-labeled
trastuzumab and ABY-025 in these cell lines.
Materials and methods
Materials
Affibody molecule ABY-025 was provided by Affibody
AB (Stockholm, Sweden) in a freeze-dried form. The monoclonal
antibody trastuzumab (Herceptin®) was from Roche Pharma
AG (Germany). Before use, trastuzumab was purified using the NAP-5
size exclusion column (Amersham Biosciences, Uppsala, Sweden),
pre-equilibrated and eluted with MilliQ-water and freeze dried.
Isothiocyanate-CHX-A″DTPA was purchased from Macrocyclics (Dallas,
TX, USA).
Buffers including 0.1 M phosphate-buffered saline
(PBS), pH 7.5, 0.07 M sodium borate, pH 9.3, and 0.2 M ammonium
acetate, pH 5.5, were prepared using common methods from chemicals
supplied by Merck (Darmstadt, Germany). High-quality
Milli-Q© water (resistance >18 MΩ cm) was used to
prepare the solutions. NAP-5 size-exclusion columns were from GE
Healthcare (Uppsala, Sweden). Buffers used for conjugation and
labeling were purified from metal contamination using Chelex 100
resin (Bio-Rad Laboratories, Richmond, CA, USA).
[111In]-indium chloride was purchased from Covidien
(Hazelwood, MO, USA).
Cell lines
Three different prostate cancer cell lines were
evaluated for HER2 expression, and their HER2-receptor levels were
quantified. The cell lines, all originating from prostate cancer
metastases, were DU-145 (brain metastasis), PC3 (bone metastasis)
and LNCaP (lymph node metastasis). All cell lines were purchased
from the American Type Culture Collection (ATCC).
The cells were cultivated in complete RPMI-medium
supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamate,
100 IU/ml penicillin and 100 μg/ml streptomycin. For the LNCaP
cells, the medium was supplemented with Na-pyruvate and HEPES. All
reagents, including the medium and trypsine-EDTA, were from
Biochrom KG (Berlin, Germany). Bottles and Petri dishes for cell
cultivation were from Nunclon Surface (Roskilde, Denmark). The
cells were incubated in a humidified incubator with 5%
CO2 at 37˚C, unless stated otherwise.
Instrumentation
Radioactivity was measured using an automated
γ-counter with a 3-inch NaI(Tl) detector (1480 WIZARD; Wallac Oy,
Turku, Finland). Indium-111 was measured using both photo peaks and
the summation peak (energy setting from 140 to 507 keV). The
distribution of radioactivity along the ITLC strips was measured on
a Cyclone™ Storage Phosphor system (further referred to as
Phosphorimager) and analyzed using OptiQuant™ image analysis
software. Cells were counted using an electronic cell counter
(Beckman Coulter).
Labeling chemistry
For labeling, ABY-025 was reconstituted in 0.2 M
ammonium acetate buffer, pH 5.5, to a concentration of 1 mg/ml. For
typical labeling, 30 μl of ABY-025 solution was mixed with 50 μl
0.2 M ammonium acetate buffer, pH 5.5, and 30–70 MBq of
[111In]-indium chloride (80-160 μl solution in 0.05 M
HCl). The reaction mixture was incubated at 60˚C for 30 min and the
radiochemical purity was evaluated using Tec-Control Chromatography
150–771 strips eluted with 0.2 M citric acid.
Trastuzumab was labeled with
[111In]-indium chloride using the CHX-A″DTPA chelator.
Coupling (∼4 chelators per antibody) was performed similarly to a
previously described method (21).
In brief, 1.4 mg purified antibody was reconstituted in 200 μl 0.07
M sodium borate buffer, pH 9.3, and a freshly prepared solution of
isothiocyanate-CHX-A″DTPA (27 μl, 1 mg/ml in 0.07 M sodium borate,
pH 9.3) was added. The mixture was incubated at 37˚C for 4 h, and
then CHX-A″DTPA-trastuzumab was purified from unreacted chelator
using the NAP-5 column, equilibrated and eluted with 0.2 M ammonium
acetate, pH 5.5. The CHX-A″DTPA-trastuzumab was divided in aliquots
of 100 μg in 64 μl 0.2 M ammonium acetate, pH 5.5, each, and stored
frozen at −20˚C.
For typical labeling, an aliquot of
CHX-A″DTPA-trastuzumab was mixed with 10 MBq
[111In]-indium chloride (12 μl solution in 0.05 M HCl).
The mixture was incubated for 60 min at ambient temperature and
analyzed using Tec-Control Chromatography 150–771 strips.
In vitro binding specificity test
Pre-cultivated cells from the DU-145, PC3 or LNCaP
cell lines were incubated for 2 h with a 150 pM solution of
[111In]ABY-025 in medium. Simultaneously, another set of
dishes was treated in the same way, but with the inclusion of a
blocking amount of ABY-025 before the addition of the solution
containing [111In]ABY-025. The experiment was also
performed with [111In]CHX-A″DTPA-trastuzumab at a 1-nM
concentration. Both experiments were performed in triplicate.
Subsequently, the incubation media were collected
and the cell cultures were trypsinized with 0.5 ml trypsin-EDTA for
10 min at 37˚C. To each dish, 0.5 ml of medium was added, and the
cells were resuspended. The cell suspension was also collected.
Medium and cell samples were measured for
radioactivity content, and cell-associated radioactivity was
calculated as follows: (Cell-associated radioactivity, CPM) ×
100%/[(Cell-associated radioactivity, CPM) + (Radioactivity in
media, CPM)]. The significance of the blocking was analyzed by the
t-test.
Quantification of HER2 receptor
expression in prostate cancer cell lines
DU-145, PC3 or LNCaP cells were incubated for 4 h at
4˚C with [111In]CHX-A″DTPA-trastuzumab at concentrations
of 0.2–33 nM in complete medium. For each data point, four dishes
were used, including one pre-saturated with unlabeled trastuzumab
at a 3-μM concentration. For each data point, a sample of the added
solution was obtained for concentration calculations. After
incubation, the medium was aspirated and the cells were washed once
with cold serum-free medium. The cells were treated with trypsin
for 10–15 min, and the cells in each dish were resuspended after
the addition of 1 ml medium. The cell suspension (0.5 ml) was used
for cell counting and for radioactivity measurements (1 ml).
Samples were measured for radioactivity content in
an automated γ-counter, and data were analyzed using GraphPad Prism
version 4.0 for Windows (GraphPad Software, San Diego, CA,
USA).
Cellular binding and processing of
radiolabeled conjugates
Internalization of [111In]ABY-025 and
[111In]CHX-A″DTPA-trastuzumab was evaluated as described
by Wållberg and Orlova (22).
DU-145, PC3 or LNCaP cells were incubated with 0.1 nM of
[111In]ABY-025 or 1 nM
[111In]CHX-″DTPA-trastuzumab in complete medium at 37˚C.
At designated times during the incubation (0.5, 1, 2, 3, 4, 8 and
24 h), one group of 3 dishes was analyzed for cell-associated
radioactivity. The incubation medium was collected. Membrane-bound
radioactivity was determined by acid wash. Cells were treated with
0.5 ml 4 M urea solution in a 0.1 M glycine buffer, pH 2.5, for 5
min on ice. The acid fraction was collected, and the cells were
washed with an additional 0.5 ml acid solution, which was added to
the acid fraction. Subsequently, the cells were lysed and collected
for the measurement of internalized radioactivity. For lysis, 0.5
ml of 1 M sodium hydroxide solution was added, and the cells were
incubated at 37˚C for at least 0.5 h. The basic solution was
collected. Dishes were washed with an additional 0.5 ml basic
solution, and the basic fractions were pooled.
The radioactivity content of the samples was
measured using an automated γ-counter, and data were normalized to
the maximum uptake.
Results
Conjugation and labeling chemistry
In agreement with previous results,
[111In]-labeling of ABY-025 provided a yield exceeding
95% with specific activities up to 7 GBq/μmol. Labeling of
CHX-A″DTPA-trastuzumab was as efficient; all yields were >98%,
and the maximum specific radioactivity of 26.6 GBq/μmol was
obtained. Since the labeling yield was consistently >95%, no
further purification was required. The labeled proteins were
diluted with PBS for further experiments.
In vitro binding specificity
The results of the in vitro specificity tests
for [111In]CHX-A″DTPA-trastuzumab and [111In]
ABY-025 are presented in Fig. 1. A
pre-saturation of HER2 receptors using non-labeled molecules
reduced the cell-bound radioactivity of
[111In]CHX-A″DTPA-trastuzumab from 6.5±0.3 to 1.89±0.08%
for LNCaP, from 3.31±0.07 to 1.06±0.51% for PC3, and from 2.72±0.14
to 0.92±0.08% for the DU145 prostate cancer cell line. Similarly,
pre-saturation of HER2 with non-labeled Affibody molecule reduced
the binding of [111In] ABY-025 from 15.22±0.31 to
0.37±0.12%, from 11.00±1.39 to 0.42±0.36%, and from 5.59±0.37 to
0.88±0.09%, for the same cell lines, respectively. In all cases,
the binding reduction was highly significant (p<0.0005). This
demonstrated that the binding of all tested conjugates to prostate
cancer cells was specific, and that all tested cell lines expressed
HER2.
Quantification of HER2 receptor
expression in prostate cancer cell lines
The HER2 expression level was quantified using
saturation experiments with
[111In]CHX-A″DTPA-trastuzumab. The results of a typical
saturation experiment are presented in Fig. 2. All cell lines demonstrated
moderate, yet detectable, expression levels. Quantitative data
concerning HER2 expression are shown in Table I.
 | Table I.Expression of HER2 receptors by
prostate cell lines in vitro. |
Table I.
Expression of HER2 receptors by
prostate cell lines in vitro.
| Cell line | Receptors per
cell |
|---|
| LNCaP | 30,000±8,000 |
| PC3 | 23,600±8,500 |
| DU145 | 51,000±14,000 |
Cellular binding and processing of
radiolabeled conjugates
The data concerning the binding and cellular
processing of [111In]CHX-A″DTPA-trastuzumab and
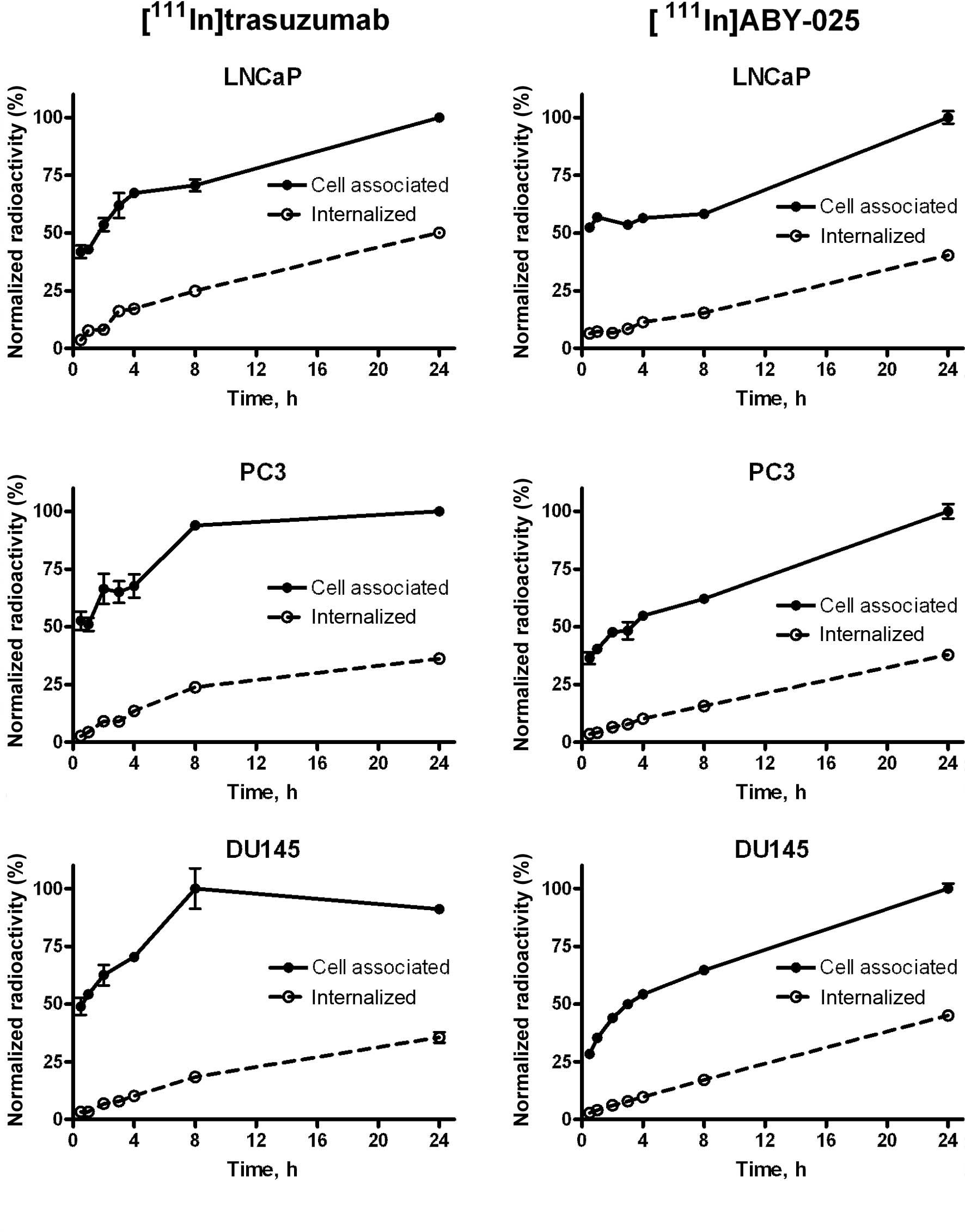
[111In]ABY-025 are presented in Fig. 3. The binding pattern was somewhat
different for the two conjugates. For Affibody molecules, an
initial rapid binding was followed by slow but continuous growth of
the uptake up to 24 h of incubation. Such a pattern may be due to
continuous cell proliferation and an absence of HER2
down-regulation. A similar pattern was also observed with
[111In]CHX-A″DTPA-trastuzumab in the LNCaP cells.
However, in the DU-145 and PC3 cells, a rapid initial binding of
[111In]CHX-A″DTPA-trastuzumab was followed by a plateau
after 8 h of incubation.
The internalization of both conjugates demonstrated
continued growth during the entire incubation period. The
internalization rate was approximately equal for both the
monoclonal antibody and the Affibody molecule. In both cases,
internalization was moderately rapid. In the case of
[111In]ABY-025, ∼10% of cell-associated radioactivity
was internalized at 4 h after the initiation of incubation. At 24
h, the percentage of internalized radioactivity was 38–45%. The
internalization rate of [111In]CHX-A″DTPA-trastuzumab
was slightly higher than that of [111In]ABY-025, with a
greater difference between the cell lines. For example, 50.1±2.3%
of [111In]CHX-A″DTPA-trastuzumab-delivered radioactivity
was internalized by LNCaP cells at 24 h, while the corresponding
value for DU145 cells was only 35.5±3.9%.
Discussion
Involvement of HER2 in the transition to androgen
independence renders it a possible target for the therapy of
disseminated prostate cancer. A common choice of therapy for breast
cancer with documented overexpression of HER2 is the use of
trastuzumab (Herceptin), a humanized monoclonal antibody that binds
specifically to the HER2 receptor. Since a well-established drug is
already in use and has been proven effective for
HER2-overexpressing breast cancer tumors, the investigation of the
possible use of this drug in other HER2-overexpressing tumors is
warranted.
Agus et al demonstrated that trastuzumab is
effective in androgen-independent tumor xenografts when used in
combination with paclitaxel (23).
The EGFR/HER2 tyrosine kinase inhibitor lapatinib has also
demonstrated a promising effect in a pre-clinical in vitro
model (24). Nevertheless,
clinical trials concerning the treatment of prostate cancer with
trastuzumab or lapatinib have failed to demonstrate their efficacy
(25–27). It must be emphasized that
unselected patients were enrolled in these trials and, at best,
only biopsies from primary tumors were analyzed. An absence of
patient stratification according to HER2 expression level was
suggested as one possible reason for failure (25). However, the detection of HER2
expression in prostate cancer metastases remains a challenge. There
have been numerous attempts to establish HER2 expression levels in
prostate cancer using immunohistochemistry (IHC). The results have
varied from no (28) to detectable
(29) overexpression in all tested
samples. IHC has the downside of being a technique that requires
high sample quality, well-established procedures and experienced
staff to produce trustworthy data. It is difficult to compare data
obtained from different studies, as some studies employ the
antibody of the established HerceptTest kit (Dako Inc.), but use
their own protocols, while others use the HerceptTest kit, even
though the test is known to perform with less reliability in
prostate tissue. Moreover, IHC requires that a biopsy be performed,
and it is difficult to obtain a biopsy from bone metastases, which
are the most common form in disseminated prostate cancer. Obtaining
biopsies only from amenable sites is associated with the risk of
false negative or positive findings, since information concerning
all metastases is required for the determination of personalized
treatment. The use of radionuclide molecular imaging may be a
solution in this case. The present study is an initial step in the
characterization of the potential imaging probes
[111In]ABY-025 and
[111In]CHX-A″DTPA-trastuzumab for the detection of HER2
overexpression in vivo.
The need to establish pre-clinical models for the
in vivo detection of HER2 expression in prostate cancer
cells is clear. One step towards this is the proper
characterization of prostate cancer cell lines to establish the
number of HER2 receptors on the cells. During this study, such a
characterization was carried out for the prostate cancer cell lines
DU-145, PC3 and LNCaP. Expression of HER2 was demonstrated and
quantified in all three of the prostate cancer cell lines tested
(Table I). As these cell lines
have different degrees of androgen dependence, together they
constitute a panel that can be used for the measurement of changes
in HER2 expression in response to different treatments. Although
the expression is moderate (20,000–50,000 receptors per cell), our
previous experience with LS174T cells having similar expression
level shows that such xenografts can be clearly visualized in
vivo (30,31).
Another significant factor involved in tracer
development is the internalization rate. Internalization is
followed by transfer to the lysosomal compartment, where targeting
proteins undergo proteolytic degradation. Charged radiocatabolites
of radiometal labels remain trapped intracellularly, while
lipophilic catabolites of radiohalogen labels leak from cells,
decreasing tumor-associated radioactivity. Previous studies using
breast and ovarian carcinoma cell lines suggested relatively slow
internalization of Affibody molecules (20,22,30),
but could not exclude a priori that internalization of
Affibody molecules proceeds at a different rate in prostate cancer
cells.
Previously reported data concerning the
internalization of radiolabeled trastuzumab are conflicting.
Certain authors have claimed that internalization is low (32), while others have suggested a much
higher internalization rate (33).
Our previous data on the cellular processing of radioiodinated
tracers by the gastric adenocarcinoma NCI-N87 cell line suggested a
somewhat more rapid internalization of trastuzumab in comparison to
Affibody molecules (34). This
study demonstrated that the internalization of Affibody molecules
by a prostate cancer cell line was somewhat more rapid than that of
ovarian and breast cancer cell lines (22). The internalization rates of
Affibody molecules and trastuzumab were rather similar, with a
small variation between cell lines. Such features would definitely
favor the use of radiometal labels for trastuzumab and, most
likely, for Affibody molecules.
Acknowledgements
This study was supported by the
Swedish Cancer Society (Cancerfonden) and the Swedish Research
Council (Vetenskapsrådet). The authors thank Affibody AB,
Stockholm, for providingABY-025, and Apoteket Farmaci AB
(Cytostatikaberedniongen, Sjukhusapoteket, Uppsala) for assistance
in obtaining the Herceptin.
References
|
1.
|
Taichman RS, Loberg RD, Mehra R and Pienta
KJ: The evolving biology and treatment of prostate cancer. J Clin
Invest. 117:2351–2361. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pienta KJ and Smith DC: Advances in
prostate cancer chemotherapy: a new era begins. CA Cancer J Clin.
55:300–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Signoretti S, Montironi R, Manola J, et
al: Her-2-neu expression and progression toward androgen
independence in human prostate cancer. J Natl Cancer Inst.
92:1918–1925. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Scher HI and Sawyers CL: Biology of
castration-resistant prostate cancer: directed therapies targeting
the androgenreceptor signaling axis. J Clin Oncol. 23:8253–8261.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
So A, Gleave M, Hurtado-Col A and Nelson
C: Mechanisms of the development of androgen independence in
prostate cancer. World J Urol. 23:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Culig Z, Hobisch A, Cronauer MV, et al:
Androgen receptor activation in prostatic tumor cell lines by
insulin-like growth factor-I, keratinocyte growth factor and
epidermal growth factor. Cancer Res. 54:5474–5478. 1994.
|
|
10.
|
Craft N, Shostak Y, Carey M and Sawyers
CL: A mechanism for hormone-independent prostate cancer through
modulation of androgen receptor signaling by the HER-2/neu tyrosine
kinase. Nat Med. 5:280–285. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gioeli D, Ficarro SB, Kwiek JJ, et al:
Androgen receptor phosphorylation. Regulation and identification of
the phosphorylation sites. J Biol Chem. 277:29304–29314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/ neu proto-oncogene in human breast and
ovarian cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tolmachev V: Imaging of HER-2
overexpression in tumors for guiding therapy. Curr Pharm Des.
14:2999–3011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Behr TM, Behe M and Wormann B: Trastuzumab
and breast cancer. N Engl J Med. 345:995–996. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tolmachev V and Orlova A: Update on
affibody molecules for in vivo imaging of targets for cancer
therapy. Minerva Biotechnologica. 21:21–30. 2009.
|
|
16.
|
Nygren PA: Alternative binding proteins:
affibody binding proteins developed from a small three-helix bundle
scaffold. FEBS J. 275:2668–2676. 2008. View Article : Google Scholar
|
|
17.
|
Orlova A, Magnusson M, Eriksson TL, et al:
Tumor imaging using a picomolar affinity HER2 binding affibody
molecule. Cancer Res. 66:4339–4348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Baum RP, Prasad V, Müller D, et al:
Molecular imaging of HER2-expressing malignant tumors in breast
cancer patients using synthetic 111In- or
68Ga-labeled Affibody molecules. J Nucl Med. 51:892–897.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Feldwisch J, Tolmachev V, Lendel C, et al:
Design of an optimized scaffold for Affibody molecules. J Mol Biol.
398:232–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ahlgren S, Orlova A, Wållberg H, et al:
Targeting of HER2-expressing tumors using 111In-ABY-025,
a second generation Affibody molecule with a fundamentally
re-engineered scaffold. J Nucl Med. 51:1131–1138. 2010.PubMed/NCBI
|
|
21.
|
Almqvist Y, Steffen AC, Tolmachev V, Divgi
CR and Sundin A: In vitro and in vivo characterization of
177Lu-huA33: a radioimmunoconjugate against colorectal cancer. Nucl
Med Biol. 33:991–998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: implications for development
of labeled tracers. Cancer Biother Radiopharm. 23:435–442.
2008.
|
|
23.
|
Agus DB, Scher HI, Higgins B, et al:
Response of prostate cancer to anti-Her-2/neu antibody in
androgen-dependent and -independent human xenograft models. Cancer
Res. 59:4761–4764. 1999.PubMed/NCBI
|
|
24.
|
Liu Y, Majumder S, McCall W, et al:
Inhibition of HER-2/neu kinase impairs androgen receptor
recruitment to the androgen responsive enhancer. Cancer Res.
65:3404–3409. 2005.PubMed/NCBI
|
|
25.
|
Ziada A, Barqawi A, Glode LM, et al: The
use of trastuzumab in the treatment of hormone refractory prostate
cancer; phase II trial. Prostate. 60:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lara PN Jr, Chee KG, Longmate J, et al:
Trastuzumab plus docetaxel in HER-2/neu-positive prostate
carcinoma: final results from the California Cancer Consortium
Screening and Phase II Trial. Cancer. 100:2125–2131. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sridhar SS, Hotte SJ, Chin JL, et al: A
multicenter phase II clinical trial of lapatinib (GW572016) in
hormonally untreated advanced prostate cancer. Am J Clin Oncol.
33:609–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Visakorpi T, Kallioniemi OP, Koivula T,
Harvey J and Isola J: Expression of epidermal growth factor
receptor and ERBB2 (HER-2/Neu) oncoprotein in prostatic carcinomas.
Mod Pathol. 5:643–648. 1992.PubMed/NCBI
|
|
29.
|
Gu K, Mes-Masson AM, Gauthier J and Saad
F: Overexpression of HER-2/neu in human prostate cancer and benign
hyperplasia. Cancer Lett. 99:185–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ahlgren S, Wållberg H, Tran TA, et al:
Targeting of HER2-expressing tumors with a site-specifically
99mTc-labeled recombinant affibody molecule, ZHER2:2395, with
C-terminally engineered cysteine. J Nucl Med. 50:781–789. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Tran TA, Rosik D, Abrahmsén L, et al:
Design, synthesis and biological evaluation of a multifunctional
HER2-specific Affibody molecule for molecular imaging. Eur J Nucl
Med Mol Imaging. 36:1864–1873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Austin CD, de Maziere AM, Pisacane PI, et
al: Endocytosis and sorting of ErbB2 and the site of action of
cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell.
15:5268–5282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Lub-de Hooge MN, Kosterink JG, Perik PJ,
et al: Preclinical characterisation of 111In-DTPA-trastuzumab. Br J
Pharmacol. 143:99–106. 2004.PubMed/NCBI
|
|
34.
|
Orlova A, Wållberg H, Stone-Elander S and
Tolmachev V: On the selection of a tracer for PET imaging of
HER2-expressing tumors: direct comparison of a 124I-labeled
affibody molecule and trastuzumab in a murine xenograft model. J
Nucl Med. 50:417–425. 2009. View Article : Google Scholar
|