Contents
Introduction
Materials and methods
Malignant melanoma micrometastases
Stroma immunohistochemistry beneath malignant
melanoma
Discussion
Conclusion
Introduction
Cutaneous malignant melanoma (MM) is basically an
uncontrolled overgrowth of neoplastic melanocytes. At some stage of
its progression, the neoplasm exhibits a high metastatic potential.
It proves to be resistant to drug-induced apoptosis, which is
believed to underlie the resistance of MM to conventional
chemotherapy and radiotherapy (1,2).
Various interactions exist between MM cells and other biological
systems, including immune cells, vascularity, contiguous stromal
cells and the dermal extracellular matrix (ECM). Certain aspects of
MM-stroma interactions are thought to be associated with disease
prognosis (3). In addition,
environmental influences, including ultraviolet (UV) light, are
probably responsible for MM initiation and may support its
progression along with the intervention of diverse autocrine and
paracrine factors (4). In
particular, a number of growth factors and specific enzymes are
released in the MM microenvironment (5–7).
The participation of the host in the ‘cancer
micro-ecosystem’ basically involves the microvasculature, stromal
cells and specific immune reactions (8–10).
Angiogenesis is a typical host-mediated response to many cancers.
It appears crucial for cancer progression, as blood vessels deliver
nutrients and oxygen to neoplastic cells (11). Furthermore, the microvasculature
likely allows communication between the primary MM and its
metastases. Pro-angiogenic molecules originate from cancer cells as
well as from the stroma. The relative contribution of both
compartments is likely to change with MM type and site, and is
balanced by other factors as well (11,12).
Cross-talk between MM and stromal cells may be
mediated through direct heterotypic cell-cell contacts, adhesion
molecules, signaling factors, and other secreted molecules
consisting of growth factors, cytokines, chemokines, ECM proteins,
proteinases, proteinase inhibitors and lipid products (13). Conceptually, the MM
microenvironment is crucial for the maintenance of cellular
functions and tissue integrity, suggesting that a cancer-induced
change in the ECM may contribute to cancer invasion (14). Any alteration in the MM stroma may
be due to an imbalance in the cytokine profile, resulting from
oncogenic changes in the cancer cells. In particular, experimental
animal models have demonstrated that cancer invasion is stimulated
by the wound-healing stroma (15).
Both stromal cells and the ECM located beneath
primary MM lesions are therefore likely involved in the process of
invasion of the neoplasm and in the early dissemination of
micrometastases associated or not with neoangiogenesis (2,10,16–19).
These characteristics are possibly associated with phenotypic
changes in the stromal cells in the MM vicinity. In recent years,
tumor growth regulation by ECM components has been one of the main
topics of neoplastic biology research.
Materials and methods
This study is a review of current peer-reviewed
publications admixed with personal original findings from a series
of 400 MM cases with a thickness ranging between 0.4 and 1.0 mm
(median 0.83) that were retrieved from our files. The microscopic
diagnosis was previously established by a group of three
dermatopathologists. Immunohistochemistry was performed as
previously described (20–23). In short, samples were fixed in
buffered formalin and embedded in paraffin. A series of 6-μm
sections were prepared for immunohistochemistry. The avidin-biotin
peroxidase method was used with the antibodies listed in Table I. After 1 h of incubation with each
primary antibody, the slides were washed in Tris-buffered saline
(TBS) and incubated for 30 min with the secondary antibody
(biotinylated swine anti-rabbit, 1:300; Dakopatts Glostrup,
Denmark). Slides were rinsed in TBS and covered by the EnVision
(Dakopatts) polymer-based revelation system. After TBS washings,
Fast Red (Dakopatts) was used as the chromogen substrate. The final
steps consisted of counter-staining with Mayer's hemalum and
mounting in glycerin mounting medium (Dakopatts). Negative
immunohistochemical controls were performed by omitting or
substituting the primary and the secondary antibodies from the
laboratory procedure.
 | Table I.Panel of antibodies. |
Table I.
Panel of antibodies.
| Antigen | Dilution | Source |
|---|
| α1 (IV)
collagen | 1:25 | Arnold I
Caplan |
| α5 (IV)
collagen | 1:25 | Weiselab |
| Elafin | 1:500 | Santa Cruz
Biotechnology |
| Factor XIIIa | 1:100 | Neomarkers |
| Lysozyme | 1:300 | Dako |
| Versican | 1:500 | Seikagaku
Corp. |
Malignant melanoma micrometastases
A typical biological feature of human MM is the
tremendous impact of the primary lesion thickness on prognosis.
Primary MM <1 mm in thickness is associated with a high cure
rate, sharply contrasting with thicker lesions associated with
poorer prognosis. The apparent breakpoint beyond an ∼1-mm thickness
is a discouraging factor in disease outcome. One possible reason
appears to be linked to vascularization patterns of MM (11,12).
Such an anatomic argument is persuasive, but it is by no means the
only one.
MM cells apparently fail to form metastases unless
they present the genotypic and phenotypic information allowing them
to effectively migrate in the ECM, intravasate, extravasate, cross
interstitial basement membranes and proliferate in distant tissue
sites. These characteristics are expressed by variant
subpopulations of metastatically competent MM cells present in
primary neoplasms (2). These
subpopulations probably acquire a growth advantage at the primary
site over time, so that they become a dominant proliferating
population. At this stage, the MM truly expresses overt malignancy.
As a result of this process of clonal dominance of metastatically
competent cells, it is possible that most thin primary MM lesions
contain very few, if any, metastatically competent cells, whereas
thicker MM lesions may contain significant proportions of such
cells. It is possible that the stromal microenvironment plays a
role in such a shift in the biological profile of MM (24).
The four metastatic routes
In order to form metastases at distant sites, MM
cells must acquire certain functions and properties in an ordered
sequence referred to as the MM metastatic cascade. In order to form
overt metastasis, this process must encompass hyperproliferation,
detachment from the primary neoplasm, invasion into the peritumoral
stroma and possibly penetration into blood and lymphatic vessels,
survival in the circulation, adhesion to a vessel wall at the site
of the final metastatic deposition, extravasation and proliferation
(3).
The specific function involved in the metastatic
cascade combines intrinsic characteristics of various MM cells and
regulatory influences from the miroenvironment. Indeed, MM cells
and their surrounding stroma jointly form a microecosystem
receptive or not to early inconspicuous metastatic spread.
Early MM micrometastases are not discernable upon
regular clinical or dermoscopic examination. They are disclosed
under the microscope, particularly after highlighting their
presence using immunohistochemistry (2,17).
They are found in four distinct locations, namely i) inside lymph
vessels, ii) inside blood vessels, iii) in a perivascular location
just adjacent to the outer area of the endothelial lining, and iv)
dispersed inside the stroma (10,16,18).
The latter eventuality is not infrequently associated with
neoangiogenesis, and an enhanced neoplastic germinative pool is
commonly found (10,19).
The active migration of metastatic MM cells in the
peritumoral stroma is probably a complex process. It involves the
active mobility of MM cells and changes in the neoplastic cell
adherence systems with ECM components.
Melanoma stem cells
The presence of MM stem cells is an important
consideration when investigating the characteristics of MM
micrometastases and their relationship with the peritumoral stroma
(10,22,25–27).
Similar to physiological stem cells, cancer stem cells are capable
of self-renewal and differentiation, and have the potential for
indefinite proliferation, a function linked to MM growth (2,22,28).
Although conventional anticancer treatments may eradicate most
malignant cells, they are potentially ineffective against
chemoresistant cancer stem cells, which are ultimately responsible
for tumor recurrence and progression (2,10,25).
MM shows tumor heterogeneity, undifferentiated molecular signatures
and increased tumorigenicity of MM subsets with embryonic-like
differentiation plasticity. This strongly suggests the presence and
involvement of MM stem cells in the initiation and propagation of
this malignancy (25–27,29–32).
The ECM structure and biologic activity may influence the
invasiveness and propagation of MM stem cells.
Micrometastases and the peri-melanoma
stroma
When present, interstitial unicellular MM
micrometastases are frequently found and confined to the
perineoplastic stroma. Their presence is significantly correlated
with the risk of involvement of the sentinel lymph node (17).
Stroma immunohistochemistry beneath
malignant melanoma
Upon standard histopathological examination, stromal
cells appear normal underneath primary MM lesions when partial
regression is not operative. However, their differentiation as
revealed by immunohistochemistry appears altered when compared to
the surrounding skin. In particular, phenotypic changes are noted
when identifying the transglutaminase Factor XIIIa, α (IV) collagen
chains, as well as elafin and versican. It is possible, although
not yet proven, that transforming growth factor (TGF)-β1 and
platelet-derived growth factor (PDGF) may play a role in the
alteration of the stromal host compartment in MM.
Factor XIIIa-enriched stromal cells
Factor XIIIa-enriched stromal cells are commonly
identified as dermal dendrocytes (DDs). They are preferentially
found adjacent to superficial microvasculature (24,33–35).
Increased numbers of Factor XIIIa-positive DDs are often found in
the vicinity of most invasive cutaneous neoplasms. In our
experience, Factor XIIIapositive DDs are numerous; they neighbor
and infiltrate most thin MM lesions (24). By contrast, they are present in few
numbers or even absent in thick primary MM lesions and their
metastases (36–38). Circumstantial evidence indicates
that the density of Factor XIIIa-positive DDs is correlated with a
low proliferative rate of MM cells. Thus, Factor XIIIa-positive DDs
may not be passive bystanders in MM (24,34,36).
Their function may differ based on whether they are located in the
stroma or inside the neoplasm (24). Intratumoral DDs may be associated
with a growth-restricting role. By contrast, stromal DDs may help
in the invasiveness and metastatic spread of MM cells.
Collagen IV-enriched stromal cells
In malignant neoplasms, basement membranes (BMs) are
composite structures synthesized by tumor cells or stromal cells;
either by one of these two cell types yet dependent on the
interactions between them, or a mixture from both origins. These
tumoral BMs are often abnormal in their composition and
ultrastructural features (39,40).
BM material appears to accompany malignant cells rather than to
prevent invasion as a physical barrier. Nevertheless, active
interactions between neoplastic cells and stroma, in particular the
ECM, play a key role in neoplastic progression leading to invasion
and metastasis (41). Several BM
components have been identified surrounding MM cells, including
collagen IV (39,42–44).
In the skin, collagen IV represents an assembly of α1 (IV) and α5
(IV) collagen chains. In MM, some neoplastic and stromal cells
exhibit intracytoplasmic immunolabeling for α1 (IV) chains
(44). The pattern is
heterogeneous. BM components, including collagen IV, gradually
disappear during the dermal ingrowth of MM cells. Notably, a
minority of MM cases without any identifiable micrometastasis and a
majority of MM with cutaneous micrometastasis show discrete
cytoplasmic positivity for the α5 (IV) collagen chain (44).
Distribution of the α1 (IV) collagen chain in MM
highlights the heterogeneity in both cell differentiation and
stroma-MM interactions. Thus, MM cells appear to have their own
individual potential to be enclosed by a BM and to interact with
the stroma. This biological aspect may be related to neoplastic
progression and may influence inconspicuous metastatic
potential.
Versican-enriched stromal cells
Versican is a large proteoglycan normally present
inside the stromal cells of the skin. The molecule belongs to the
chondroitin sulfate family of the hyalectan group, named for its
ability to bind hyaluronan (45).
In mammals, versican appears as four possible spliced isoforms, V0
to V3. Little is known concerning the differential regulation of
the isoforms or about their respective roles in the ECM either
normal or peritumoral. Versican production is deregulated in
several types of human cancer (46). As it is largely expressed in
rapidly growing neoplastic cells, it has been suggested that
versican plays a direct role in cell proliferation and other cell
functions (45). It appears to be
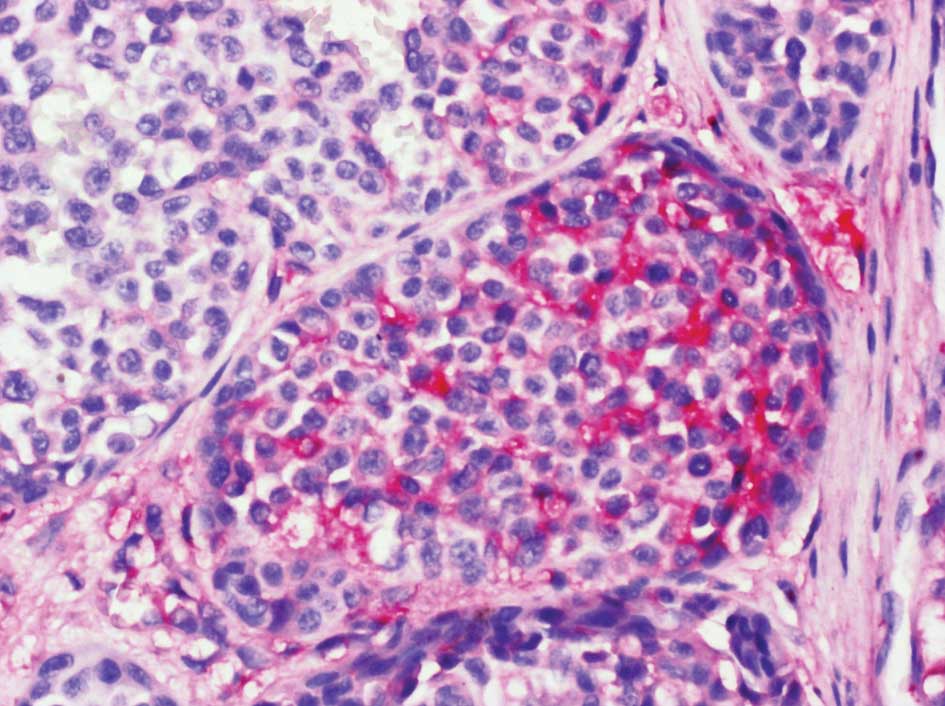
particularly abundant in the stromal cell population underlying MM
(Fig. 1) (46–49).
Versican overexpression was found to sharply circumscribe to a
cup-shaped structure cuffing the bottom of MM lesions. In addition,
some nests of MM cells were found to be strongly labeled with the
anti-versican antibody (Fig. 2).
This finding contrasts with another study reporting the absence of
versican immunoreactivity in neoplastic melanocytes (49). In addition, versican expression is
not correlated with Breslow tumor thickness and Clark's level
(49).
Elafin-, versican- and lysozyme-loaded
ECM
Elastic fibres are coated with distinct molecules
following chronic UV exposure (50–53).
The serine anti-leukoprotease elafin, as well as versican and
lysozyme, bind to elastin preventing elastolytic degradation by
elastases on sun-exposed areas exhibiting solar elastosis (51,53,54).
Under these conditions, the labeling was found to range from
partial, moderate to strong. In addition, inhibition of elastase
may decrease the adhesion of cancer cells to endothelial cells
(55).
In addition, elafin was reported to elicit
p53-dependent apoptosis in cultured MM cells transfected by a
plasmidproducing elafin under doxycycline boosting (56). In contrast to these in vitro
experiments, immunohistochemistry did not disclose an intratumoral
cell presence of elafin in human MM. Rather, keratinocytes covering
MM overexpressed elafin in their cytoplasm. Of note, Western
blotting and reverse transcription analyses indicated
transcriptional elafin repression in MM cells (56).
The implication of elafin in other diseases, such as
psoriasis and graft-versus-host reaction, indicates its distinct
importance in skin biology (57,58).
Discussion
This review highlights the existence of a distinct
region of the dermis adjacent to the base of a primary MM lesion.
Stromal cells exhibit particular phenotypic features suggesting
altered functionality. The involved territory appears to be
conducive to micrometastatic spread. Some of these cells survive
singly, and due to their manner of migration to other organs may
represent MM stem cells.
In addition to the importance of MM vascularization
for tumor growth, invasiveness and metastatic spread (10–12,19,59),
numerous other roles are ascribed to the tumoral stroma. This
structure is involved in a constant remodeling following
degradation and repair of the ECM. Notably, immunohistochemistry
highlights the direct implication of MM cells in the synthesis
and/or storage of certain ECM molecular components.
The immunohistochemical characterization of MM cells
is important (20,22,60–64),
yet should be extended to the peritumoral stroma, including the
microvasculature (10–12,59)
and other ECM components. A comprehensive mapping of MM
immunohistochemical characteristics should aid in identifying
relevant targeted therapies (63–66).
Inflammatory cells and immunocytes represent another
class of host cells that are regulated by the balance of cytokines.
They perform counter-current invasion, from the circulation into
the tumor, and provide routes for MM cell invasion. It is important
for our understanding of MM stroma turnover that tumor-infiltrating
leukocytes produce proteinases.
Conclusion
Interaction between MM and its stroma is evident
during the invasive and metastatic stages of disease progression.
Stromal cells are known to secrete metalloproteinases and their
inhibitors, growth factors, the scatter factor/hepatocyte growth
factor and other factors, as well as participate in the growth and
mobility of MM cells. In addition, other molecules are synthesized
and overexpressed by stromal cells and/or MM cells.
Immunohistochemistry has identified Factor XIII-a, α1 and α5 (IV)
collagen chains, versican, elafin and lysozyme. These possibly
influence the migration of MM cells, including their stem
cells.
While MM cell motility cannot be directly assessed,
there is circumstantial evidence indicating that motility is
essential to MM progression and possibly of prognostic
significance. Apart from the secretion and activation of enzymes
altering the ECM, a variety of stromal alterations occur following
overexpression of diverse ECM components. Molecular morphology
yields evidence suggesting that the MM stroma plays an integral
role in MM. Although much remains to be determined, the findings as
described in the present review may have diagnostic and prognostic
significance, which warrant further investigation.
Acknowledgements
This study was supported by a grant
from the ‘Fonds d'Investissement de la Recherche Scientifique’ of
the University Hospital of Liège. No other sources of funding were
used to assist in the preparation of this manuscript. The authors
appreciate the excellent secretarial assistance of Mrs. Ida
Leclercq and Mrs. Marie Pugliese.
References
|
1.
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
2.
|
Quatresooz P and Piérard GE: Malignant
melanoma: from cell kinetics to micrometastases. Am J Clin
Dermatol. Dec 13–2010.(E-pub ahead of print).
|
|
3.
|
Smolle J, Hofmann-Wellenhof R and
Fink-Puches R: Melanoma and stroma: an interaction of biological
and prognostic importance. Semin Cutan Med Surg. 15:326–335. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bennett DC: Ultraviolet wavebands and
melanoma initiation. Pigment Cell Melanoma Res. 21:520–524. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Brenner M, Degitz K, Besch R and Berking
C: Differential expression of melanoma-associated growth factors in
keratinocytes and fibroblasts by ultraviolet A and ultraviolet B
radiation. Br J Dermatol. 153:733–739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Philips N, Keller T and Holmes C:
Reciprocal effects of ascorbate on cancer cell growth and the
expression of matrix metalloproteinases and transforming growth
factor-beta. Cancer Lett. 256:49–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Philips N, Conte J, Chen YJ, Natrajan P,
Taw M, Keller T, Givant J, Tuason M, Dulaj L, Leonardi D and
Gonzalez S: Beneficial regulation of matrix-metalloproteinases and
their inhibitors, fibrillar collagens and transforming growth
factor-β by Polypodium leucotomos, directly or in dermal
fibroblasts, ultraviolet-radiated fibroblasts, and melanoma cells.
Arch Dermatol Res. 301:487–495. 2009.
|
|
8.
|
Dvorak HF: Tumors: wounds that do not
heal. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447.
2003.PubMed/NCBI
|
|
10.
|
Quatresooz P, Piérard-Franchimont C,
Paquet P and Piérard GE: Angiogenic fast-growing melanomas and
their micrometastases. Eur J Dermatol. 20:302–307. 2010.PubMed/NCBI
|
|
11.
|
Piérard GE and Piérard-Franchimont C:
Stochastic relationship between the growth fraction and vascularity
of thin malignant melanomas. Eur J Cancer. 33:1888–1892.
1997.PubMed/NCBI
|
|
12.
|
Piérard-Franchimont C, Henry F, Heymans O
and Piérard GE: Vascular retardation in dormant growth-stunted
malignant melanomas. Int J Mol Med. 4:403–406. 1999.PubMed/NCBI
|
|
13.
|
Wernert N: The multiple roles of tumour
stroma. Virchows Arch. 430:433–443. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Dingemans KP, Zeeman-Boeschoten IM, Keep
RF and Das PK: Transplantation of colon carcinoma into granulation
tissue induces an invasive morphotype. Int J Cancer. 54:1010–1016.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lugassy C and Barnhill RL: Angiotropic
malignant melanoma and extravascular migratory metastasis:
description of 26 cases with emphasis on a new mechanism of tumor
spread. Pathology. 36:485–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Claessens N, Piérard GE,
Piérard-Franchimont C, Arrese JE and Quatresooz P:
Immunohistochemical detection of incipient melanoma
micrometastases. Relationship with sentinel lymph node involvement.
Melanoma Res. 15:107–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lugassy C and Barnhill RL: Angiotropic
melanoma and extravascular migratory metastasis. A review. Adv Anat
Pathol. 14:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Quatresooz P, Piérard GE,
Piérard-Franchimont C, Humbert P and Piérard S: Introduction to the
spectral analysis of microvasculature in primary cutaneous
melanoma. Pathol Biol. Feb;2010.(E-pub ahead of print).
|
|
20.
|
Quatresooz P, Arrese JE,
Piérard-Franchimont C and Piérard GE: Immunohistochemical aid at
risk stratification of melanocytic neoplasms. Int J Oncol.
24:211–216. 2004.PubMed/NCBI
|
|
21.
|
Quatresooz P, Piérard-Franchimont C and
Piérard GE: Highlighting the immunohistochemical profile of
melanocytomas. Oncol Rep. 19:1367–1372. 2008.PubMed/NCBI
|
|
22.
|
Quatresooz P, Piérard GE and
Piérard-Franchimont C; the Mosan Study Group of Pigmented Tumors:
Molecular pathways supporting the proliferation staging of
malignant melanoma. Int J Mol Med. 24:295–301. 2009.PubMed/NCBI
|
|
23.
|
Quatresooz P, Piérard-Franchimont C and
Piérard GE; the Mosan Study Group of Pigmented Tumors: Molecular
histology on the diagnostic cutting edge between malignant
melanomas and cutaneous melanocytomas. Oncol Rep. 22:1263–1267.
2009.PubMed/NCBI
|
|
24.
|
Piérard-Franchimont C, Arrese JE, Nikkels
AF, Al Saleh W, Delvenne P and Piérard GE: Factor XIIIa-positive
dermal dendrocytes and proliferative activity of cutaneous cancers.
Virchows Arch. 429:43–48. 1996.
|
|
25.
|
Schatton T and Franck MH: Cancer stem
cells and human malignant melanoma. Pigment Cell Melanoma Res.
21:39–55. 2007. View Article : Google Scholar
|
|
26.
|
Rappa G, Fodstad O and Lorico A: The stem
cell-associated antigen CD133 (Prominin-1) is a molecular
therapeutic target for metastatic melanoma. Stem Cells.
26:3008–3017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Schatton T, Murphy GF, Frank NY, et al:
Identification of cells initiating human melanomas. Nature.
451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Cramer SF: Stem cells for epidermal
melanocytes. A challenge for students of dermatopathology. Am J
Dermatopathol. 31:331–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Grichnik JM, Burch JA, Schulteis RD, Shan
S, Liu J, Darrow TL, Vervaert CE and Seigler HF: Melanoma, a tumor
based on a mutant stem cell? J Invest Dermatol. 126:142–153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Buac K and Pavan WJ: Stem cells of the
melanocyte lineage. Cancer Biomark. 3:203–209. 2007.PubMed/NCBI
|
|
32.
|
Klein WM, Wu BP, Zhao S, Wu H,
Klein-Szanto AJ and Tahan SR: Increased expression of stem cell
markers in malignant melanoma. Mod Pathol. 20:102–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Arrese Estrada J and Piérard GE: Factor
XIIIa-positive dendrocytes and the dermal microvascular unit.
Dermatologica. 180:51–53. 1990.PubMed/NCBI
|
|
34.
|
Quatresooz P, Paquet P, Hermanns-Lê T and
Pierard GE: Molecular mapping of Factor XIIIa-enriched dendrocytes
in the skin. Int J Mol Med. 22:403–409. 2008.PubMed/NCBI
|
|
35.
|
Quatresooz P and Piérard GE:
Immunohistochemical clues at aging of the skin microvascular unit.
J Cutan Pathol. 36:39–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Fullen DR and Headington JT: Factor
XIIIa-positive dermal dendritic cells and HLA-DR expression in
radial versus vertical growth-phase melanomas. J Cutan Pathol.
25:553–558. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Denton KJ, Cotton DW, Wright A and Hird P:
Factor XIIIa in nodular malignant melanoma and Spitz naevi. Br J
Dermatol. 12:783–786. 1990.PubMed/NCBI
|
|
38.
|
Polak ME, Johnson P, Di Palma S, Higgins
B, Hurren J, Borthwick NJ, Jager MJ, Mccormick D and Cree IA:
Presence and maturity of dendritic cells in melanoma lymph node
metastases. J Pathol. 207:83–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lugassy C, Eyden BP, Christensen L and
Escande JP: Angiotumoral complex in human malignant melanoma
characterized by free laminin: ultrastructural and
immunohistochemical observations. J Submicrosc Cytol Pathol.
29:19–28. 1997.
|
|
40.
|
Schaumburg-Lever G, Lever I, Fehrenbacher
B, Möller H, Bischof B, Kaiserling E, Garbe C and Rassner G:
Melanocytes in naevi and melanomas synthesize basement membrane and
basement membrane-like material. An immunohistochemical and
electron microscopic study including immunoelectron microscopy. J
Cutan Pathol. 27:67–75. 2000. View Article : Google Scholar
|
|
41.
|
Van Duinen CM, Fleuren GJ and Bruijn JA:
The extracellular matrix in pigmented skin lesions: an
immunohistochemical study. Histopathology. 24:33–40.
1994.PubMed/NCBI
|
|
42.
|
Lugassy C, Kickersin GR, Christensen L,
Karaoli T, Le Charpeniter M, Escande JP and Barnhill RL:
Ultrastructural and immunohistochemical studies of the
periendothelial matrix in malignant melanoma: evidence for an
amorphous matrix containing laminin. J Cutan Pathol. 26:78–83.
1999. View Article : Google Scholar
|
|
43.
|
Lugassy C, Shahsafaei A, Bonitz P, Busam
KJ and Barnhill RL: Tumor microvessels in melanoma express the
beta-2 chain of laminin. Implications for melanoma metastasis. J
Cutan Pathol. 26:222–226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Quatresooz P and Piérard GE:
Immunohistochemical investigation of α 1 (IV) and α 5 (IV) collagen
chains in a broad spectrum of melanocytic tumours. Melanoma Res.
15:161–168. 2005.
|
|
45.
|
Wight TN: Versican: a versatile
extracellular matrix proteoglycan in cell biology. Curr Opin Cell
Biol. 14:617–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Serra M, Miquel L, Domenzain C, Docampo
MJ, Fabra A, Wight TN and Bassols A: V3 versican isoform expression
alters the phenotype of melanoma cells and their tumorigenic
potential. Int J Cancer. 114:879–886. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Touab M, Villena J, Barranco C, Arumi-Uria
M and Bassols A: Versican is differentially expressed in human
melanoma and may play a role in tumor development. Am J Pathol.
160:549–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Docampo MJ, Rabanal RM, Miquel-Serra L,
Hernandez D, Domenzain C and Bassols A: Altered expression of
versican and hyaluronan in melanocytic tumors of dogs. Am J Vet
Res. 68:1376–1385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Gambichler T, Kreuter A, Grothe S,
Altmeyer P, Brockmeyer HN and Rotterdam S: Versican overexpression
in cutaneous malgnant melanoma. Eur J Med Res. 13:500–504.
2008.PubMed/NCBI
|
|
50.
|
Seité S, Moyal D, Richard S, de Rigal J,
Lévêque JL, Hourseau C and Fourtanier A: Effects of repeated
suberythemal doses of UVA in human skin. Eur J Dermatol. 7:204–209.
1997.
|
|
51.
|
Seité S, Zucchi H, Septier D,
Igondjo-Tchen S, Senni K and Godeau G: Elastin changes during
chronological and photo-ageing: the important role of lysozyme. J
Eur Acad Dermatol Venereol. 20:980–987. 2006.PubMed/NCBI
|
|
52.
|
Piérard-Franchimont C, Uhoda I, Saint
Léger D and Piérard GE: Androgenic alopecia and stress-induced
premature senescence by cumulative ultraviolet light exposure. Exog
Dermatol. 1:203–206. 2002.
|
|
53.
|
Muto J, Kuroda K, Wachi H, Hirose S and
Tajima S: Accumulation of elafin in actinic elastosis of
sun-damaged skin: elafin binds to elastin and prevents elastolytic
degradation. J Invest Dermatol. 127:1358–1366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Williams SE, Brown TI, Roghanian A and
Sallenave JM: SLPI and elafin: one glove, many fingers. Clin Sci.
110:21–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Nozowa F, Hirota M, Okabe A, Shibata M,
Iwamura T, Haga Y and Ogawa M: Elastase activity enhances the
adhesion of neutrophil and cancer cells to vascular endothelial
cells. J Surg Res. 94:153–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Yu KS, Lee Y, Kim CM, Park EC, Choi J, Lim
DS, Chung YH and Koh SS: The protease inhibitor, elafin, induces
p53-dependent apoptosis in human melanoma cells. Int J Cancer.
127:1308–1320. 2010.PubMed/NCBI
|
|
57.
|
Kamsteeg M, Jansen PA, van Vlijmen-Willems
IM, van Erp PE, Rodij-Olthuis D, van der Valk PG, Feuth T, Zeeuwen
PL and Schalkwijk J: Molecular diagnostics of psoriasis, atopic
dermatitis, allergic contact dermatitis and irritant contact
dermatitis. Br J Dermatol. 162:568–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Paczesny S, Braun TM, Levine JE, et al:
Elafin is a biomarker of graft-versus-host disease of the skin. Sci
Transl Med. 2:13ra22010. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Marcoval J, Moreno A, Graells J, Vidal A,
Escriba JM, Garcia-Ramirez JM and Fabra A: Angiogenesis and
malignant melanoma. Angiogenesis is related to the development of
vertical (tumorigenic) growth phase. J Cutan Pathol. 24:212–218.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Alonso S, Ortiz P, Pollan M, Pérez-Gomez
B, Sanchez L, Acuna MJ, Pajares R, Martinez-Tello FJ, Hortelano CM,
Piris MA and Rodriguez-Peralto JL: Progression in cutaneous
malignant melanoma is associated with distinct expression profiles.
A tissue microarray-based study. Am J Pathol. 164:193–203. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Fecher LA, Cummings SD, Keefe MJ and Alani
RM: Toward a molecular classification of melanoma. J Clin Oncol.
25:1606–1620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Plaza JA, Suster D and Perez-Montiel D:
Expression of immunohistochemical markers in primary and metastatic
malignant melanoma: a comparative study in 70 patients using a
tissue microarray technique. Appl Immunohistochem Mol Morphol.
15:421–425. 2007. View Article : Google Scholar
|
|
63.
|
Ohsie SJ, Sarantopoulos GP, Cochran AJ and
Binder SW: Immunohistochemical characteristics of melanoma. J Cutan
Pathol. 35:433–444. 2008. View Article : Google Scholar
|
|
64.
|
Hamza S: Prognostic parameters of
malignant melanoma. Diagn Histopathol. 16:330–336. 2010. View Article : Google Scholar
|
|
65.
|
Schopfer G, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Piérard GE, Quatresooz P, Rorive A and
Piérard-Franchimont C; Groupe Mosan d'Etude des Tumeurs
Pigmentaires: Malignant melanoma: conceptual and therapeutic
innovations based on translational research. Rev Med Liège.
63:579–584. 2008.PubMed/NCBI
|
|
67.
|
Basu B, Biswas S, Wrigley J, Sirohi B and
Corrie P: Angiogenesis in cutaneous malignant melanoma and
potential therapeutic strategies. Exp Rev Anticancer The.
9:1583–1598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Sullivan RJ and Atkins MB:
Molecular-targeted therapy in malignant melanoma. Exp Rev
Anticancer Ther. 9:567–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Kerbel RS, Kobayashi H, Graham CH and Lu
C: Analysis and significance of the malignant ‘eclipse’ during the
progression of primary cutaneous human melanomas. J Invest Dermatol
Symp Proc. 1:183–187. 1996.
|