Introduction
Nosocomial infections caused by opportunistic
infections or microbial biofilms with resistance to antibiotics may
occur during hospitalization or treatment at healthcare service
units. Recently, the increase in microbial resistance to
antibiotics, for example, that of methicillin-resistant
Staphylococcus aureus (MRSA), has threatened public health
on a global scale as it reduces the efficacy of treatments and
results in increased patient morbidity, mortality and health care
costs (1).
An opportunistic infection is an infection caused by
pathogens (bacterial, viral, fungal or protozoan) that do not
usually cause disease in a healthy host with a functioning immune
system. However, in patients with a compromised immune system, the
pathogen has the ‘opportunity’ to infect through skin injury, a
chronic disease, cancer or a drug-induced abnormality.
Opportunistic infections are a potential cause of nosocomial
infection, and may be resistant to antibiotics. Microbial biofilms,
which are polymer-dipped communities of cells responsible for a
number of chronic infections, also have extremely high resistance
to antibiotics and host defense systems (2,3).
However, there are no commercially available specific biofilm
inhibitors. Natural compounds with antibiotic properties,
particularly plant-derived ones, are therefore preferred to
artificial compounds. However, few of these natural compounds have
been identified. Biotechnology companies are currently focused on
identifying natural plant compounds with potential use as
antimicrobial and antibiofilm drugs (4).
The sugar 1,5-anhydro-D-fructose (1,5-AF) is a
recently identified monosaccharide that is formed directly from
starch or glycogen through an α-1,4-glucan lyase reaction (EC
4.2.2.13), during which its carbonyl group does not undergo
hemiacetal bonding; however, when fully hydrated in an aqueous
solution it may play a metabolically active role (5). The compound 1,5-AF has been found in
fungi, red algae, Escherichia coli and rat liver tissue
(6–9). A recent study reported that the
1,5-AF pathway is possibly operative only when the organism is
subjected to biotic and abiotic stresses (10). 1,5-AF is likely to act as an
antioxidant (11) and a precursor
of antibiotics (5). However, the
functionality and physiological role of 1,5-AF are largely
unknown.
In the present study, the antimicrobial activity of
1,5-AF against a wide range of pathogens, including
coagulase-negative staphylococci (CNS), Staphylococcus
epidermidis and MRSA biofilm formation, was investigated.
Materials and methods
Preparation of 1,5-AF solution
1,5-AF was provided by Nihon Starch Co., Ltd.
(Kagoshima, Japan) as a gift. Fresh 1,5-AF solutions were prepared
in sterile H2O at a concentration of 1 mg/ml.
Determination of the effect of 1,5-AF on
coagulase-negative staphylococci
Hands contaminated with coagulase-negative
staphylococci were treated as follows: 75% ethanol was spread on
one hand and 1% 1,5-AF + 75% ethanol on the other. After 1 min,
each hand was placed onto an agar plate. The specimens were
incubated at 37°C for 24 h.
Determination of the effect of 1,5-AF on
S. epidermidis
All the keys on computer keyboards at the clinical
laboratory of Kagoshima University Hospital were swabbed with
sterile cotton swabs moistened with saline. The specimens were
incubated at 37°C for 24 h, then the strain of S.
epidermidis was identified. S. epidermidis from the swab
was washed in 1 ml of saline solution, and 0.05 ml of the resulting
suspension was spread on either 5% sheep blood agar, 5% sheep blood
agar combined with 75% ethanol, or 5% sheep blood agar combined
with 1% 1,5-AF + 75% ethanol. The specimens were incubated at 37°C
for 16 h.
Determination of the effect of 1,5-AF on
MRSA biofilm
A microtiter plate assay (12,13)
was employed to determine the effect of 1,5-AF on biofilm
formation. MRSA was obtained from the clinical laboratory of
Kagoshima University Hospital and was cultured on sheep blood agar
plates for 18 h at 37°C. Briefly, overnight cultures of MRSA
strains were inoculated in tryptic soy broth (TSB; BD Microbiology
Systems, Sparks, MD, USA) with 0.25% glucose for 18 h at 37°C. A
0.5 McFarland standard was used to create inoculum densities of
1.5×108 CFU/ml in PBS using the direct suspension method
(14) for the biofilm assay. The
biofilm assay was performed in sterile 96-well flat-bottom
polystyrene microtiter plates. A volume of 5 μl of the bacterial
suspensions containing 1.5×108 CFU/ml was added to the
test wells, which already contained 200 μl TSB with 0.25% glucose.
The wells were subsequently treated with 2 or 4 μl of 1,5-AF (1
mg/ml), resulting in a final concentration of 10 or 20 μg/ml,
respectively. To allow bacteria to form a biofilm, the microplates
were incubated for 18 h at 37°C without shaking. The cells were
then decanted, and the wells were washed gently four times with tap
water. The cells that remained in the wells were stained with 0.1%
crystal violet for 5 min. The wells were then washed four times
with tap water. The stained cells were resolved by the addition of
200 μl of 95% ethanol. Absorbance was measured at 570 nm using an
ELISA plate reader (ImmunoMini NJ-2300, Japan). Negative controls
(bacteria + TSB), vehicle controls (bacteria + TSB +
H2O) and media controls (TSB) were also included. 1,5-AF
activity was defined as the ratio of the absorbance of the biofilm
remaining after 1,5-AF treatment in comparison to the negative
control (15). All experiments
were performed in quintuplicate.
Statistical analysis
Results are expressed as the means ± SE. Differences
between the means were evaluated using an unpaired two-sided
Student’s t-test. A value of P<0.05 was considered
significant.
Results
Growth inhibition of coagulase-negative
staphylococci by 1,5-AF
Hand washing is the most fundamental method for
preventing nosocomial infections (16). Alcohol-based hand rubs are the
international gold standard method for hand washing (16), but may nonetheless prove
ineffective. We therefore examined whether 1,5-AF affects the
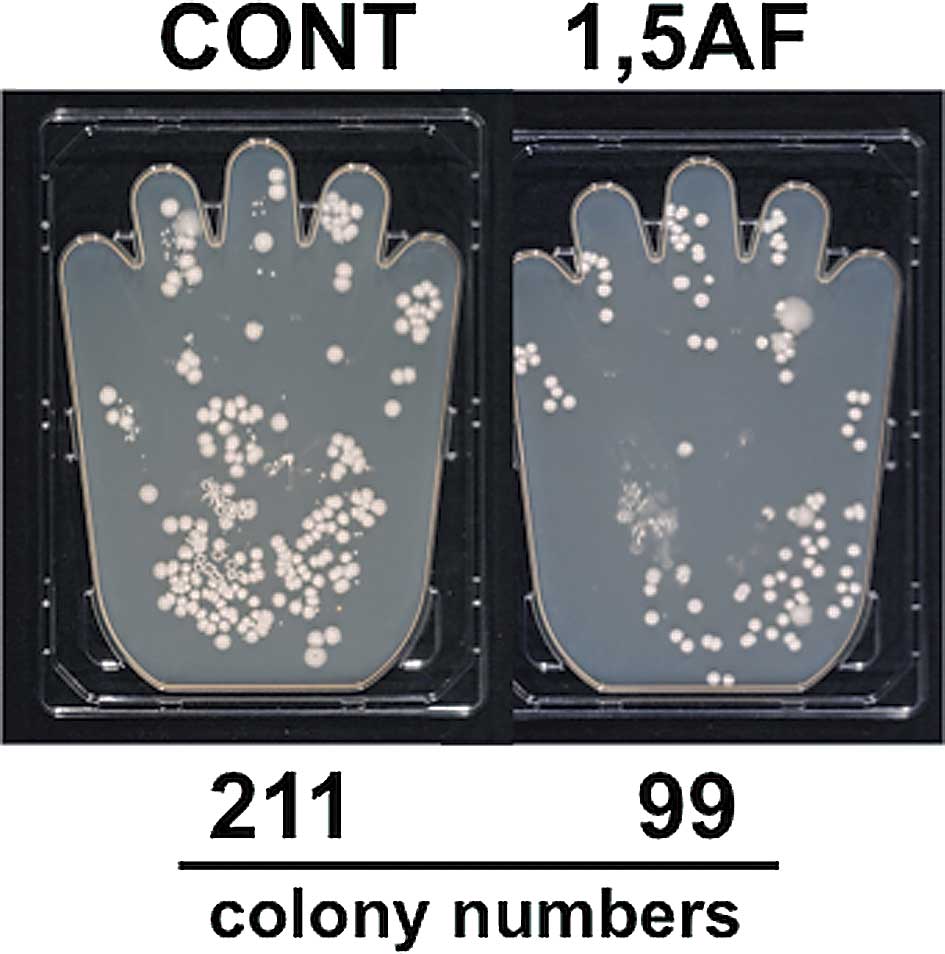
growth of CNS on CNS-contaminated hands. As shown in Fig. 1, 1,5-AF inhibited the growth of CNS
as compared to the control. Colony numbers of CNS from the hand
spread with 1,5-AF and 75% ethanol were 99, versus 211 from the
control hand spread with 75% ethanol alone.
Growth inhibition of S. epidermidis by
1,5-AF
To further investigate the antimicrobial effect of
1,5-AF, bacterial counts were collected from computer keyboards
S. epidermis was detected in a specimen from a computer
keyboard. (Fig. 2A). We then
examined whether 1,5-AF affected the growth of S.
epidermidis. The strain of S. epidermidis was incubated
with a 75% ethanol solution with or without the addition of 1%
1,5-AF. In the specimen treated with 1,5-AF (Fig. 2B, right), the growth of S.
epidermidis was inhibited as compared to the control (Fig. 2B, left).
Inhibition of MRSA biofilm formation by
1,5-AF
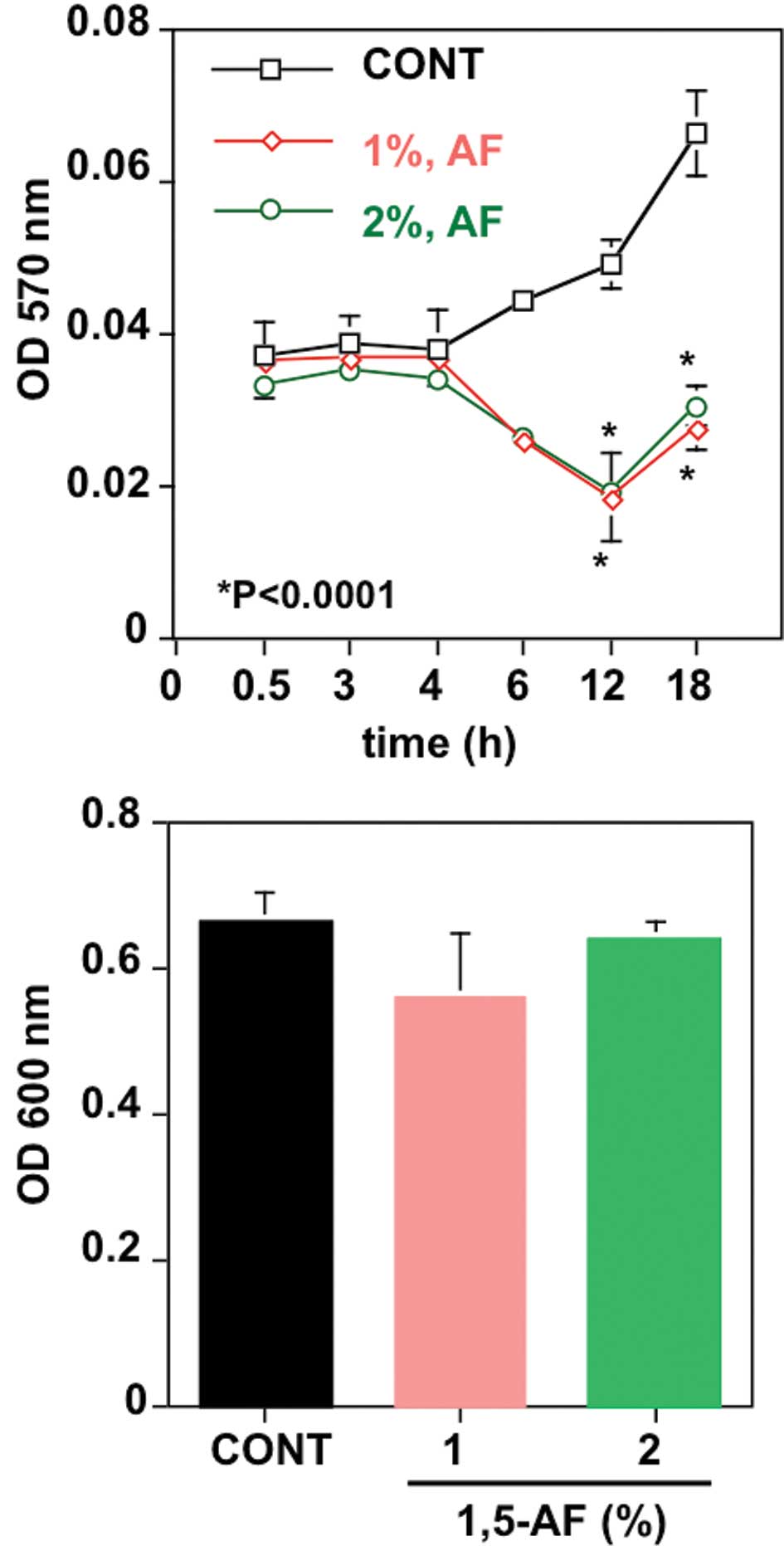
Lastly, we examined whether 1,5-AF inhibited the
biofilm formation of MRSA by means of a microtiter plate assay.
1,5-AF was found to significantly inhibit the biofilm formation of
MRSA (Fig. 3A). However, further
investigation revealed that 1,5-AF failed to induce the death of
MRSA (Fig. 3B).
Discussion
In the present study, we demonstrated that the
monosaccharide 1,5-AF had an antimicrobial effect on the
proliferation of CNS and S. epidermidis. Furthermore, 1,5-AF
significantly suppressed the formation of MRSA biofilm. These
results suggest that the antibiotic properties of 1,5-AF may be
clinically effective in preventing opportunistic infection and the
formation of biofilm, such as MRSA, with microbial resistance to
antibiotics.
In healthy subjects, internal tissues, such as the
blood, brain and muscle, are normally free of microorganisms.
However, surface tissues, such as the skin and mucous membranes,
are constantly in contact with environmental organisms and readily
become colonized by various microbial species. The normal flora of
humans consists of a few eukaryotic fungi and protists, with
bacteria being the most numerous and obvious microbial components.
CNS are among the normal flora of the human skin and mucous
membrane. CNS that are aerobic and gram-positive cocci are likely
to consist mainly of S. epidermidis. Opportunistic
infections have previously been shown to be caused by S.
epidermidis (16), and result
in nosocomial infections. Thus, inhibition of the increase in
opportunistic infections as well as overcoming microbial resistance
to antibiotics is essential for preventing nosocomial
infections.
It is believed that 1,5-AF may inhibit opportunistic
infections and microbial resistance to antibiotics. Recent studies
have found that 1,5-AF has multifunctional properties, including
acting as antioxidant for scavenging reactive oxygen species
induced by phorbol myristate acetate in THP-1 cells,
copper-mediated LDL oxidation, antiplatelet aggregation by thrombin
and anti-inflammation, and inhibition of the following:
translocation of nuclear factor-κB by lipopolysaccharide
stimulation, expression of inducible nitric oxide synthesis protein
in vitro, cytokines, including macrophage chemoattractant
protein, interleukin-6, and tumor necrosis factor (10,11,17,18).
1,5-AF contains pre-antimicrobial substances. These are first
converted to the intermediate enolone ascopyrone M, which is then
converted to the antimicrobial microthecin in fungi belonging to
morels, such as Morchella (M) costata and
M. vulgaris, and in the red algae Gracilariopsis
lemaneiformis (10). Our
results, which are consistent with those of previous studies
(10,11,17,18),
indicate that treatment with 1,5-AF suppresses the growth of CNS
and S. epidermidis. Additionally, pre-treatment with 1,5-AF
may suppress the growth of MRSA (data not shown). We also found
that, 1,5-AF inhibited MRSA biofilm formation, though it did not
inhibit MRSA growth. Thus, 1,5-AF has both a suppressive
(pre-treatment) and therapeutic (inhibition of biofilm formation)
effect. These findings suggest that 1,5-AF has important antibiotic
effects that could aid in the prevention of nosocomial
infections.
The present findings suggest that 1,5-AF may serve
as a novel natural antibiotic compound with properties including
the suppression of gram-positive bacteria growth and MRSA biofilm
formation. This novel function of 1,5-AF may be useful in the
treatment of infectious diseases.
Acknowledgements
We thank Nobue Uto and Tomomi Morizono
for excellent technical assistance. This study was supported by
research grants from the Ministry of Education, Culture, Sports,
Science and Technology of Japan (ID: 21390483, K.-I. K.).
References
|
1.
|
Coast J, Smith RD and Millar MR:
Superbugs: should antimicrobial resistance be included as a cost in
economic evaluation? Health Econ. 5:217–226. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lewis K: Riddle of biofilm resistance.
Antimicrob Agents Chemother. 45:999–1007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fux CA, Stoodley P, Hall-Stoodley L and
Costerton JW: Bacterial biofilms: a diagnostic and therapeutic
challenge. Expert Rev Anti Infect Ther. 1:667–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schachter B: Slimy business – the
biotechnology of biofilms. Nat Biotechnol. 21:361–365. 2003.
|
|
5.
|
Yu S, Bojsen K, Svensson B and Marcussen
J: α-l,4-Glucan lyases producing 1,5-anhydro-D-fructose from starch
and glycogen have sequence similarity to α-glucosidases. Biochim
Biophys Acta. 1433:1–15. 1999.
|
|
6.
|
Baute M-A, Baute R and Deffieux G: Fungal
enzymic activity degrading 1,4-a-D-glucans to
1,5,-D-anhydrofructose. Phytochemistry. 27:3401–3403. 1988.
View Article : Google Scholar
|
|
7.
|
Broberg A, Kenne L and Pedersen M:
Analysis of 1,5-anhydro-D-fructose, microthecin, and
4-deoxy-glycero-2,3-diulose in algae using gas chromatography-mass
spectrometry in selected ion monitoring mode. Anal Biochem.
268:35–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shiga Y, Kametani S, Kadokura T and
Akanuma H: 1,5-Anhydroglucitol promotes glycogenolysis in
Escherichia coli. J Biochem. 125:166–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kametani S, Shiga Y and Akanuma H: Hepatic
production of 1,5-anhydrofructose and 1,5-anhydroglucitol in rat by
the third glycogenolytic pathway. Eur J Biochem. 242:832–838. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yu S and Fiskesund R: The anhydrofructose
pathway and its possible role in stress response and signaling.
Biochim Biophys Acta. 1760:1314–1322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yamaji K, Sarker KP, Maruyama I and
Hizukuri S: Antioxidant effects of 1,5-anhydro-D-fructose, a new
natural sugar, in vitro. Planta Med. 68:16–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Christensen GD, Simpson WA, Younger JJ,
Baddour LM, Barrett FF, Melton DM and Beachey EH: Adherence of
coagulase-negative staphylococci to plastic tissue culture plates:
a quantitiative model for the adhesion of staphylococci to medical
devices. J Clin Microbiol. 22:996–1006. 1985.PubMed/NCBI
|
|
13.
|
Mango K, Nishi J, Wakimoto N, et al:
Biofilm formation by and accessory gene regulator typing of
methicillin-resistant Staphylococcus aureus strains
recovered from patients with nosocomial infections. Infect Control
Hosp Epidemiol. 27:188–190. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Isenberg HD: Clinical Microbiology
Procedures Handbook. ASM Press; Washington, DC: pp. 1.5.1–1.5.18.
2004
|
|
15.
|
Djordjevic D, Wiedmann M and
McLandsborough LA: Microtiter plate assay for assessment of
Listeria monocytogenes biofilm formation. Appl Environ Microbiol.
68:2950–2958. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Darmstadt GL, Nawshad Uddin Ahmed AS, Saha
SK, et al: Infection control practices reduce nosocomial infections
and mortality in preterm infants in Bangladesh. J Perinatol.
25:331–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Meng X, Kawahara K, Nawa Y, et al:
1,5-Anhydro-D-fructose attenuates lipopolysaccharide-induced
cytokine release via suppression of NF-kappaB p65 phosphorylation.
Biochem Biophys Res Commun. 380:343–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Meng X, Kawahara K, Matsushita K, et al:
Attenuation of LPS-induced iNOS expression by
1,5-anhydro-D-fructose. Biochem Biophys Res Commun. 387:42–46.
2009. View Article : Google Scholar : PubMed/NCBI
|