Introduction
Aldehyde dehydrogenase (ALDH) 1 is a predominant
isoform of the ALDH family in mammals, which oxidizes retinol to
retinoic acid at the early stage of stem cell differentiation
(1). ALDH1 is expressed in various
stem/progenitor cells, such as hematopoietic and neural stem cells
(1–3). In addition to normal tissues, a small
population of tumor cells expresses ALDH1 at a high level. The
ALDH1-high population of tumor cells is resistant to antitumor
agents and exhibits a tumorigenic potential, suggesting that ALDH1
is one of the markers for cancer-initiating cells (CICs) (1). The presence of a large number of
ALDH1-high cells is indicative of poor prognosis in cancers of the
breast (1,4), lung (5), pancreas (6), bladder (7) and prostate (8).
CICs were first identified in leukemia (9). Although lymphoma is the most common
disease among hematologic malignancies, CICs have not yet been
determined in lymphoma. In the present study, ALDH1 expression was
examined in tissues involved by diffuse large B-cell lymphoma
(DLBCL) to investigate the presence of CICs. Unexpectedly, no
lymphoma cells expressed ALDH1. Instead, ALDH1 expression was
detected in surrounding non-tumorigenic cells. Stromal cells
consist of vasculature, fibroblasts and inflammatory cells, such as
lymphocytes, macrophages and dendritic cells (DCs). It is well
known that stromal cells affect the character of tumors (10). For example, the macrophage
subpopulation named M1 produces interleukin (IL)-12 which promotes
tumoricidal responses, whereas another subpopulation, named M2,
produces IL-10 which aids in tumor progression (11). In the present study, ALDH1
expression in non-tumorigenic stromal cells of tissues involved by
DLBCL was evaluated, and its clinical implications were
examined.
Materials and methods
Clinical samples
Forty-six patients, who were diagnosed with DLBCL at
Osaka University Hospital during the period from April 2006 to
December 2009 and whose follow-up data could be accessed, were
examined. Clinicopathological findings in these 46 patients are
summarized in Table I. There were
19 men and 27 women with ages ranging from 29 to 78 years (median
age, 63). Location of DLBCL was nodal in 25 cases and extranodal in
21 cases. Immunohistochemically, the large lymphoid cells were
CD20+ and CD3−. According to the criteria
proposed by Hans et al (12), DLBCL is categorized into germinal
center-type B cell lymphoma (GCB) (CD10+ or
CD10−/bcl-6+/MUM1−) and non-GCB
(CD10−/bcl-6−/MUM1+) type. The
subtype of the studied DLBCL cases was GCB in 11 cases and non-GCB
in 35 cases. Although the number of females and non-GCB cases was
higher than that of males and GCB cases, there was no collection
bias. Histological specimens were fixed in 10% formalin and
routinely processed for paraffin-embedding. Paraffin-embedded
specimens were stored in a dark room at room temperature at the
Department of Pathology of Osaka University Hospital, and sectioned
(4 μm) at the time of staining and stained with H&E and
immunoperoxidase procedure.
 | Table I.Characteristics of the 46 DLBCL
patients. |
Table I.
Characteristics of the 46 DLBCL
patients.
| No. of patients |
|---|
| Gender | |
| Male | 19 |
| Female | 27 |
| Location | |
| Nodal | 25 |
| Extranodal | 21 |
| Subtype | |
| GCB | 11 |
| Non-GCB | 35 |
| Stage | |
| I | 18 |
| II | 7 |
| III | 4 |
| IV | 17 |
| Treatment | |
|
Chemo-radiotherapy | 10 |
| Chemotherapy
alone | 28 |
| Radiotherapy
alone | 1 |
| Chemotherapy after
surgery | 2 |
|
Transplantation | 4 |
| No treatment | 1 |
| Response to
therapies | |
| CR | 36 |
| PR/NC | 8 |
| PD | 1 |
Based on physical examination records, surgical
notes and pathological assessments of the specimens, the Ann Arbor
staging system was applied. Eighteen cases were at stage I, 7 at
stage II, 4 at stage III and 17 at stage IV. Ten patients received
a combination of radiotherapy and chemotherapy, 28 received
chemotherapy only, 2 received chemotherapy after surgery, 4
transplantation (3 auto- and 1 allo-transplantation), 1 received
radiation therapy only and 1 received no therapy. The clinical
outcome was evaluated according to the Guidelines of the
International Workshop to Standardize Response Criteria for
non-Hodgkin Lymphoma (13). The
characteristics of the patients, such as age, tumor location and
stage, in the present study were similar to those in previous
reports (14,15). The proportion of male DLBCL
patients in general was ∼55%, indicating that the proportion of
female patients was slightly high in the present study. The study
was approved by the Ethics Review Board of the Graduate School of
Medicine, Osaka University.
Immunohistochemistry
ALDH1 expression was immunohistochemically examined
using the anti-ALDH1 antibody (BD Biosciences, Franklin Lakes, NJ,
USA). The sections were incubated with anti-ALDH1 diluted at x100,
and subsequently processed using the ChemMate EnVision kit (Dako
A/S, Glostrup, Denmark). DAB (Dako) was used as a chromogen. As the
negative control, staining was carried out in the absence of the
primary antibody. For immunophenotyping of DLBCL, the following
monoclonal antibodies were used: CD20, CD3, Bcl-6, CD10 and MUM1
(Dako; dilution at 1:400, 1:50, 1:50, 1:100 and 1:100,
respectively). Stained sections were evaluated independently by two
pathologists (S.F. and E.M.).
Double staining of ALDH1 with CD20,
fascin and CD68
Double staining of ALDH1 with CD20 (a marker for
DLBCL cells), fascin (a marker for DCs; Dako) and CD68 (a marker
for macrophages; Dako) was carried out using the EnVision G/2
doublestain system (Dako) according to the manufacturer’s protocol.
For double staining with CD20, sections were first incubated with
the anti-CD20 antibody (1:100), colored with DAB and, subsequently,
ALDH1 expression was detected with Permanent Red (Dako). For double
staining with CD68 or fascin, sections were initially incubated
with ALDH1, colored with DAB and, subsequently, antigen retrieval
was carried out using a Pascal pressurized heating chamber (Dako).
After incubation with the anti-CD68 (1:100) or anti-fascin (1:100)
antibody, sections were colored with Permanent Red. Since red
fluorescence is released from Permanent Red, the red-color signal
was detected with a fluorescence microscope (Biozero, Keyence,
Osaka, Japan).
Statistical analysis
The Chi-square test was used to analyze the
correlation between ALDH1 expression and clinicopathological
characteristics of the lymphoma cases. Kaplan-Meier methods were
used to calculate the overall survival (OS) rate, and differences
in survival curves were evaluated with the log-rank test. p-values
of <0.05 were considered statistically significant.
Results
Immunohistochemical findings
The expression of ALDH1 was examined in 46 DLBCL
cases. The signals for ALDH1 were detected in two types of cells:
cells with ample cytoplasm and fine chromatin and those with
reticular structure (Fig. 1A). To
confirm the absence of ALDH1 signals in DLBCL cells, double
staining of ALDH1 and CD20 was performed; CD20+ large
cells (brown-colored cells) did not express ALDH1 (Fig. 1B and C).
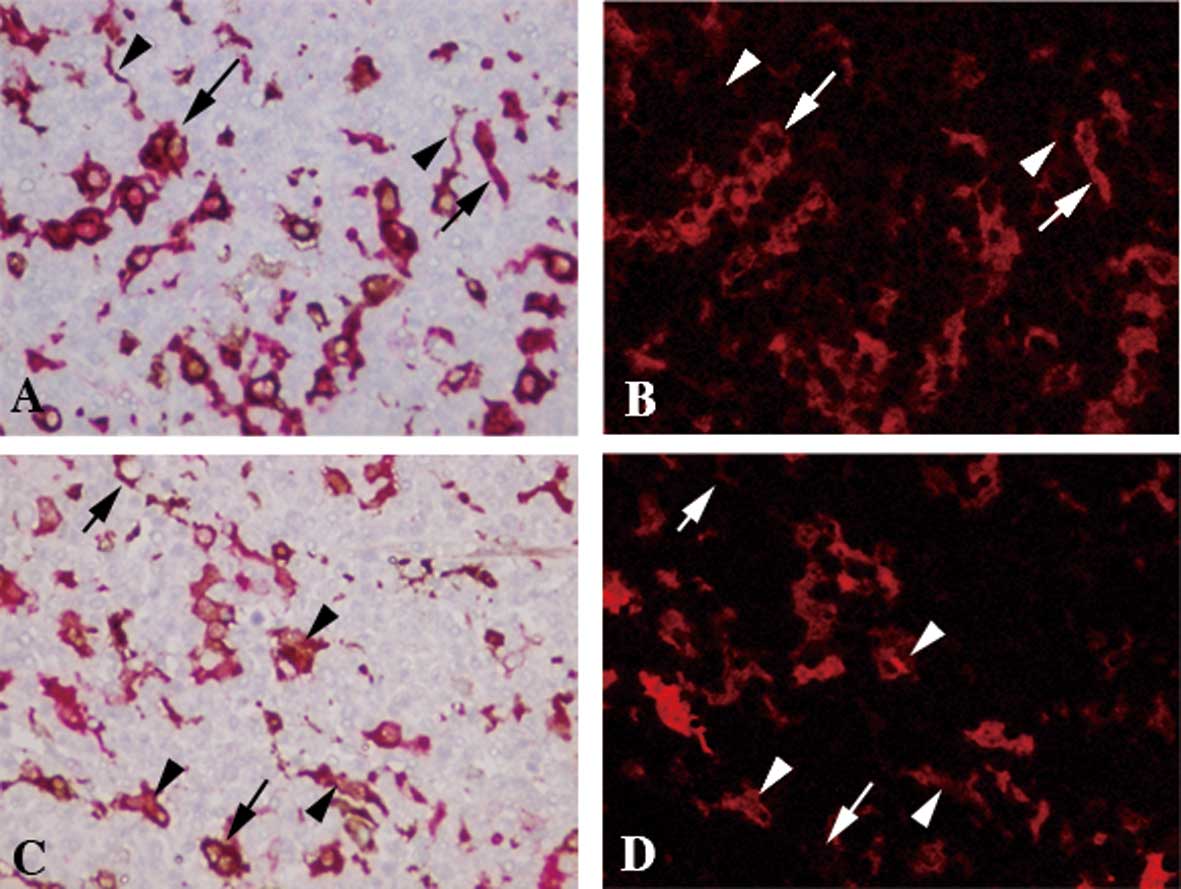
Double staining of ALDH1 and CD68
Since ALDH1+ cells with ample cytoplasm
and fine chromatin morphologically resembled macrophages, the
double staining of ALDH1 and CD68, a marker for macrophages, was
carried out. ALDH1+ cells with ample cytoplasm and fine
chromatin were positive for CD68 (Fig.
2A and B). By contrast, ALDH1+ cells with reticular
morphology were negative for CD68 (Fig. 2A and B).
Double staining of ALDH1 and fascin
Reticular morphology in ALDH1+ cells
suggested that a portion of the DCs was ALDH1+. To
confirm this, double staining of ALDH1 and fascin was performed.
ALDH1+ DC-like cells were positive for fascin (Fig. 2C and D).
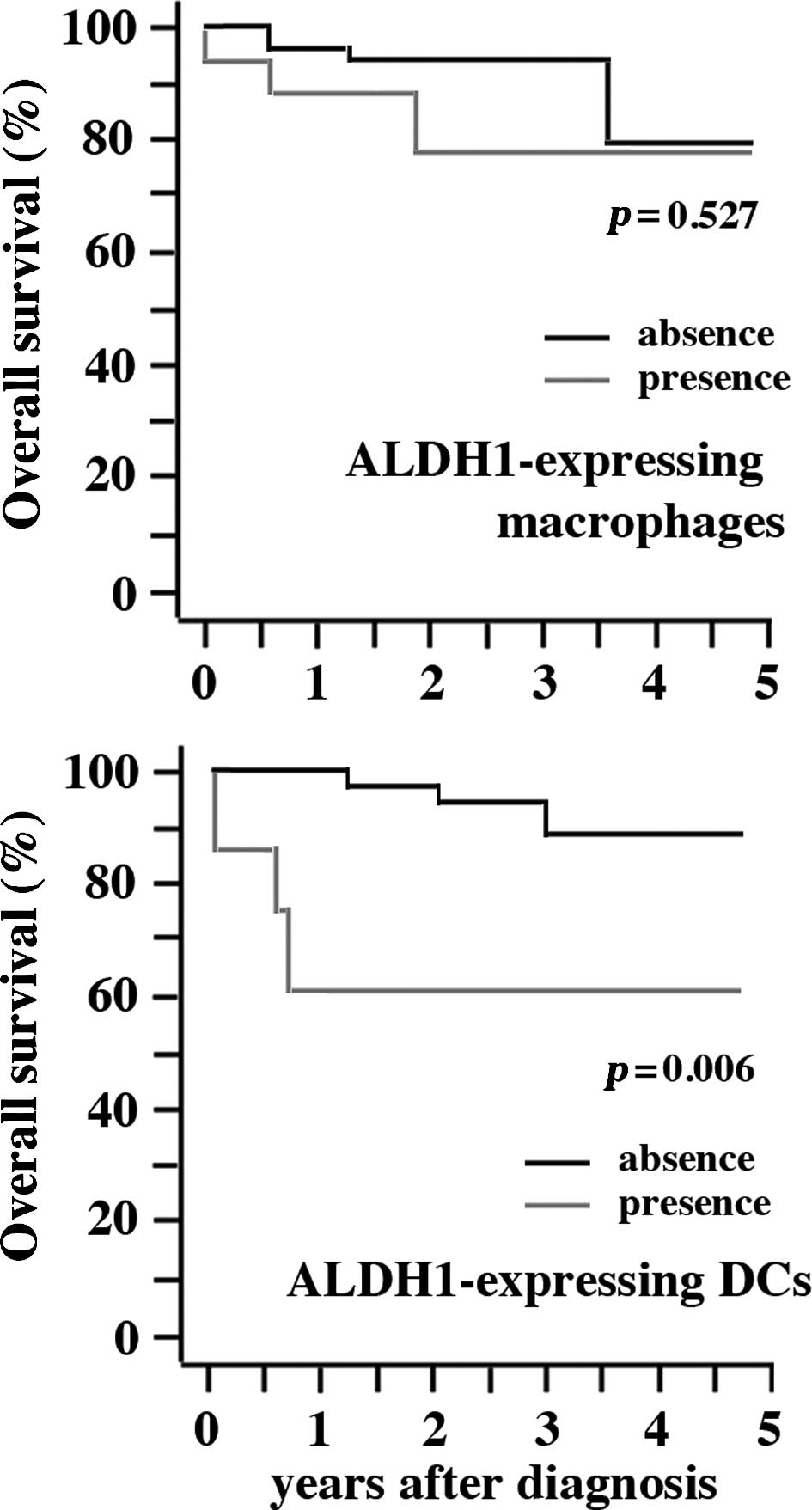
Clinical implication of ALDH1+
cells in DLBCL
Clinical significance of the presence of
ALDH1+ cells in DLBCL was examined. Seventeen and 7 of
46 cases possessed ALDH1+ macrophages and
ALDH1+ DCs, respectively. The presence of
ALDH1+ macrophages did not show any correlation with
gender, location, subtype, stage of diseases and response to
therapies (Table II). By contrast,
the presence of ALDH1+ DCs was correlated with location
(p=0.038) and response to therapies (p=0.039), although no
correlation was detected in gender, subtype and stage of disease
(Table III). There was a
statistically significant difference in OS between patients with
and without ALDH1+ DCs (p=0.006), although no difference
was detected between patients with and without ALDH1+
macrophages (Fig. 3).
 | Table II.Correlation between the presence of
ALDH1+ macrophages and clinicopathological
parameters. |
Table II.
Correlation between the presence of
ALDH1+ macrophages and clinicopathological
parameters.
| ALDH1+
macrophages
| p-value |
|---|
| Absent | Present | |
|---|
| Gender | | | 0.544 |
| Male | 11 | 8 | |
| Female | 18 | 9 | |
| Location | | | 0.090 |
| Nodal | 13 | 12 | |
| Extranodal | 16 | 5 | |
| Subtype | | | 0.964 |
| GCB | 7 | 4 | |
| Non-GCB | 22 | 13 | |
| Stage | | | 0.717 |
| I | 10 | 8 | |
| II | 5 | 2 | |
| III | 2 | 2 | |
| IV | 12 | 5 | |
| Response to
therapies | | | 0.752 |
| CR | 23 | 13 | |
| PR/NC | 5 | 3 | |
| PD | 1 | 0 | |
 | Table III.Correlation between the presence of
ALDH1+ DCs and clinicopathological parameters. |
Table III.
Correlation between the presence of
ALDH1+ DCs and clinicopathological parameters.
| ALDH1+
DCs
| p-value |
|---|
| Absent | Present | |
|---|
| Gender | | | 0.180 |
| Male | 14 | 5 | |
| Female | 24 | 3 | |
| Location | | | 0.038 |
| Nodal | 18 | 7 | |
| Extranodal | 20 | 1 | |
| Subtype | | | 0.938 |
| GCB | 9 | 2 | |
| Non-GCB | 29 | 6 | |
| Stage | | | 0.555 |
| I | 15 | 3 | |
| II | 7 | 0 | |
| III | 3 | 1 | |
| IV | 13 | 4 | |
| Response to
therapies | | | 0.039 |
| CR | 32 | 4 | |
| PR/NC | 6 | 2 | |
| PD | 0 | 1 | |
Discussion
ALDH1 is reported to be one of the markers for CICs
(1,8,16).
To search for the presence of CICs in DLBCL, the ALDH1+
cells were immunohistochemically evaluated in lymphoid tissues
involved by DLCBL. Unexpectedly, there were no ALDH1+
cells noted among the DLBCL cells. Instead, two types of cells in
the stromal tissues expressed ALDH1: one with ample cytoplasm and
fine chromatin, and another with reticular morphology. The former
was stained with anti-CD68, and the latter with anti-fascin,
indicating that ALDH1+ cells were macrophages and DCs,
respectively.
Recently, ALDH1 expression has been reported in
murine DCs derived from the gut (17), lung (5) and dermis (18). In humans, there have been no
reports on ALDH1 expression in DCs. To our knowledge, this is the
first report on ALDH1 expression in human DCs. Murine gut and
dermal ALDH1+ DCs induce regulatory T cells (18,19),
and thus may inhibit antitumorigenic immunity. The present study
revealed that DLBCL patients with ALDH1+ DCs were
associated with an unfavorable prognosis compared to those without,
suggesting that human ALDH1+ DCs may induce regulatory T
cells and inhibit antitumorigenic immunity. Further studies on the
function of human ALDH1+ DCs may clarify their precise
role in antitumorigenic immunity.
Recently, Guilliams et al found expression of
ALDH1 in macrophages in intestinal lamina propria, but did not
report on their function (18). To
our knowledge, this is the first report on ALDH1+
macrophages in humans, but failed to show the clinical implication.
The role of ALDH1 in macrophage function may be limited, at least
in lymphoid tissues involved by DLBCL.
In conclusion, ALDH1+ macrophages and
ALDH1+ DCs were found in the stromal tissues of DLBCL.
Although no clinical implications were found to be associated with
the presence of ALDH1+ macrophages, cases with
ALDH1+ DCs demonstrated a less favorable prognosis
compared to those without.
Acknowledgements
The authors thank Ms. Megumi Sugano,
Ms. Etsuko Maeno and Ms. Takako Sawamura for the technical
assistance. This study was supported by grants from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
References
|
1.
|
Ginestier C, Hur MH, Charafe-Jauffret E,
et al: ALDH1 is a marker of normal and malignant human mammary stem
cells and a predictor of poor clinical outcome. Cell Stem Cell.
1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Liu S, Ginestier C, Charafe-Jauffret E,
Foco H, Kleer CG, Merajver SD, Dontu G and Wicha MS: BRCA1
regulates human mammary stem/progenitor cell fate. Proc Natl Acad
Sci USA. 105:1680–1685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ibarra I, Erlich Y, Muthuswamy SK,
Sachidanandam R and Hannon GJ: A role for microRNAs in maintenance
of mouse mammary epithelial progenitor cells. Genes Dev.
21:3238–3243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Dave B and Chang J: Treatment resistance
in stem cells and breast cancer. J Mammary Gland Biol Neoplasia.
14:79–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak
J, Xing L, Wang H, Liu Z, Su Y, Stass SA and Katz RL: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rasheed ZA, Yang J, Wang Q, et al:
Prognostic significance of tumorigenic cells with mesenchymal
features in pancreatic adenocarcinoma. J Natl Cancer Inst.
102:340–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu
Z, Stass SA and Jiang F: Aldehyde dehydrogenase 1 A1-positive cell
population is enriched in tumor-initiating cells and associated
with progression of bladder cancer. Cancer Epidemiol Biomarkers
Prev. 19:327–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z,
Stass SA and Jiang F: ALDH1A1 is a marker for malignant prostate
stem cells and predictor of prostate cancer patients’ outcome. Lab
Invest. 90:234–244. 2010.PubMed/NCBI
|
|
9.
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Udagawa T and Wood M: Tumor-stromal cell
interactions and opportunities for therapeutic intervention. Curr
Opin Pharmacol. 10:369–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ma J, Liu L, Che G, Yu N, Dai F and You Z:
The M1 form of tumor-associated macrophages in non-small cell lung
cancer is positively associated with survival time. BMC Cancer.
10:1122010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hans CP, Weisenburger DD, Greiner TC, et
al: Confirmation of the molecular classification of diffuse large
B-cell lymphoma by immunohistochemistry using a tissue microarray.
Blood. 103:275–282. 2004. View Article : Google Scholar
|
|
13.
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin’s lymphomas. NCI Sponsored International
Working Group. J Clin Oncol. 17:1244–1253. 1999.PubMed/NCBI
|
|
14.
|
The Non-Hodgkin’s Lymphoma Classification
Project: A Clinical Evaluation of the International Lymphoma Study
Group Classification of Non-Hodgkin’s Lymphoma. Blood.
89:3909–3918. 1997.
|
|
15.
|
Armitage JO and Weisenburger DD: New
approach to classifying non-Hodgkin’s lymphomas: clinical features
of the major histologic subtypes. Non-Hodgkin’s Lymphoma
Classification Project. J Clin Oncol. 16:2780–2795. 1998.
|
|
16.
|
Huang EH, Hynes MJ, Zhang T, et al:
Aldehyde dehydrogenase 1 is a marker for normal and malignant human
colonic stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar
|
|
17.
|
Saurer L, McCullough KC and Summerfield A:
In vitro induction of mucosa-type dendritic cells by all-trans
retinoic acid. J Immunol. 179:3504–3514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Guilliams M, Crozat K, Henri S, et al:
Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic
cells that constitutively produce retinoic acid and induce Foxp3(+)
regulatory T cells. Blood. 115:1958–1968. 2010.PubMed/NCBI
|
|
19.
|
Coombes JL and Powrie F: Dendritic cells
in intestinal immune regulation. Nat Rev Immunol. 8:435–446. 2008.
View Article : Google Scholar : PubMed/NCBI
|