Introduction
Interleukin (IL)-8, also known as neutrophil
activating peptide, CXCL8, small inducible cytokine subfamily B
(SCY88) and granulocyte chemotactic protein-1 (GCP-1), is a
cytokine that belongs to the CXC chemokine family (1,2).
IL-8 is a small protein that is encoded by the IL-8 gene,
which is located on chromosome 4q12-q21 (3). IL-8 has been shown to be linked to a
number of conditions including viral bronchiolitis (4–6),
cystic fibrosis related pathophysiology of the lungs, infection and
other inflammatory diseases, including rheumatoid arthritis
(7). It plays a pivotal role in
the pathophysiology of sepsis. IL-8 is a powerful chemoattractant
for neutrophils, basophils and T cells, but not monocytes, although
the latter is a rich source of IL-8.
The action of IL-8 is mediated by its receptor,
IL-8R. IL-8R has two forms, IL-8RA and IL-8RB. IL-8RA is also known
as chemokine, CXC motif, receptor-1 (CXCR1), or IL-8R1.
IL-8RA is located on chromosome 2q35 (8,10).
IL-8RB is also known as chemokine, CXC motif, receptor-2 (CXCR2) or
IL-8R2 (8,9), and is located on chromosome 2q35. In
the body, monocytes and fibroblasts are rich sources of IL-8. It
was recently reported that the healing of diabetic and non-diabetic
leg ulcers following treatment with Lactobacillus plantarum,
increased such that, to a certain degree, it coincided with the
increase in the IL-8 production of the polymorphonuclear cells in
the wound base (11). It has also
been shown that IL-8 has an impact on the migration of fibroblasts
and endothelial cells.
IL-8 has been found to be highly stained in
keratinocytes (12). Keratinocytes
increased the expression of IL-8 in response to inflammatory
stimuli such as tissue plasminogen activator (tPA) (13). Other agents, include trypsin, which
increases and salbutamol, which inhibits the production of IL-8
from keratinocytes (14). Gingko
biloba extract also inhibits IL-8 secretion from keratinocytes
(15). IL-8RA and IL-8RB have been
found to be present in keratinocytes of the skin. IL-8 and IL-8R
have also been shown to be co-expressed in gingival keratinocytes
(16). In psoriasis, IL-8R has
been found to be overexpressed in keratinocytes, which may be
inhibited by FK-506, an anti-psoriasis compound (17).
The potential role of IL-8 as a cell migration and
chemotaxis factor indicates a significant role in wound healing.
Indeed, it has been demonstrated that IL-8 may be chemotactic for
human keratinocytes, which express IL-8Rs (18). In animal models using IL-8RB
knockout mice, there was a delayed rate of wound healing (19). This appears to be associated with a
delay in monocyte recruitment to the wounding space and a delay in
re-epithelialisation and angiogenesis.
The impact of IL-8 and the IL-8R in the context of
chronic wounds and clinical wound healing have otherwise not been
well investigated. Chronic wounds represent a major health issue.
Derived from multiple aetiology, including diabetes mellitus,
pressure sores and circulation conditions, chronic wounds are
costly to treat for the healthcare system. For example, in the UK,
there are approximately 200,000 patients with chronic wounds at any
given time, the care of which consumes more than 3% of the budget
of the NHS. However, the biology of wound healing and, in
particular, chronic wound healing are not yet fully understood.
Wound healing is a complex biological process that requires
temporal, special and co-ordinated action of multiple cell types,
including immune cells for combating possible pathogens,
fibroblasts for matrix deposition and remodelling, endothelial
cells for the angiogenic process and keratinocytes for
re-epithelialisation. IL-8 appears to play multiple roles in these
processes.
In the present study, we investigated the transcript
expression of IL-8RA and IL-8RB in a cohort of human wound tissues,
and report the significant link between the cytokine complex and
the fate of the wound. Furthermore, we have shown that IL-8 has a
direct effect on the migration of human keratinocytes.
Materials and methods
Cells and tissues
The human keratinocyte cell line, HaCaT, was
purchased from the German Cancer Institute and routinely maintained
in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10%
fetal calf serum (FCS) and antibiotics. A cohort of fresh, frozen
human wound tissues, acute wound tissues (n=10) and chronic wound
tissues from venous ulcers (n=14), were collected under an Ethics
Approval from clinics in the University Hospital of Wales, and used
for histology and gene expression analysis, as previously reported
(20,21).
Recombinant human IL-8 (rhIL-8) was obtained from
NBSB (Potters Bar, England, UK). Antibodies to human IL-8RA and
IL-8RB were from Santa Cruz Biotechnologies Inc. (Santa Cruz, CA,
USA). ERK inhibitor II (FR180204) and PLC-γ inhibitor (U73122) were
purchased from Calbiochem (Merck Chemicals Ltd, Nottingham, UK).
FAK inhibitor was from Tocris (Bristol, England), and Arp2/3
inhibitor was from Chemidiv Ltd (San Diego, CA, USA).
Tissue processing and preparation of
materials for protein and molecular investigations
Tissues were frozen and sectioned on a Leica
cryostat at 5–10 μM thickness. The sections were divided into two
portions: one section was processed for histological investigation
and the other for extraction of genetic materials. For histological
analysis, sections were laid on slides, prior to fixing and
blocking by hydrogen peroxide and methanol. The section was then
processed for H&E staining.
A large number of frozen sections were combined and
homogenised using a homogenizer in a RNA isolation buffer
(Triagent; Sigma-Aldrich, Poole, Dorset, UK). Total RNA was
subsequently separated and quantified using a
spectrophotometer.
Quantitative analysis of IL-8RA and
IL-8RB transcripts in cells and tissues
Total RNA was extracted from cell pellets using
Triagent obtained from Sigma-Aldrich. Fresh frozen wound tissues
were combined, resuspended in Triagent and homogenised using a
hand-held homogeniser (Fisher Scientific). Total RNA was extracted
and purified from the cells and tissue sections according to
manufacturer’s instructions. Equal amounts of RNA were reverse
transcribed into complementary DNA (cDNA) using a first-strand
reverse transcription (RT) kit from Bio-Rad (Hemel Hemstead, UK).
For quantitative analysis of the IL-8R transcripts, we employed a
real-time quantitative PCR assay, as previously reported (22–24).
Briefly, primers (sequences shown in Table I) were designed using the Beacon
Designer software and satisfied the following criteria: the
amplified region sits on an exon-exon joint region and is specific
to the respective target; the amplicon is smaller than 150 bp; a
sequence that was complementary to the probe was added to one of
the primers (routinely the reverse primer); and the annealing
temperature is, or is close to being, 55°C. The Ampliflor Uniprimer
system was used as the probe system. cDNA from cells and tissues,
together with a set of standards were amplified simultaneously, on
a IcyclerIQ5 system (Bio-Rad). The concentration of the
respective transcript was calculated from the standard curve, which
was simultaneously generated. The levels of the transcripts are
shown here as the respective transcript/glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) ratio.
 | Table I.Primers used in the quantitative
analysis of gene transcripts. |
Table I.
Primers used in the quantitative
analysis of gene transcripts.
| Molecule name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| IL-8RA |
TGGGGACTGTCTATGAATCT |
ACTGAACCTGACCGTACACATTTCCCAGGACCTCATA |
| IL-8RB |
TCAAATTCATATGTCTCAGCA |
ACTGAACCTGACCGTACAGTTGCCCATGTCCTCATA |
| IL-8 |
TCTCTTGGCAGCCTTCCT |
ACTGAACCTGACCGTACATGTCTTTATGCACTGACATCT |
| GAPDH |
CTGAGTACGTCGTGGAGTC |
ACTGAACCTGACCGTACACAGAGATGATGACCCTTTTG |
Electric cell-substrate impedance sensing
(ECIS)-based cell adhesion and cell migration assays
ECIS Ztheta and 96W1E arrays were used (Applied
Biophysics Inc, Troy, NY, USA) (23,25,26).
Following treatment of the array surface with a cysteine solution,
the arrays were incubated with complete medium for 1 h. The same
number of HaCaT cells were added into each well, with or without
IL-8 and small inhibitors. Cell adhesion was recorded immediately
following addition of the cells, at multiple frequencies, for up to
6 h. For the cell migration assay, confluent HaCaT cells with or
without IL-8, and small signalling inhibitors, were wounded for 20
sec at 6 V. The migration of the cells was immediately traced
following wounding for up to 18 h, again with multiple frequencies.
Cell behaviour was modelled using the Rb method, using a cell-free
well as a reference unit. Cell migration and adhesion are shown
here as the change in resistance.
Statistical analysis was carried out using Minitab.
For normality test, the Anderson-Darling test was used, and for
statistical differences the Student’s t-test and Mann-Whitney U
test were used as appropriate.
Results
IL-8 markedly increased the migration of
keratinocytes
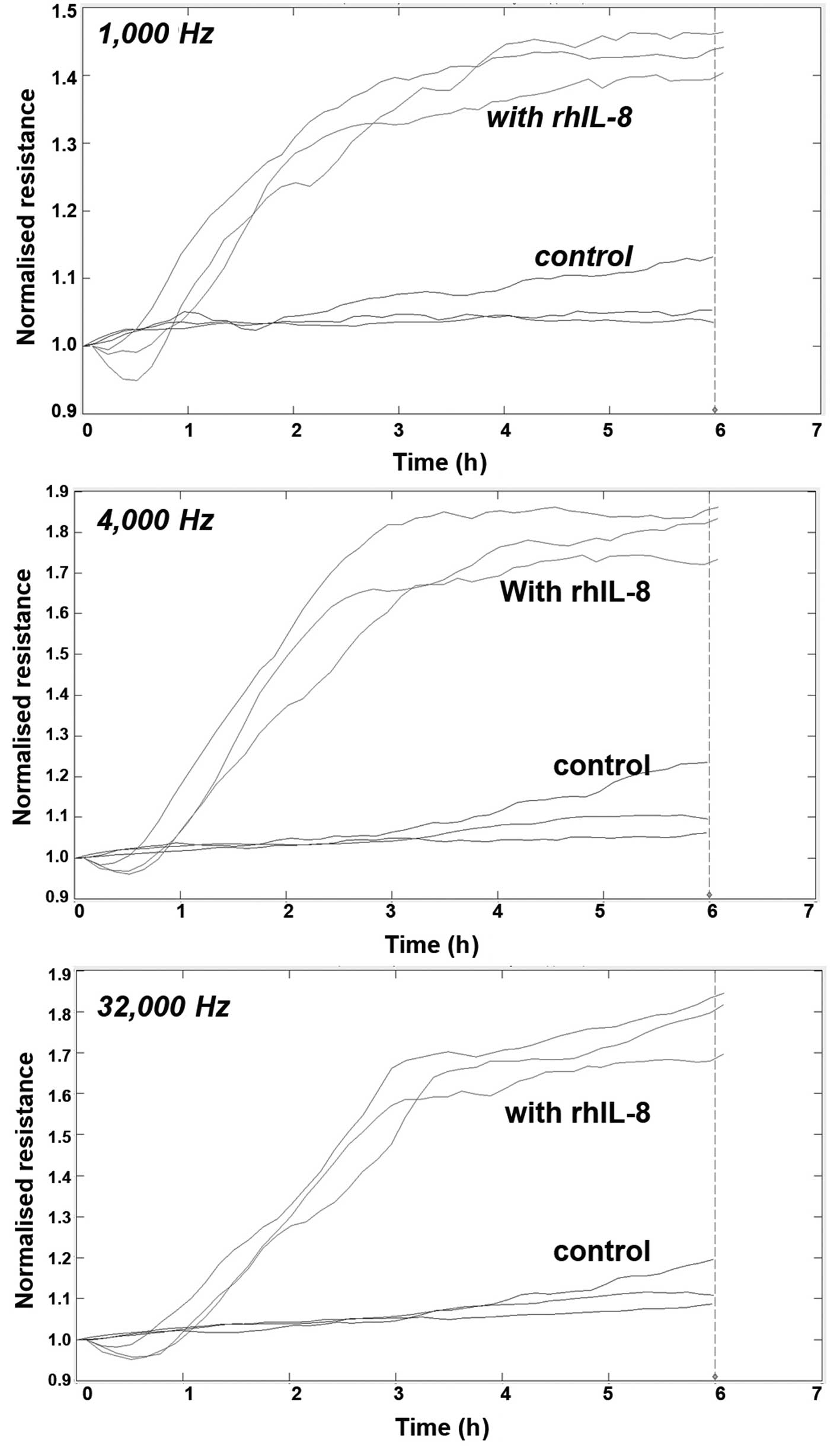
Using electrical wounding methods, we first tested
the impact of rhIL-8 on the migration of HaCaT cells following
wounding. As seen in Fig. 1, IL-8
markedly increased the migration of the cells (Fig. 1).
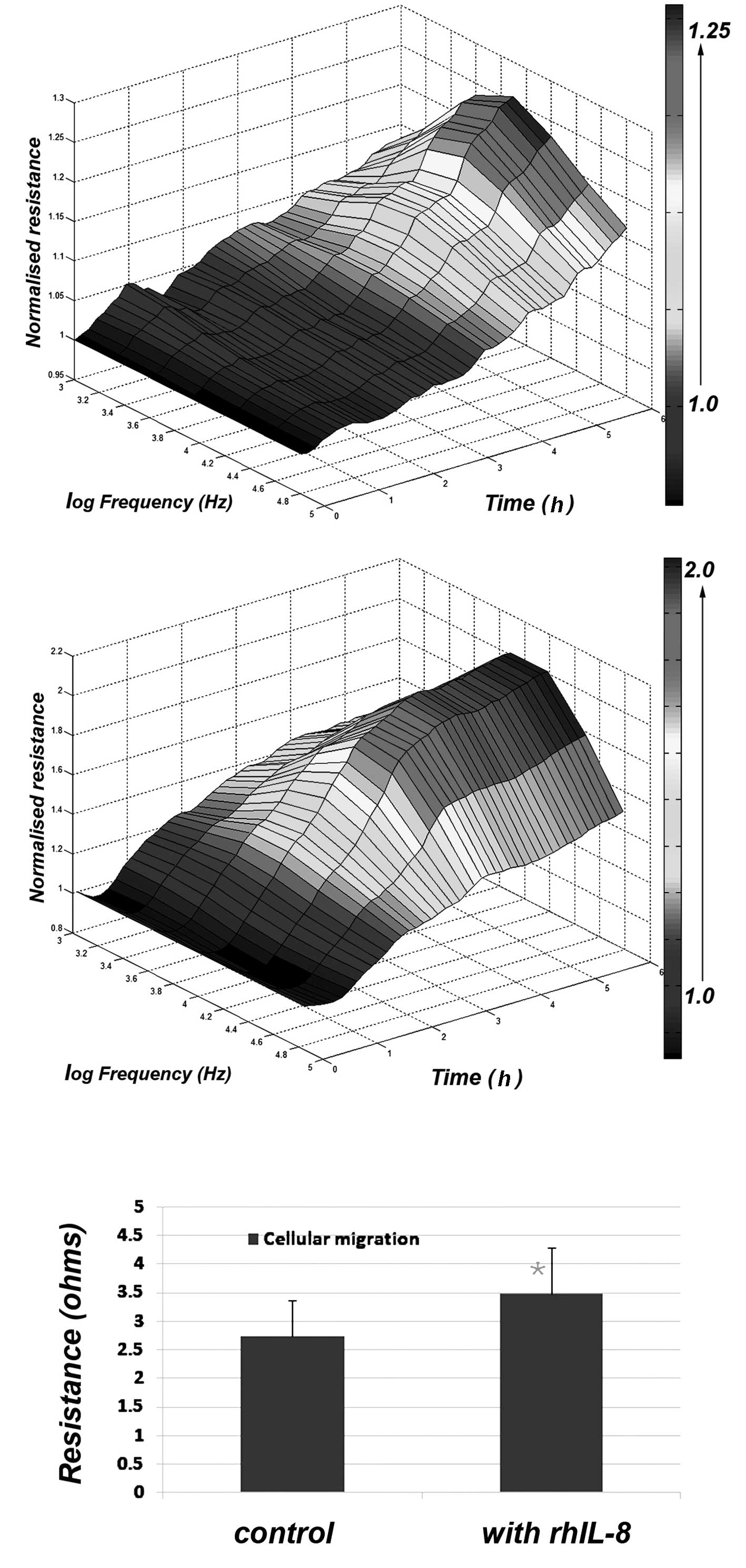
Using 3-D cellular modelling, the differences
between control and IL-8-treated cells were clearly visible
(Fig. 2) with the maximum effects
observed at a frequency of 4,000 Hz. This difference was
statistically significant (p<0.01) (Fig. 2).
IL-8 had a significant effect on the
adhesion of keratinocytes
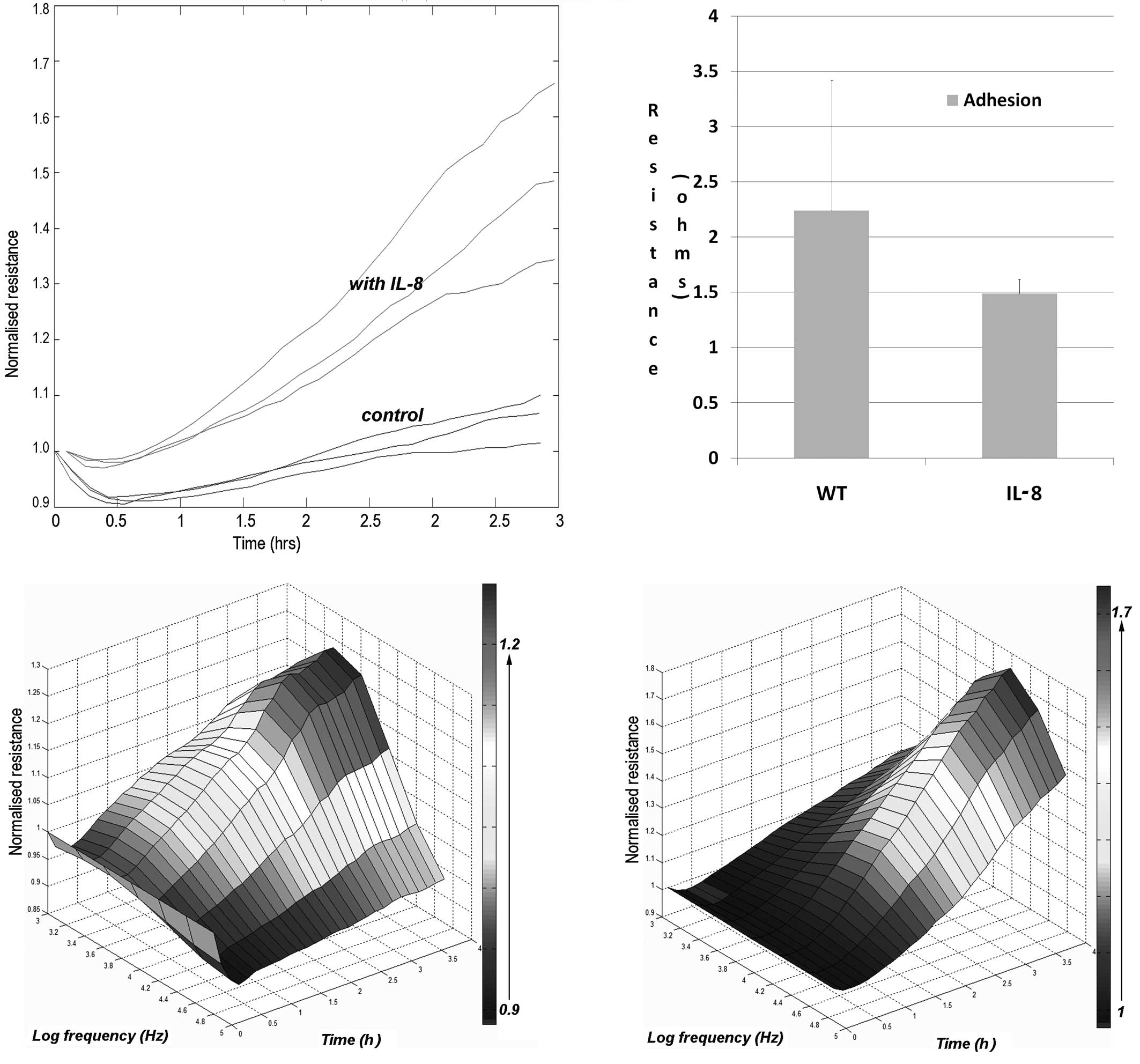
We further tested to see whether IL-8 had an effect
on the adhesion of keratinocytes to the matrix. Again, using the
ECIS attachment method, we evaluated the rate of cell adhesion. As
shown in Fig. 3, the presence of
IL-8 markedly increased the adhesion of the cells.
Potential signalling pathways in the
action of IL-8
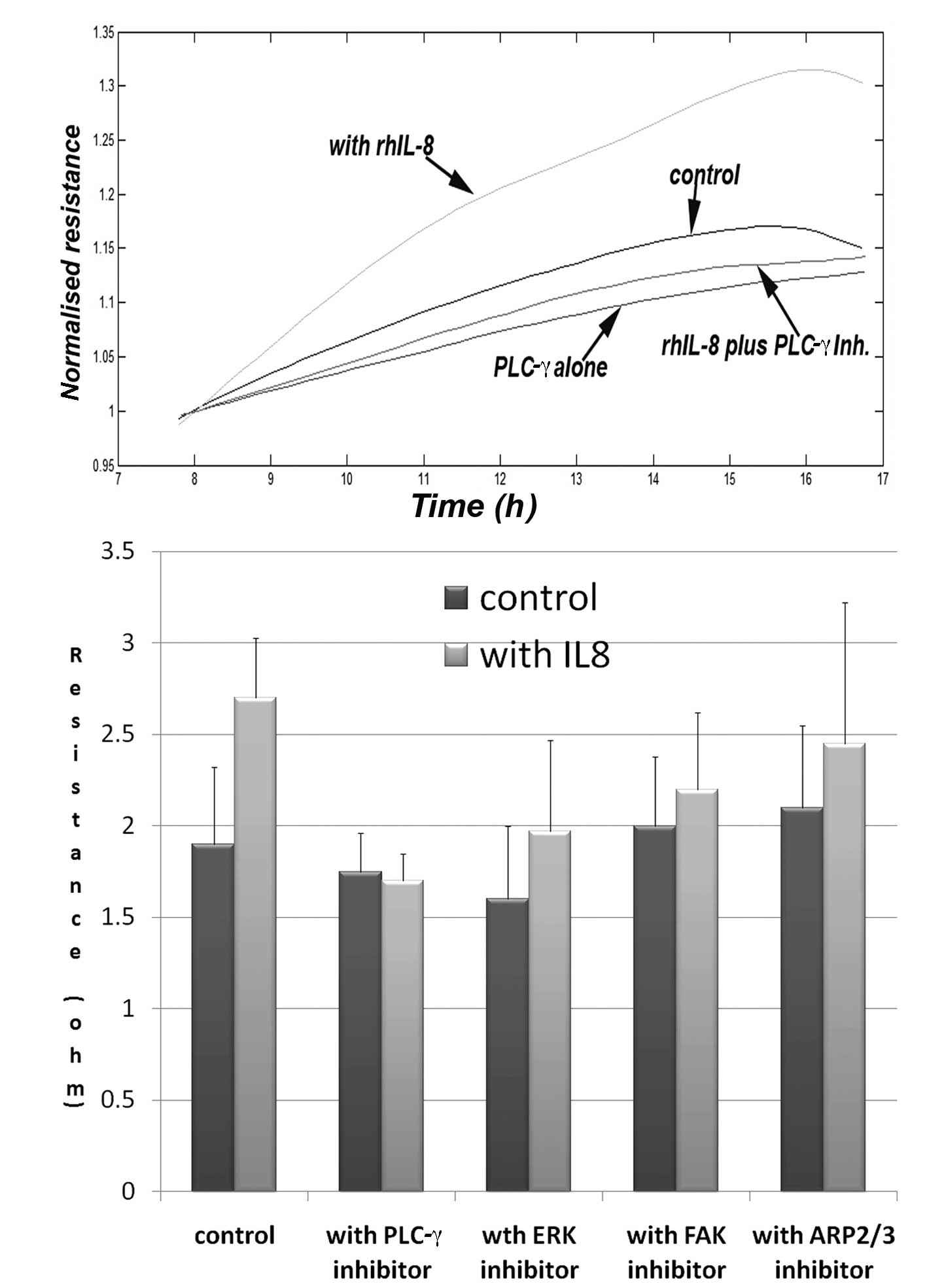
In order to explore potential pathways involved in
the aforementioned action, we used a panel of small inhibitors to
some of the known pathways downstream of the IL-8R. We have
demonstrated that the PLC-γ inhibitor markedly inhibited the
increased migration induced by IL-8, and brought the migration to
the control level. The same inhibitor has an inhibitory effect on
cell adhesion, although this is yet to reach significance (data not
shown). The ERK inhibitor also had an inhibitory effect on the
migration of keratinocytes, but did not completely eliminate the
action of IL-8. The FAK and ARP2/3 inhibitors had marginal effects
(Fig. 4).
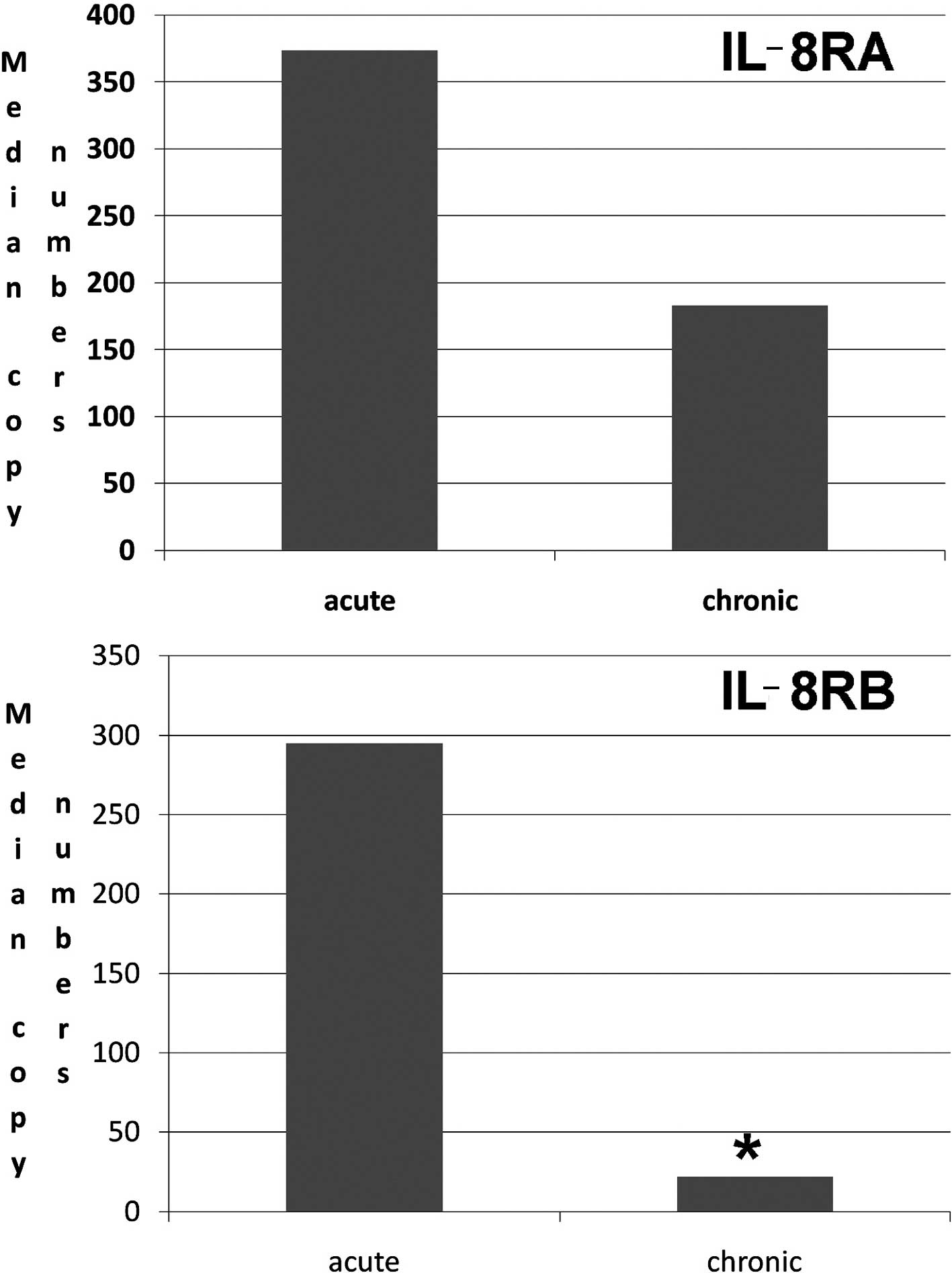
Human chronic wound tissues had a marked
low level of IL-8RB
We investigated the transcript levels of the IL-8Rs,
IL-8RA and IL-8RB in acute and chronic wound tissues. As shown in
Fig. 5, there were significantly
lower levels of IL-8RB in chronic wound tissues than in acute
tissues (Fig. 5). Although the
levels of IL-8RA tended to be lower, this was not statistically
significant.
Discussion
The present study reports that the status of the
IL-8R, IL-8RB, is linked to clinical outcome in chronic wounds.
Furthermore, this study further demonstrates that IL-8 has a direct
impact on the migration of human keratinocytes.
We show that IL-8 has a profound effect on the
migration of keratinocytes. It has previously been shown that IL-8
may act as a chemoattractant to keratinocytes, in that
keratinocytes may respond to the gradient change of IL-8. In the
present study, we used a novel method, ECIS, and an electrical
wounding model. In this model, cells were wounded by an electric
current, and the migration of cells from the surrounding areas was
traced in the prescence of IL-8. The data presented here,
therefore, clearly indicate that IL-8 also induces migration, most
likely in the form of chemokinesis, of keratinocytes, another
crucial form of cell migration.
CXC chemokine-induced chemotaxis involves a number
of intracellular signalling pathways, classically the JAK,
Src/PI3K, FAK and PLC pathways (27–30).
The present study clearly demonstrates that the IL-8-induced
migration of HaCaT cells (chemokinesis) critically requires the
PLC-γ pathway, in that the PLC-γ inhibitor suppressed IL-8-induced
migration and completely blocked the action of IL-8. By contrast,
FAK, ARP2/3 and ERK inhibitors do not appear to signficantly affect
the action of IL-8.
It has previously been shown that IL-8 is associated
with wound healing in vivo (19). In an IL-8RB knockout mouse model,
it was found that the rate of wound healing was reduced, thought to
be the result of a reduction in monocyte recruitment to the
wounding space, reduced angiogenesis and re-epithelialisation. In
this study, we provide further evidence that IL-8 itself has a
direct impact on the re-epithelialisation of keratinocytes.
Despite the evidence, in vitro and in
vivo, that IL-8 and IL-8Rs have an impact on wound healing, it
is not known how the IL-8Rs are linked to the healing of human
wounds. The present study provides first-line evidence that in
chronic wounds, the levels of IL-8RA and, in particular, IL-8RB are
significantly lower when compared with acute wound tissues. This is
noteworthy, as it indicates that a loss or reduction of IL-8Rs may
be an essential part of the mechanisms of why wounds are not
healing or responding to IL-8. It is highly plausible that the low
levels of IL-8Rs rendered cells necessary for wound healing
irresponsive or poorly responsive to IL-8, a cytokine that appears
to be involved in the wound healing process. Together with in
vitro data and evidence from animal studies, it is suggested
that IL-8 and IL-8Rs are a useful indicator for the nature of
healing of a given wound. This further suggests that IL-8 may be a
means to manipulate the healing of wound tissues and may have a
therapeutic value. This is likely to be manifested by the direct
effect of IL-8 on keratinocytes, on endothelial cells, through the
induction of angiogenesis, on immune cells for combating infection
and fibroblasts during the tissue modelling process of wound
healing. Of course, IL-8 is not at all a clear wound healer without
concerns. It has been shown that IL-8RB may mediate skin
carcinogenesis when overexpressed or repeatedly induced (30).
In conclusion, IL-8 has a profound impact on
keratinocytes by increasing the rate of cell migration. Together
with the observation that IL-8Rs are markedly lower in chronic than
in acute wounds, it is suggested that IL-8 and its receptors have
prognostic and therapeutic value in difficult-healing wounds.
Acknowledgements
The authors wish to thank Dr Kevin
Conway for his help in the collection of tissues. The authors also
wish to thank the Welsh Government (A4B programme), Cardiff
Partnership Fund, Cancer Research Wales and Albert Hung Foundation
for their support.
References
|
1.
|
J Van DammeB DecockR ConingsJP LenaertsG
OpdenakkerA BilliauThe chemotactic activity for granulocytes
produced by virally infected fibroblasts is identical to
monocyte-derived interleukin 8Eur J
Immunol191189119419892668011
|
|
2.
|
SD WolpeA CeramiMacrophage inflammatory
proteins 1 and 2: members of a novel superfamily of cytokinesFASEB
J32565257319892687068
|
|
3.
|
WS ModiZQ ChenLocalization of the human
CXC chemokine subfamily on the long arm of chromosome 4 using
radiation
hybridsGenomics47136139199810.1006/geno.1997.51009465307
|
|
4.
|
J HullA ThomsonD KwiatkowskiAssociation of
respiratory syncytial virus bronchiolitis with the interleukin 8
gene region in UK
familiesThorax5510231027200010.1136/thorax.55.12.102311083887
|
|
5.
|
RL SmythK MobbsU O’HeaD AshbyCA HartThe
association between disease severity, cytokines and virus genotype
in infants with respiratory syncytial virus (RSV) bronchiolitisArch
Dis Child82Suppl 1A4A52000
|
|
6.
|
J HullK RowlandsE LockhartM SharlandC
MooreN HanchardDP KwiatkowskiHaplotype mapping of the bronchiolitis
susceptibility locus near IL8Hum
Genet114272279200410.1007/s00439-003-1038-x14605870
|
|
7.
|
M SrivastavaO EidelmanJ ZhangC PaweletzH
CaohuyQ YangKA JacobsonE HeldmanW HuangC JozwikBS PollardHB
PollardDigitoxin mimics gene therapy with CFTR and suppresses
hypersecretion of IL-8 from cystic fibrosis lung epithelial
cellsProc Nat Acad Sci
USA10176937698200410.1073/pnas.040203010115136726
|
|
8.
|
SW MorrisN NelsonMB ValentineDN ShapiroAT
LookCJ KozloskyMP BeckmannDP CerrettiAssignment of the genes
encoding human interleukin-8 receptor types 1 and 2 and an
interleukin-8 receptor pseudogene to chromosome
2q35Genomics14685691199210.1016/S0888-7543(05)80169-71427896
|
|
9.
|
C MollereauF MuscatelliMG MatteiG VassartM
ParmentierThe high-affinity interleukin 8 receptor gene (IL8RA)
maps to the 2q33-q36 region of the human genome: cloning of a
pseudogene (IL8RBP) for the low-affinity
receptorGenomics16248251199310.1006/geno.1993.11678486366
|
|
10.
|
PM MurphyHL TiffanyCloning of
complementary DNA encoding a functional human interleukin-8
receptorScience25312801283199110.1126/science.1891716
|
|
11.
|
MC PeralMM RachidNM GobbatoMA Huaman
MartinezJC ValdezInterleukin-8 production by polymorphonuclear
leukocytes from patients with chronic infected leg ulcers treated
with Lactobacillus plantarumClin Microbiol
Infect16281286201010.1111/j.1469-0691.2009.02793.x19519855
|
|
12.
|
M SticherlingE BornscheuerJM SchröderE
ChristophersLocalization of neutrophil-activating
peptide-1/interleukin-8-immunoreactivity in normal and psoriatic
skinJ Invest
Dermatol962630199110.1111/1523-1747.ep125146891702820
|
|
13.
|
C CataissonAJ PearsonMZ TsienF MasciaJL
GaoS PastoreSH YuspaCXCR2 ligands and G-CSF mediate
PKCalpha-induced intraepidermal inflammationJ Clin
Invest11627572766200610.1172/JCI2751416964312
|
|
14.
|
FR WetteyL XueR PettipherSalbutamol
inhibits trypsin-mediated production of CXCL8 by
keratinocytesCytokine362934200610.1016/j.cyto.2006.10.00817161617
|
|
15.
|
S TrompezinskiM BonnevilleI PernetA DenisD
SchmittJ ViacGingko biloba extract reduces VEGF and CXCL-8/ IL-8
levels in keratinocytes with cumulative effect with
epigallocatechin-3-gallateArch Dermatol
Res302183189201010.1007/s00403-009-0979-x19597830
|
|
16.
|
A SfakianakisCE BarrDL
KreutzerLocalization of the chemokine interleukin-8 and
interleukin-8 receptors in human gingiva and cultured gingival
keratinocytesJ Periodontal
Res37154160200210.1034/j.1600-0765.2002.00024.x12009185
|
|
17.
|
BS SchulzG MichelS WagnerR SüssA BeetzRU
PeterL KeményT RuzickaIncreased expression of epidermal IL-8
receptor in psoriasis. Down-regulation by FK-506 in vitroJ
Immunol1514399440619937691948
|
|
18.
|
G MichelL KeményRU PeterA BeetzC RiedP
ArenbergerT RuzickaInterleukin-8 receptor-mediated chemotaxis of
normal human epidermal cellsFEBS
Lett305241243199210.1016/0014-5793(92)80677-91299623
|
|
19.
|
RM DevalarajaLB NanneyJ DuQ QianY YuMN
DevalarajaA RichmondDelayed wound healing in CXCR2 knockout miceJ
Invest
Dermatol115234244200010.1046/j.1523-1747.2000.00034.x10951241
|
|
20.
|
K ConwayG HarrisonP PriceKG HardingWG
JiangExpression of HGF, HGF receptor and the JHGF regulators in the
acute and chronic wounds in the lower limbWound Repair
Reg156836922007
|
|
21.
|
K ConwayG HarrisonP PriceKG HardingWG
JiangVascular endothelial growth inhibitor represents an important
target for therapeutic angiogenesis in the lower limbInt Wound
J455642007
|
|
22.
|
IA NazarenkoSK BhatnagarRJ HohmanA closed
tube format for amplification and detection of DNA based on energy
transferNucleic Acids
Res2525162521199710.1093/nar/25.12.25169171107
|
|
23.
|
WG JiangL YeG PatelKG HardingExpression of
WAVES, the WASP (Wiskott-Aldrich Syndrome Protein) family of
Verprolin Homologous proteins, in human wound tissues determines
the fate of wound healingWound Repair
Reg18594604201010.1111/j.1524-475X.2010.00630.x
|
|
24.
|
WG JiangG DaviesTA MartinC ParrG WatkinsMD
MasonRE ManselExpression of membrane type-1 matrix
metalloproteinase, MT1-MMP in human breast cancer and its impact on
invasiveness of breast cancer cellsInt J Mol
Med17583590200616525713
|
|
25.
|
CR KeeseJ WegenerSR WalkerI
GiaeverElectrical wound-healing assay for cells in vitroProc Natl
Acad Sci USA10115541559200410.1073/pnas.030758810014747654
|
|
26.
|
WG JiangTA MartinJ Russell-LewisL YeA
Douglas-JonesRE ManselEplin-alpha expression in human breast
cancer, the impact on cellular migration and clinical outcomeMol
Cancer771200810.1186/1476-4598-7-7118796137
|
|
27.
|
WE HolmesJ LeeWJ KuangGC RiceWI
WoodStructure and functional expression of a human interleukin-8
receptorScience25312781280199110.1126/science.1840701
|
|
28.
|
LC BorishJW SteinkeCytokines and
chemokinesJ Allergy Clin Immunol111Suppl
2S460S475200310.1067/mai.2003.10812592293
|
|
29.
|
H SprengerAR LloydLL LautensTI BonnerDJ
KelvinStructure, genomic organization, and expression of the human
interleukin-8 receptor B geneJ Biol
Chem269110651107219947512557
|
|
30.
|
C CataissonR OhmanG PatelA PearsonM TsienS
JayL WrightH HenningsSH YuspaInducible cutaneous inflammation
reveals a protumorigenic role for keratinocyte CXCR2 in skin
carcinogenesisCancer
Res69319328200910.1158/0008-5472.CAN-08-249019118017
|