Introduction
Over the years, advances in surgical resection
procedures, chemotherapeutic agents and molecular-targeted drugs
have led to an increase in the number of patients undergoing
hepatectomy following neoadjuvant chemotherapy (NAC) for multiple
liver metastases from colorectal cancer. No recurrence is generally
observed in the remnant liver following complete surgical tumor
resection. However, recurrence occurs in the remnant liver in a
number of patients, indicating incomplete cancer resection or the
persistence of micrometastases not visualized by imaging studies
(1). Furthermore, if the future
remnant liver volume and hepatic functional reserve are
insufficient for the resection of multiple liver metastases,
two-stage hepatectomy for initially unresectable multiple
colorectal hepatic metastases is occasionally performed, in which
case the cancer is knowingly left behind (2). Such patients at high risk of remnant
liver recurrence need to undergo chemotherapeutic therapy as
adjuvant chemotherapy (AC) and molecular-targeted therapy in the
early post-operative period, as the levels of tumor growth factors,
such as the vascular endothelial growth factor (VEGF), basic
fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF),
are higher in the liver tissue of patients with colorectal liver
metastases than in that of healthy controls, and become even higher
following hepatectomy, due to liver regeneration (3,4). It
is well-known that when chemotherapy or molecular-targeted therapy
is withheld following hepatectomy, the residual tumor enlarges
(5). VEGF plays a central role in
angiogenesis, and the VEGF-neutralizing antibody, bevacizumab, is
one of the most useful drugs in suppressing tumor growth. However,
VEGF has also been shown to increase the production of growth
factors, such as HGF, and induce bone marrow cells, including
vascular endothelial precursor cells, at sites of injury. This
means that VEGF is involved not only in angiogenesis but also in
liver cell regeneration following hepatectomy (6). In other words, there is concern that
the administration of bevacizumab in the early period following
hepatectomy may negatively affect the hypertrophy of the remnant
liver and recovery of the liver function. The purpose of this study
was to evaluate the effect of bevacizumab on the regeneration of
the remnant liver following hepatectomy and to investigate whether
bevacizumab exhibits an antitumor effect on the residual tumor
during the phase of liver regeneration, during which various growth
factors are secreted.
Materials and methods
Cell line and assay
The murine colon cancer cell line, CT26, which is an
N-nitroso-N-methylurethane-induced undifferentiated adenocarcinoma
of the colon, syngeneic with the BALB/c mouse was purchased from
the American Type Culture Collection (Manassas, VA, USA) for the
purposes of this study. It was grown in cell culture as monolayers
in RPMI-1640 medium with 2 mM L-glutamine (Gibco Life Technologies
Japan Ltd., Tokyo, Japan) supplemented with 10% fetal calf serum
(FCS; Sigma, St. Louis, MO, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified atmosphere containing 5%
CO2. To evaluate the VEGF protein expression of each
mouse, we used a Quantikine mouse VEGF immunoassay (R&D Systems
Inc., Minneapolis, MN). The fluorescence of VEGF and the protein
expression of the cells were normalized to those of the quantity of
these proteins at the known level.
Animals
Animals were maintained at the Animal Care and Use
Facilities at Tokyo Medical University Hospital under specific
pathogen-free conditions. All experiments were approved by the
Animal Care and Ethics Committee of Tokyo Medical University.
Female BALB/c mice (CLEA Japan Inc., Tokyo, Japan) with a body
weight (BW) of 20–22 g were used. The animals were housed in single
cages at a room temperature of 22–24°C and a relative humidity of
60–65% with a 12-h light/dark cycle environment. For all the
operative procedures, animals were anesthetized by intraperitoneal
(i.p.) injection of 50 mg/kg BW pentobarbital sodium. Mice were
placed on heated pads and the transverse upper abdominal incision
was used to expose all liver lobes. A medium-large hemostatic clip
(Ligaclip MCA, Ethicon Endo-surgery, Inc., Cincinnati, OH, USA) was
applied across the pedicle of the right anterior (RA) and the left
anterior (LA) lobes. These lobes were cut distal to the allied
clip. The RA and LA lobes, which were equivalent to a hepatectomy
of approximately 33% of the total liver mass, were removed. The
abdomen was irrigated with 10ml of sterile saline and closed
(7). The cell suspension of CT26,
which included 2×105 cell/25 μl phosphate-buffered
saline (PBS), was mixed with 25μl in Matrigel matrix (BD
Biosciences, San Jose, CA, USA) on ice in a 1:1 ratio. The mixture
was implanted into the surface of the left posterior (LP) lobe by
using a 27 G needle under direct visualization. The puncture site
was compressed using a cotton swab for 3 min to stop the bleeding.
The negative control group was injected with Matrigel alone.
Bevacizumab (4 mg/kg) or human IgG (Sigma) was administered by i.p.
injection 3 times a week from the day prior to (−1 day) to 10 days
after CT26 cell implantation (6 times in total) (Fig. 1). On day 14, each lobe [LP and
remnant lobes (RLs)] was removed and weighed. The number of mice
undergoing the experimental procedures was 4–10 in each group.
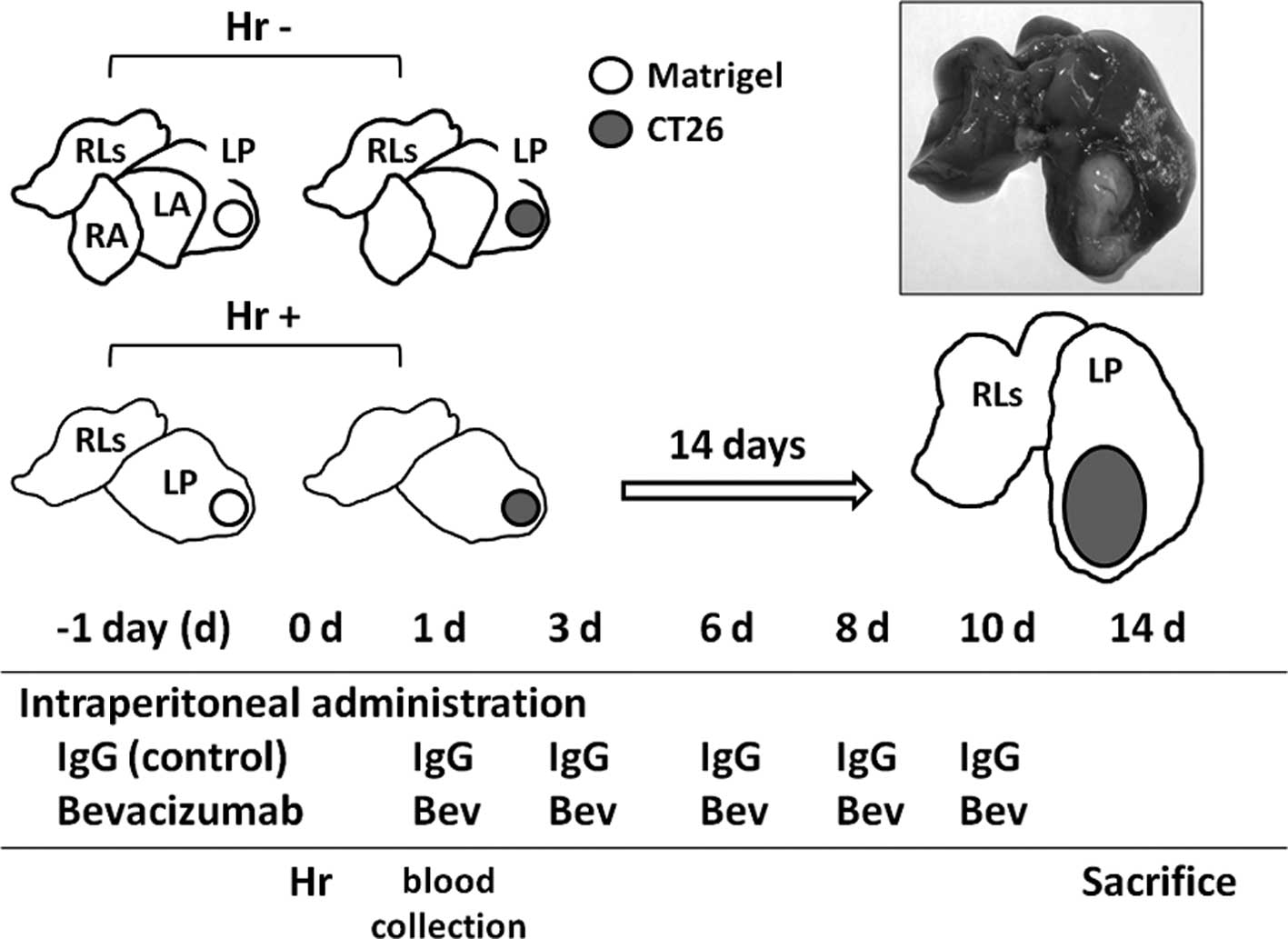 | Figure 1.The uper left section shows the mouse
liver consisting of 7 lobes (contribution of each lobe to the total
liver weight): RA, right anterior (22%); LA, left anterior (12%);
LP, left posterior (37%); RLs, remnant lobes which consist of right
posterior, right middle and two omental lobes (29%). Hepatic
resection group (Hr+), mice undergoing the RA and the LA lobectomy;
Hr−, mice without hepatic resection (Hr−). Open circle, matrigel
injection; gray circle, CT26 injection. The upper right section
shows the murine liver at 14 days after undergoing the RA and the
LA lobectomy, and CT26 transplantation into the LP lobe. The lower
section shows the schedule of administrations of bevacizumab (Bev)
or IgG as the control, hepatic resection, blood collection and
sacrifice. |
Hepatic regeneration and tumor
progression
We reviewed the following points as regards hepatic
regeneration and tumor progression: i) Changes in serum VEGF level,
LP lobe volume and RL volume by undergoing or not undergoing
hepatectomy in the cancer-bearing mice or the non-cancer-bearing
mice; ii) Changes in serum VEGF level, LP lobe volume, estimated
tumor volume and the RL volume by undergoing or not undergoing
hepatectomy, and by treating the cancer-bearing mice with or
without bevacizumab. The estimated tumor volume was calculated as
follows: The average volume of CT26 transplant LP lobe - the
average volume of Matrigel transplant LP lobe.
Statistical analysis
Statistical analyses were performed using StatView
software (Abacus Concepts Inc., Berkely, CA, USA). The serum VEGF
level and the lobe volume were compared using the Mann-Whitney U
test. A two-sided p-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Effect of hepatectomy in tumor-bearing
and non-tumor-bearing mice
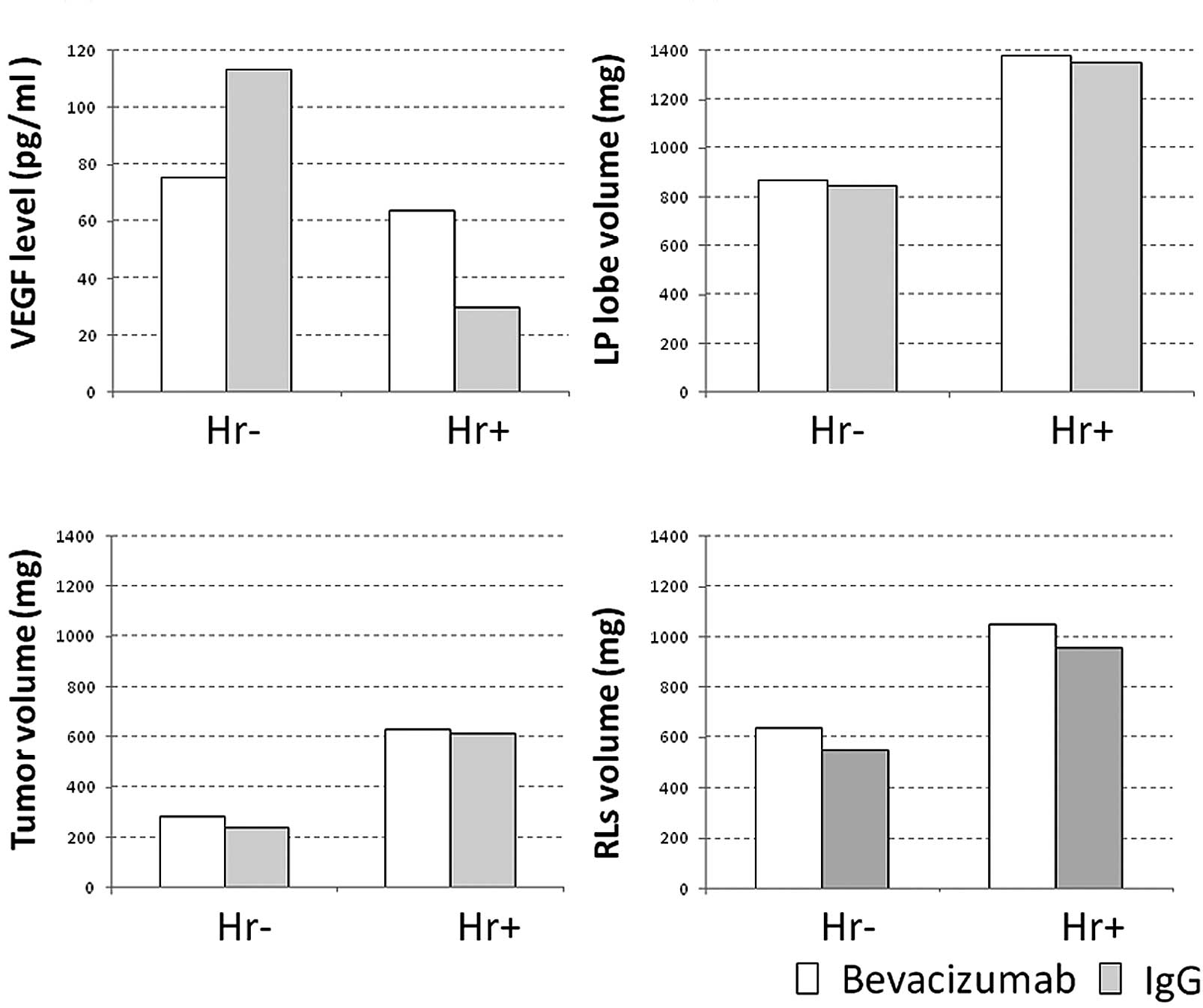
The mean serum VEGF levels (1 day after laparotomy)
of the non-hepatectomized, CT26-transplanted and
non-CT26-transplanted mice (Matrigel-transplanted mice) were
113.4±61.6 and 89.8±27.7 pg/ml, respectively. There were no
significant differences in these values between the groups [not
significant (NS)]. The mean serum VEGF levels (1 day following
hepatectomy) of the hepatectomized, CT26-transplanted and
non-CT26-transplanted mice were 29.6±11.4 and 19.3±7.7 pg/ml,
respectively (NS). The serum VEGF levels were significantly higher
in the non-hepatectomized than in the hepatectomized mice (p=0.010
in the transplanted groups vs. p<0.05 in the non-transplanted
groups) (Fig. 2A). The mean volume
of the LP lobe of the non-hepatectomized, CT26-transplanted and
non-CT26-transplanted mice was 845.3±195.7 and 606.2±41.3 mg,
respectively (NS). The increase in the volume of the LP lobe in the
CT26-transplanted mice reflected the volume of the CT26 cells
themselves. The mean volume of the LP lobe of the hepatectomized,
CT26-transplanted and non-CT26-transplanted mice was 1,349±366.5
and 735.5±62.3 mg, respectively, with a significant difference
(p=0.023). In addition, a significant difference in the mean volume
of the LB lobe was noted between the CT26 cell-transplanted,
hepatectomized and non-hepatectomized mice; however, in the
non-transplanted mice, the volume of the LP lobe increased slightly
following hepatectomy, with no significant difference (Fig. 2B). The mean volume of the residual
lobes (RLs) of the non-hepatectomized, CT26-transplanted and
non-CT26-transplanted mice was 551.9±71.0 and 736.6±101.4 mg,
respectively, and that of the RLs of the hepatectomized,
CT26-transplanted or non-CT26-transplanted mice was 957.3±195.6 and
1,025.0±163.2 mg, respectively, with no significant difference
associated with CT26 cell transplantation. By contrast, the mean
volume of the RLs was significantly larger in the hepatectomized,
CT26-transplanted mice than in their non-hepatectomized
counterparts (p=0.016), but not significantly greater than in their
non-CT26-transplanted counterparts (p=0.126) (Fig. 2C), indicating that regeneration of
the remnant liver occurs regardless of the presence or absence of
cancer.
Effect of bevacizumab administration in
CT26-transplanted mice
The mean serum VEGF level of the non-hepatectomized,
bevacizumab-administered and non-bevacizumab-administered mice was
75.2 and 113.4 ng/ml, respectively, and that of their
hepatectomized counterparts was 63.8 and 29.9 ng/ ml, respectively
(Fig. 3A; NS). The mean LP volume
of the non-hepatectomized bevacizumab-administered and
non-bevacizumab-administered mice was 871.4 and 845.3 mg,
respectively, and that of their hepatectomized mice was 1,379.0 and
1,349.0 mg, respectively (Fig. 3B;
NS). The estimated tumor volume in the non-hepatectomized,
bevacizumab-administered and non-bevacizumab-administered mice was
284.6 and 239.2 mg, respectively, and that of their hepatectomized
counterparts was 628.0 and 613.4 mg, respectively (Fig. 3C; NS). The mean volume of the RLs
in the non-hepatectomized, bevacizumab-administered and
non-bevacizumab-administered mice was 637.3 and 551.9 mg,
respectively, and that of their hepatectomized counterparts was
1,051.0 and 957.3 mg, respectively (Fig. 3D; NS).
Discussion
Marked advances in chemotherapy and hepatic surgical
procedures for liver metastases from colorectal cancer have led to
an increase in the number of patients who have undergone
hepatectomy for multiple liver metastases from colorectal cancer,
which were previously deemed unresectable (1,2). A
number of these patients have undergone NAC. At this point, it is
not clear whether NAC is capable of suppressing recurrence
following hepatectomy. NAC reportedly has advantages in that the
frequency of complete surgical tumor resection (R0) increases if
chemotherapy reduces the tumor size, and that the serum levels of
tumor growth factors, including VEGF and HGF, are higher in
tumor-bearing patients than in healthy controls (3,4). NAC
is expected to reduce the levels of tumor growth factors in
tumor-bearing patients. In the present study, the serum VEGF level
was also higher in the CT26-transplanted than in the
Matrigel-transplanted mice. In order to investigate what changes
would occur in the levels of various growth factors following
hepatectomy in tumor-bearing mice with high levels of these growth
factors, using a mouse hepatectomy model, Meredith et al
(8) measured HGF and bFGF levels
following hepatectomy, and demonstrated that their levels increased
24 h later, stating that HGF and bFGF were involved in liver
regeneration. Taniguchi et al (9) reported the overexpression of VEGF in
hepatocytes in the periportal areas of the rat, which are
considered to be the primary site of hepatocyte regeneration, at
48–72 h following hepatectomy. Yamamoto et al (10) also reported an increase in the VEGF
level in the portal blood following rat hepatectomy. These two
reports suggested that VEGF, which was secreted by periportal
hepatocytes, was closely involved in liver regeneration. In the
present study, the serum VEGF level in the peripheral blood was
lower in the hepatectomized than in the non-hepatectomized mice,
suggesting that VEGF is expressed in hepatocytes in the remnant
liver, that is, the regenerating liver itself. In the above-cited
report (10), the VEGF level
changed less in the peripheral blood than in the portal vein blood.
These two reports suggest that VEGF is involved in liver
regeneration, but that this involvement only occurs at sites of
liver regeneration, and is not directly correlated with peripheral
blood VEGF levels. In order to investigate the effect of tumor
growth factors, including VEGF, on tumors in the remnant liver,
Meredith et al (8)
transplanted tumors in the remnant liver in a mouse model of liver
resection, resulting in significantly enhanced tumor growth in
hepatectomized mice in comparison with non-hepatectomized mice,
which was ascribed to increased expression of HGF and bFGF. Similar
results were observed in the present study, where the tumor
enlarged rapidly following hepatectomy. However, we could not
demonstrate the direct involvement of peripheral blood VEGF in
tumor growth, or the antitumor effect of anti-VEGF antibodies.
Although the key tumor growth factor has not been identified, we
occasionally encounter such a phenomenon (i.e., rapid growth of
tumors in the remnant liver) in clinical practice, and, naturally,
we realize again the importance of not leaving any presence of the
tumor in the remnant liver.
Advances in chemotherapy for liver metastases from
colorectal cancer have led to a reduction in the size of liver
metastases and improved the rate of hepatectomy. However, the rate
of recurrence following hepatectomy is almost 80% (1,11),
since micrometastases and tumor scars, which were deemed a complete
response (CR) on imaging studies following chemotherapy, remain in
the remnant liver. Indeed, Benoist et al (12) reported that no viable cancer cells
were observed histopathologically in approximately 60% of lesions
from patients shown to have CR on imaging studies. This indicates
that some patients must continue to receive chemotherapy following
surgery. Bevacizumab is one of the most useful drugs for the
treatment of liver metastases from colorectal cancer. In addition,
studies have reported the effect of bevacizumab on liver
regeneration. Bockhorn et al (13) showed that the administration of
VEGF during liver regeneration following partial hepatectomy
promoted liver regeneration in the treatment group compared to the
control group, and that the administration of anti-VEGF antibody
delayed liver regeneration. In the present study, the
administration of bevacizumab in the hepatectomized, tumor-bearing
mice did not delay remnant liver regeneration, which did not
confirm the results of Bockhorn et al (13). However, at the same time, they
reported that VEGF did not improve liver regeneration and survival
following 90% subtotal liver resection (14). Therefore, it appears that the
effects of VEGF and anti-VEGF antibody alone are insufficient to
explain the mechanism of liver regeneration. The present study
showed that the volume of the non-tumor region of the
CT26-transplanted LP lobe increased following hepatectomy, but not
so rapidly as the tumor, that the volume of the RLs following
hepatectomy increased significantly regardless of the presence or
absence of cancer, and that the administration of anti-VEGF
antibody did not suppress the enlargement of the remnant liver.
In conclusion, the administration of anti-VEGF
antibody in tumor-bearing mice following hepatectomy does not delay
remnant liver regeneration. We found that the tumor in the remnant
liver following hepatectomy grew rapidly, and the administration of
anti-VEGF did not suppress its growth. Thus, the activity of
anti-VEGF antibody was assumed to be negligible compared with the
major changes caused by hepatectomy.
Acknowledgements
We thank Mr. Hiroaki Tanaka and
Hiroshi Ohta, university students who belong to the Department of
Clinical Pharmacy of Tokyo University of Pharmacy and Life Sciences
for their valuable technical assistance.
References
|
1.
|
R AdamV DelvartG PascalRescue surgery for
unresectable colorectal liver metastases downstaged by
chemotherapy: a model to predict long-term survivalAnn
Surg240644657200415383792
|
|
2.
|
R AdamR MillerM PitomboDA WichertsRJ De
HaasG BitsakouT AloiaTwo-stage hepatectomy approach for initially
unresectable colorectal hepatic metastasesSurg Oncol Clin N
Am16525536200710.1016/j.soc.2007.04.01617606192
|
|
3.
|
T FukuuraC MikiT InoueK MatsumotoH
SuzukiSerum hepatocyte growth factor as an index of disease status
of patients with colorectal carcinomaBr J
Cancer78454459199810.1038/bjc.1998.5149716026
|
|
4.
|
SS YoonSH KimM GonenProfile of plasma
angiogenic factors before and after hepatectomy for colorectal
cancer liver metastasesAnn Surg
Oncol13353362200610.1245/ASO.2006.03.06016474912
|
|
5.
|
G MenthaS TerrazP MorelDangerous halo
after neoadjuvant chemotherapy and two-step hepatectomy for
colorectal liver metastasesBr J
Surg9695103200910.1002/bjs.643619109800
|
|
6.
|
J FolkmanY ShingAngiogenesisJ Biol
Chem26710931109341992
|
|
7.
|
AK GreeneM PuderPartial hepatectomy in the
mouse: technique and perioperative managementJ Invest
Surg1699102200310.1080/0894193039019442412746193
|
|
8.
|
K MeredithD HaemmerichC QiD MahviHepatic
resection but not radiofrequency ablation results in tumor growth
and increased growth factor expressionAnn
Surg245771776200710.1097/01.sla.0000261319.51744.5917457170
|
|
9.
|
E TaniguchiS SakisakaK MatsuoK TanikawaM
SataExpression and role of vascular endothelial growth factor in
liver regeneration after partial hepatectomy in ratsJ Histochem
Cytochem49121130200110.1177/00221554010490011211118484
|
|
10.
|
C YamamotoS YagiT HoriT IidaK TaniguchiS
IsajiS UemotoSignificance of portal venous VEGF during liver
regeneration after hepatectomyJ Surg
Res1593743201010.1016/j.jss.2008.11.00719394640
|
|
11.
|
SR AlbertsWL HorvathWC
SternfeldOxaliplatin, fluorouracil, and leucovorin for patients
with unresectable liver-only metastases from colorectal cancer: a
North Central Cancer Treatment Group phase II studyJ Clin
Oncol2392439249200510.1200/JCO.2005.07.74016230673
|
|
12.
|
S BenoistA BrouquetC PennaComplete
response of colorectal liver metastases after chemotherapy: does it
mean cure?J Clin
Oncol2439393945200610.1200/JCO.2006.05.872716921046
|
|
13.
|
M BockhornM GoralskiD ProkofievVEGF is
important for early liver regeneration after partial hepatectomyJ
Surg Res138291299200710.1016/j.jss.2006.07.02717275844
|
|
14.
|
M BockhornS SchöllmannB OpitzVascular
endothelial growth factor does not improve liver regeneration and
survival after 90% subtotal liver resectionHepatol
Res373533592007
|