Spandidos Publications style
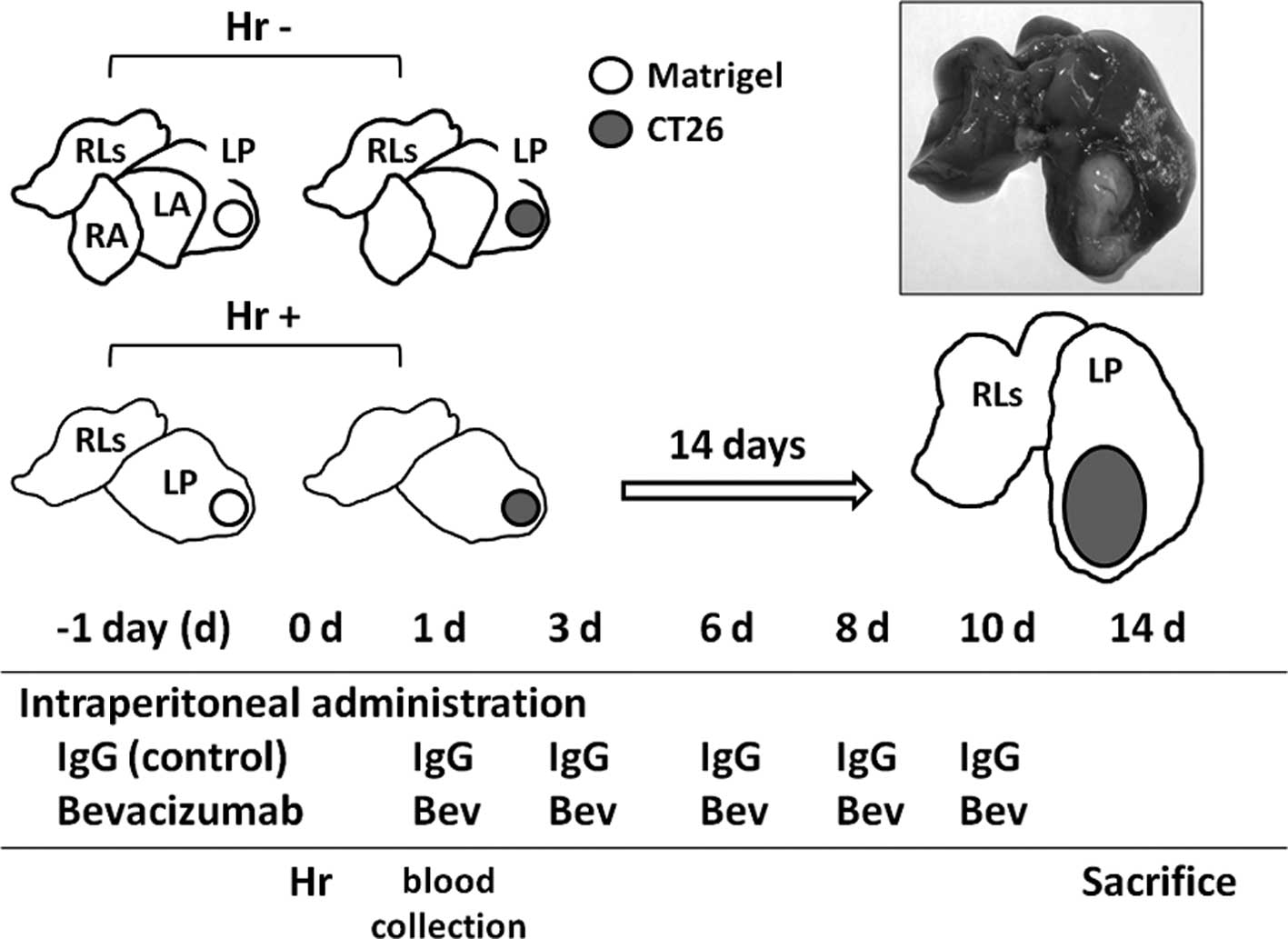
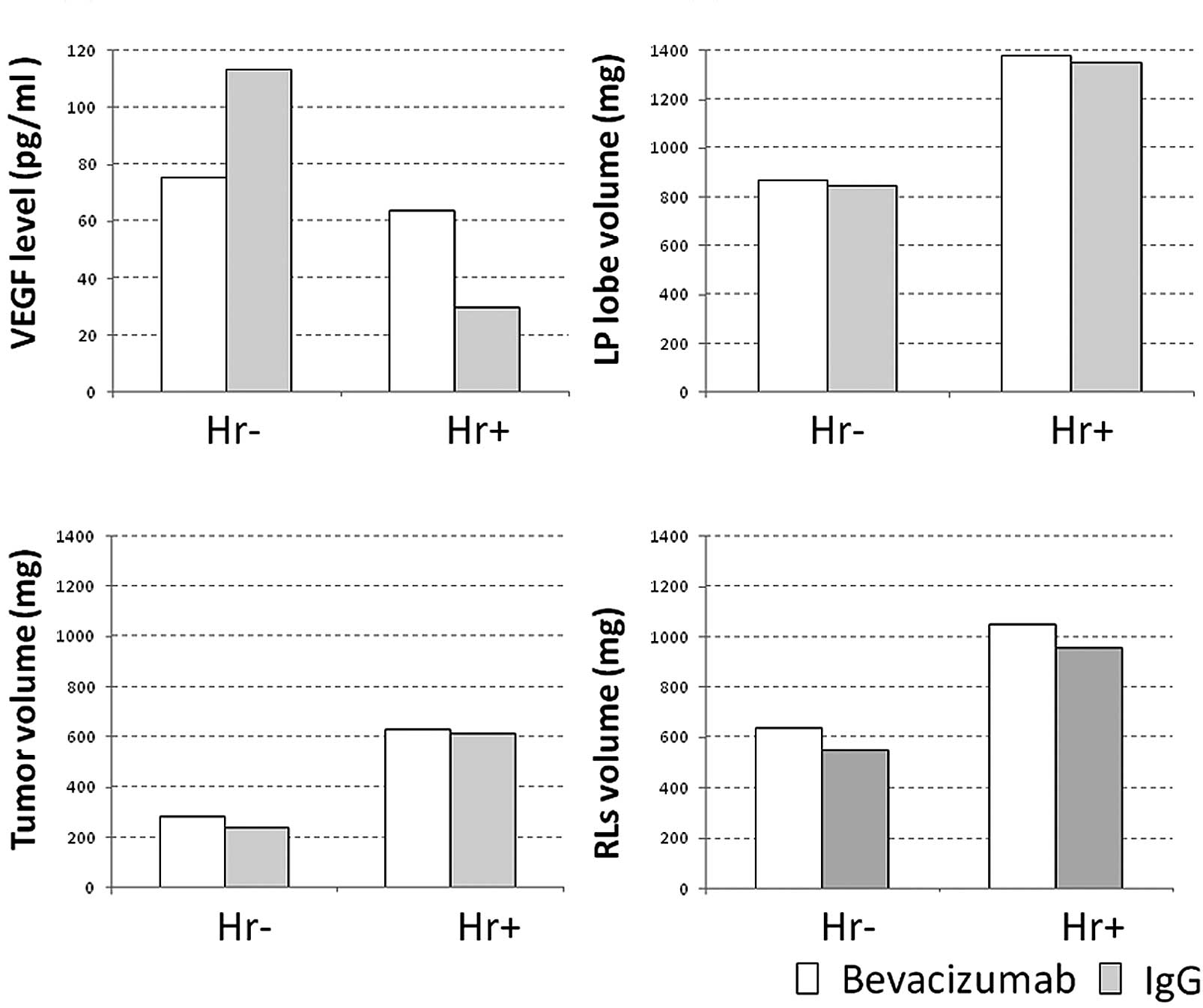
Kasuya K, Suzuki M, Nagakawa Y, Suzuki Y, Kikuchi S, Kyo B, Matsudo T, Itoi T, Tsuchida A, Aoki T, Aoki T, et al: Administration of anti-vascular endothelial growth factor antibody following hepatectomy does not inhibit remnant liver regeneration or growth of remnant metastases. Exp Ther Med 3: 347-350, 2012.
APA
Kasuya, K., Suzuki, M., Nagakawa, Y., Suzuki, Y., Kikuchi, S., Kyo, B. ... Aoki, T. (2012). Administration of anti-vascular endothelial growth factor antibody following hepatectomy does not inhibit remnant liver regeneration or growth of remnant metastases. Experimental and Therapeutic Medicine, 3, 347-350. https://doi.org/10.3892/etm.2011.409
MLA
Kasuya, K., Suzuki, M., Nagakawa, Y., Suzuki, Y., Kikuchi, S., Kyo, B., Matsudo, T., Itoi, T., Tsuchida, A., Aoki, T."Administration of anti-vascular endothelial growth factor antibody following hepatectomy does not inhibit remnant liver regeneration or growth of remnant metastases". Experimental and Therapeutic Medicine 3.2 (2012): 347-350.
Chicago
Kasuya, K., Suzuki, M., Nagakawa, Y., Suzuki, Y., Kikuchi, S., Kyo, B., Matsudo, T., Itoi, T., Tsuchida, A., Aoki, T."Administration of anti-vascular endothelial growth factor antibody following hepatectomy does not inhibit remnant liver regeneration or growth of remnant metastases". Experimental and Therapeutic Medicine 3, no. 2 (2012): 347-350. https://doi.org/10.3892/etm.2011.409