Introduction
Hepatocellular carcinoma (HCC) is one of the most
common forms of malignant tumor occurring in the liver, and is the
third leading cause of mortality from cancer worldwide (1,2).
Research has demonstrated that Southeast-Asian regions, including
China, have a higher incidence rate of HCC, due to the endemic
nature of hepatitis B and C in these countries (2,3).
Surgical interventions, including complete resection and liver
transplantation, demonstrate potentially curative treatments when
the tumor is restricted to the liver, which offer the best
prognosis for HCC (4,5). However, surgical resection is only
possible in a small proportion of patients with HCC due to advanced
liver disease, extrahepatic metastases or underlying severe liver
cirrhosis, inadequate functional hepatic reserve and poor general
condition of the patient (6,7). The
difficulties posed by HCC treatment have prompted the development
of a number of potential, non-surgical treatments, including
transcatheter arterial chemoembolization (TACE), radiofrequency
ablation (RFA), percutaneous ethanol injection therapy (PEI) and
high-intensity focused ultrasound (HIFU) therapy (8–10). A
suitable hepatic tumor animal model is crucial for the
investigation of liver cancer diagnostics and therapeutics
(11,12).
VX2 carcinoma is an animal carcinoma, first
developed by Shope and Hurst (13), and is derived from a rabbit
papilloma. Previous research has demonstrated that the rabbit VX2
hepatic tumor grows rapidly, that its arterial blood supply is
similar to that of human liver cancer and that its size is large
enough to be observed by clinical imaging (14–15).
As such, the rabbit VX2 hepatic tumor model has been widely used to
study various aspects of liver tumor behavior and is generally
accepted for the inoculation of liver cancer (16–18).
However, in terms of the inoculation method, there are a number of
different approaches to the induction of the VX2 tumor in the liver
of recipient rabbits. In previous studies, VX2 tumor cell
suspension was injected into the portal vein or hepatic artery
(19), or directly into the liver
parenchyma (20,21) in order to achieve hepatic tumor
growth. In addition, some investigators implanted small tumor
pieces into the liver of rabbits by open laparotomy (22–24).
Previous results have revealed varying degrees of success of tumor
inoculation.
In the present study, in order to identify the
preferred method for the inoculation of the VX2 tumor into the
rabbit liver, we compared the injection of VX2 tumor cell
suspension versus implantation of small, minced tumor fragment
suspension using a fine needle. To the best of our knowledge, there
is no literature comparing these two methods for inoculating VX2
tumors in the rabbit liver.
Materials and methods
Animals
This study was approved by the Animal Use and Care
Committee of Zhejiang University (China). All New Zealand White
rabbits used in this research, 2–3 months old, weighing 2–3 kg,
were provided by the experimental animal center, College of
Medicine, Zhejiang University.
Preparation of suspension of VX2 cells
and VX2 tumor fragments
Hind limb tumors of rabbits were used to propagate
and maintain the VX2 tumor. All rabbits were anesthetized using a
mixed solution of ketamine hydrochloride injection (25 mg/kg,
intramuscular) and promethazine hydrochloride injection (10 mg/kg,
intramuscular). With a fine needle, 0.2 ml of VX2 cell suspension
was injected into the muscles of the hind limbs of the rabbits.
Following implantation into the hind limbs, the tumor grew rapidly.
Two weeks following implantation, the tumor grew to a size of
approximately 3 cm in diameter. Rabbits were later sacrificed and
hind limb tumors harvested.
The VX2 tumor was aseptically resected from the hind
limb and minced with surgical scissors and knives in normal saline
(NS). The suspension was then filtered through an iron mesh with
0.08 mm2 pores to remove other tissue fragments. The
filtrate was centrifuged at 1,500 rev/min for 8 min at room
temperature and resuspended using NS at a concentration of
1x107 cells/ml.
After the tumor was stripped from the hind leg of
the rabbit, the surrounding and necrotic tissues were removed from
the tumor. The tumor was then cut into 1-mm3 fragments
and resuspended in a NS solution. The VX2 tumor fragment suspension
was then ready for injection.
Implantation of VX2 tumors into
liver
A total of 44 New Zealand White rabbits were
randomly divided into 2 groups. All inoculations were performed by
open laparotomy. To implant the VX2 tumor cell suspension or VX2
tumor fragments into the liver parenchyma, a sub-xyphoid
laparotomy, approximately 3 cm in length, was performed to expose
the left liver lobe following anaesthesia with the mixed solution
of ketamine hydrochloride injection (25 mg/kg, intramuscular) and
promethazine hydrochloride injection (10 mg/kg, intramuscular).
In group 1, a fresh VX2 tumor cell suspension
containing 2x106 cells in a volume of 0.2 ml, was
injected slowly into the liver parenchyma of the left lobe, using
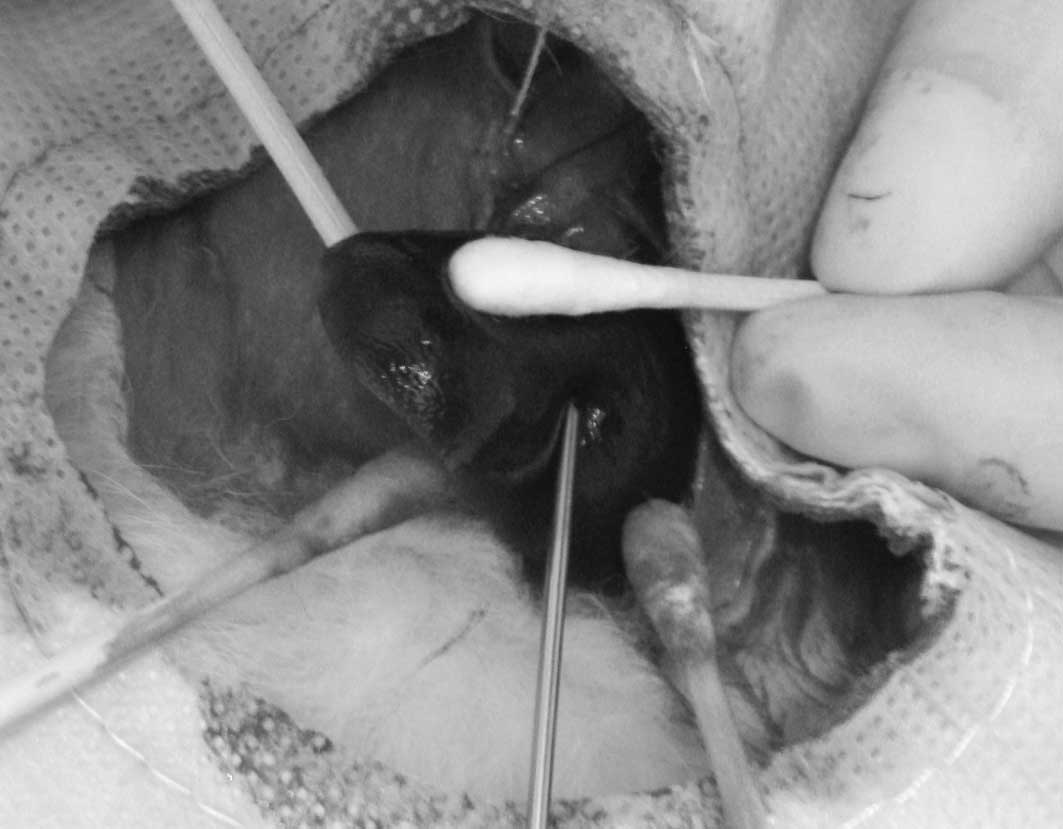
an 18-gauge needle with a 1-ml syringe. In group 2, following the
exposure of the left lobe of the liver, a suspension containing
1-mm3 fragments of VX2 tumor in a volume of 0.2 ml was
injected into the parenchyma of the left lobe of the liver, using
an 18-gauge needle with a 1-ml syringe (Fig. 1). Following removal of the needles,
all puncture places were gently compressed for 5 min in order to
prevent leakage of cancer cells and bleeding from the liver
parenchyma. Following confirmation of the absence of leakage of
cancer cells and bleeding, the liver was repositioned back to its
original abdominal space. The abdominal incision was then closed in
layers. Antibiotic ointment was applied along the suture line.
Computed tomography (CT) examination and
angiography
A total of 2 weeks following liver tumor
implantation, all rabbits were anesthetized and placed into a
helical CT scanner (General Electric Co, Milwaukee, WI, USA). Each
animal underwent a conventional CT-scanning series, including plan
scanning and enhancement scanning in order to detect tumor growth.
Immediately following CT-scanning, surgical cut-down of the right
femoral artery of the rabbits and insertion of a 2.7-F-diameter
microcatheter (Terumo, Tokyo, Japan) were performed in order to
gain access into the celiac trunk or common hepatic artery.
Selective angiography was then carried out using a digital
subtraction angiography (DSA) system (Allura Xper FD 20; Philips
Medical Systems, Best, The Netherlands) to identify the tumor.
Necropsy and histopathology
Following completion of the CT scan and angiography,
each rabbit was sacrificed and underwent a full necropsy to
determine the tumor growth in the liver and extrahepatic
metastases. All liver tumors were removed for pathological
examination using hematoxylin and eosin staining.
Statistical analyses
Statistical analyses were carried out using SPSS
software (ver. 11.0; SPSS Inc., Chicago, IL, USA). The Chi-square
test was employed to determine significant statistical differences
between the groups. However, when the sample size was too small to
use the Chi-square test, Fisher's exact test was used instead. A
P-value (two-tailed) <0.05 was considered to indicate
statistically significantly differences.
Results
All rabbits (donors and recipients) successfully
tolerated VX2 tumor inoculation. No mortality was observed at the
time of implantation. All liver tumors were confirmed upon CT-scan
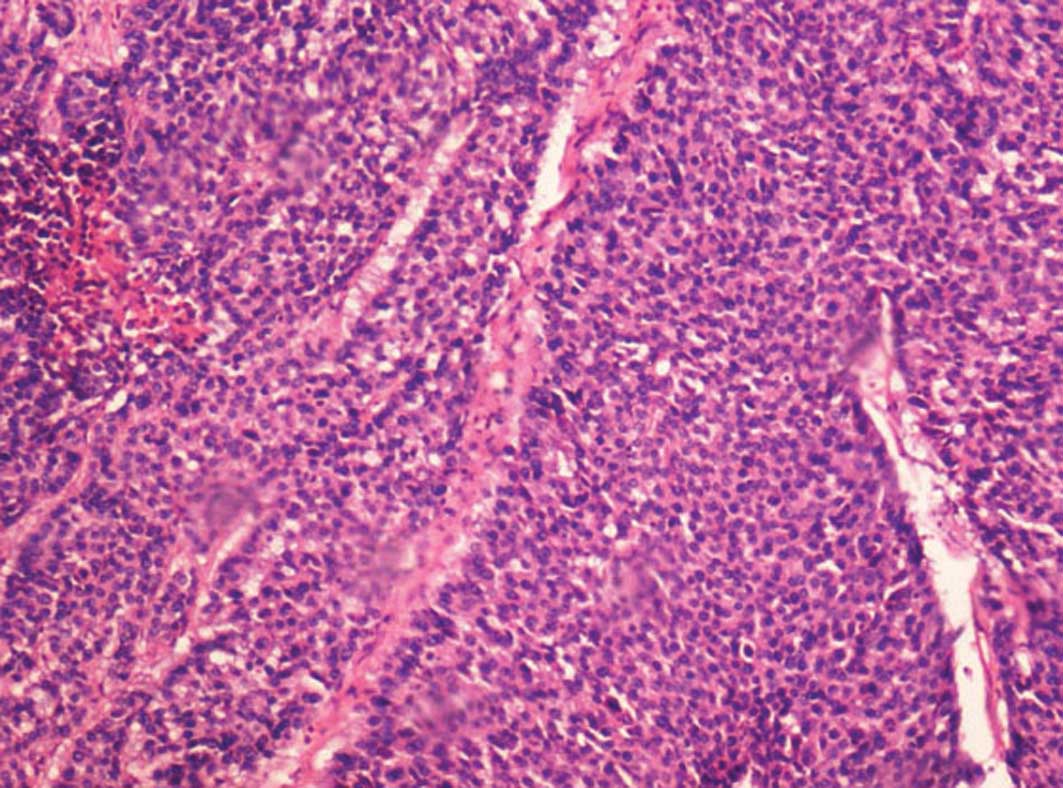
and angiography, and were further confirmed by necropsy and
pathological examination with hematoxylin and eosin staining.
In group 1, 8 rabbits displayed no evidence of liver
tumor growth at 2 weeks following implantation. Liver tumors were
grown in 14 out of 22 rabbits with an overall success rate of
63.6%. In group 2, only 1 rabbit displayed no evidence of liver
tumor growth. Successful liver tumor growth was achieved in 21 out
of the 22 rabbits with an overall success rate of 95.5%. Upon
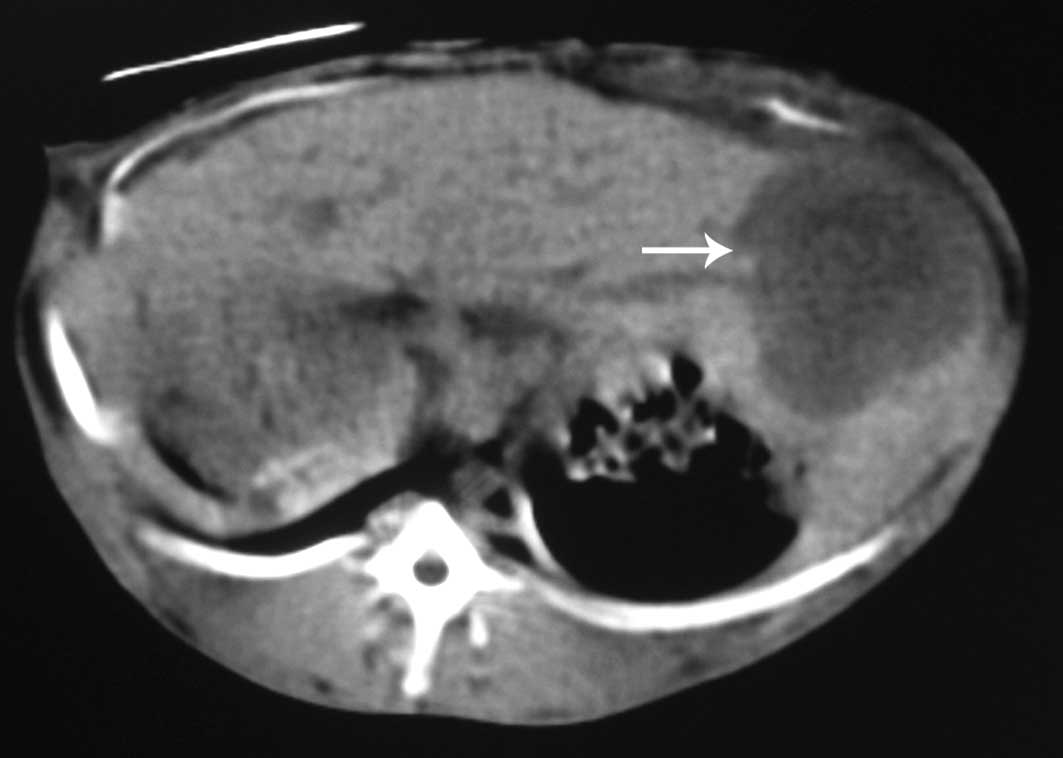
further sub-analysis, single nodular tumors in the liver were
observed in 4 out of 14 rabbits in group 1 (28.6%) and 14 out of 21
rabbits in group 2 (66.7%) (Figs.
2–5). Multinodular tumors in
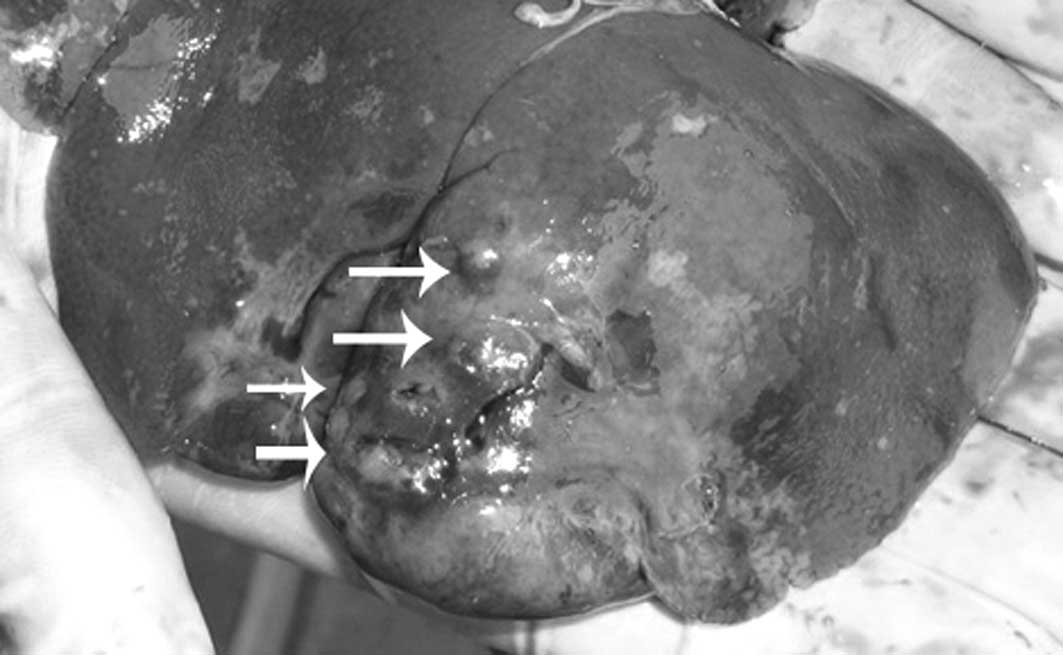
the liver were grown in 10 out of 14 rabbits in group 1 (71.4%) and
in 7 out of 21 rabbits in group 2 (33.3%) (Fig. 6). In addition, extrahepatic
metastases were observed in 5 out of 14 rabbits in group 1 (35.7%)
and in 1 of 21 rabbits in group 2 (4.9%) (Fig. 7). There were statistically
significant differences between the 2 groups. The overall liver
tumor growth rate of group 1 was significantly lower than that of
group 2 (P=0.025). Furthermore, the single nodular tumor growth
rate of group 1 was significantly lower than that of group 2, and
the multinodular tumor growth rate of group 1 was significantly
higher than that of group 2 (P=0.041). Finally, a higher incidence
of extrahepatic metastasis was observed in group 1 (P=0.028).
Discussion
Liver cancer, particularly HCC, is a form of highly
malignant tumor with a poor prognosis of only several months
survival from the time of diagnosis (1,25). A
suitable hepatic tumor animal model is essential for investigations
of HCC diagnostics and therapeutics. Over the past few decades,
despite the fact that VX2 carcinoma originated from rabbit
malignant aplastic squamous cell carcinoma, it has been widely used
as an experimental model for HCC studies of diagnosis and treatment
due to its hepatic arterial supply and rapid growth pattern
(16,19–21).
In the present study, imaging and pathological
examinations revealed that the VX2 liver tumor grew progressively 2
weeks following implantation. It is clear that successful tumor
inoculation and growth in the rabbit liver leads to successful
experimental investigations, and that incessant improvement of the
tumor implantation technique may improve our knowledge regarding
the selective use of the rabbit VX2 liver tumor. In addition,
during previous similar experiments, we noticed that the
implantation method of the tumor may not only affect the success
rate of tumor growth and the animal expenses, but also, at times,
the results of the study. Therefore, we initiated this study to
attempt to identify a more desirable liver VX2 implantation method
than previously.
Our experimental data revealed that the tumor growth
success rate was as low as 63.6% in group 1, using the method of
injecting VX2 cell suspension into the liver with a fine needle.
Furthermore, multinodular liver tumor growth rates and extrahepatic
metastasis rates were as high as 71.4 and 35.7%, respectively.
Tumor cells, injected into the liver, may not only enter the
parenchyma, but also the bile ducts and liver blood vessels.
Conversely, tumor cells may leak from the liver puncture site.
These may cause the previously mentioned results of a lower tumor
growth success rate, and a higher incidence of multinodular liver
tumor growth and extrahepatic metastases. Despite ensuring that the
needle was not in a bile duct or a vessel prior to injection of the
tumor cells and compression of the puncture site in an attempt to
achieve a better outcome, often the results remained unsatisfactory
(19,20). Conversely, the success rate of
implanting liver VX2 tumors increased from 63.6 to 95.5% when a
suspension of tumor fragments was used instead of the VX2 cell
suspension. Moreover, the use of a VX2 tumor fragment suspension
resulted in a higher liver single nodular tumor growth rate, as
well as a statistically reduced incidence of extrahepatic
metastasis.
Previously, certain investigators achieved a good
liver tumor growth rate by implanting VX2 tumor fragments via
surgical placement of these fragments in the liver parenchyma
(26–29). Surgical implantation of VX2 tumor
fragments controls the site of tumor growth more effectively, while
tending to reduce the incidence of intra- and extrahaptic
metastasis as compared to the method of injecting tumor cell
suspension (28). However, there
are also certain disadvantages of the surgical tumor fragment
placement method. Of note, the surgical technique is more traumatic
than the use of the cell suspension injection method using a fine
needle. Surgical complications, including bile leakage, hemorrhage
or abscess formation are more likely to occur (26). In addition, it is difficult to
ensure that the implanted tumor fragment from the ‘mother’ tumor is
viable tumor tissue. Unfortunately, failure of tumor induction may
occur when necrotic tissue is placed into the liver. Our data
revealed that the implantation of tumor fragment suspension into
the liver directly using a fine needle (surgically placing VX2
tumor fragments into the rabbit liver) may be a more promising
method than the inoculation method of injecting cell suspension
(injecting tumor cells).
However, there were a number of limitations to the
present study. Firstly, we did not compare all previously published
induction methods of rabbit liver VX2 tumor. Secondly, the number
of animals used in this research was small, which decreased the
statistical power of the results, although this was sufficiently
large to draw the main conclusion. Thirdly, the short-term
follow-up period up of 2 weeks prevented the determination of the
long-term fate of the hepatic tumor and the rabbits.
yIn conclusion, as a simple and effective method for
the induction of a hepatic tumor, the direct implantation of VX2
tumor fragment suspension into the liver using a fine needle
achieves a higher success tumor growth rate and a lower incidence
of intra- and extrahepatic metastasis than the method of injecting
VX2 tumor cells suspension into the liver using a fine needle. The
results from our study may prove to be significant to future
investigations and may help improve the efficiency of tumor
inoculation, thereby reducing the number of rabbits required for
study, as well as animal expenses.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 30901446), the
Program for Innovative Research Team of Science and Technology of
Zhejiang Province (No. 2009R50038), the Medical Health Fund of
Zhejiang Province (No.2008A053), the Fundamental Research Funds for
the Central Universities (2011KYJD023) and the Programme of Chinese
Medical Science of Zhejiang Province (No.2009CB040).
References
|
1.
|
DM ParkinF BrayJ FerlayP PisaniEstimating
the world cancer burden: Globocan 2000Int J
Cancer94153156200110.1002/ijc.144011668491
|
|
2.
|
J HeD GuX WuMajor causes of death among
men and women in ChinaN Engl J
Med35311241134200510.1056/NEJMsa05046716162883
|
|
3.
|
HB El-SeragHepatocellular carcinoma and
hepatitis C in the United
StatesHepatology36S74S83200210.1002/hep.184036071012407579
|
|
4.
|
Y KawanoA SasakiS KaiShort- and long-term
outcomes after hepatic resection for hepatocellular carcinoma with
concomitant esophageal varices in patients with cirrhosisAnn Surg
Oncol1516701676200810.1245/s10434-008-9880-718368453
|
|
5.
|
S SaabM YeganehK NguyenRecurrence of
hepatocellular carcinoma and hepatitis B reinfection in hepatitis B
surface antigen-positive patients after liver transplantationLiver
Transpl1515251534200910.1002/lt.2188219877207
|
|
6.
|
JM LlovetJ FusterJ BruixPrognosis of
hepatocellular carcinomaHepatogastroenterology497112002
|
|
7.
|
T TsuzukiA SugiokaM UedaS IidaT KanaiH
YoshiiK NakayasuHepatic resection for hepatocellular
carcinomaSurgery10751152019902159190
|
|
8.
|
M ScartozziGS BaroniL
FaloppiTrans-arterial chemo-embolization (TACE), with either
lipiodol (traditional TACE) or drug-eluting microspheres (precision
TACE, pTACE) in the treatment of hepatocellular carcinoma: efficacy
and safety results from a large mono-institutional analysisJ Exp
Clin Cancer Res29164201010.1186/1756-9966-29-164
|
|
9.
|
K KurokohchiT MasakiS WatanabeTime-lag
performance of radiofrequency ablation after percutaneous ethanol
injection for the treatment of hepatocellular carcinomaInt J
Oncol28971976200616525648
|
|
10.
|
K NumataH FukudaM OhtoEvaluation of the
therapeutic efficacy of high-intensity focused ultrasound ablation
of hepatocellular carcinoma by three-dimensional sonography with a
perflubutane-based contrast agentEur J
Radiol75E67E75201010.1016/j.ejrad.2009.11.022
|
|
11.
|
LE WoodardA KeravalaWE JungOL WapinskiQ
YangDW FelsherMP CalosImpact of hydrodynamic injection and phiC31
integrase on tumor latency in a mouse model of MYC-induced
hepatocellular carcinomaPLoS
One29E11367201010.1371/journal.pone.001136720614008
|
|
12.
|
P MorozC MetcalfBN GrayHistologic analysis
of liver tissue following hepatic arterial infusion of
ferromagnetic particles in a rabbit tumour
modelBiometals16455464200310.1023/A:102255543147612680709
|
|
13.
|
RE ShopeEW HurstInfectious papillomatosis
of rabbits with note on histopathologyJ Exp
Med58607624193310.1084/jem.58.5.60719870219
|
|
14.
|
P LeanderK GolmanP StrandeJ KlavenessJ
BesjakovK FältA comparison between IEEC, a new biodegradable
particulate contrast medium, and iohexol in a tumor model of
computed tomography imaging of the liverInvest
Radiol28513519199310.1097/00004424-199306000-000098320069
|
|
15.
|
EM MerkleDT BollT BoazMRI-guided
radiofrequency thermal ablation of implanted VX2 liver tumors in a
rabbit model: demonstration of feasibility at 0.2 TMagn Reson
Med42141149199910.1002/(SICI)1522-2594(199907)42:1%3C141::AID-MRM19%3E3.0.CO;2-I10398960
|
|
16.
|
A SonodaN NittaS OhtaControlled release
and antitumor effect of pluronic F127 mixed with cisplatin in a
rabbit modelCardiovasc Intervent
Radiol33135142201010.1007/s00270-009-9741-119908089
|
|
17.
|
T GuptaS VirmaniTM NeidtMR tracking of
iron-labeled glass radioembolization microspheres during
transcatheter delivery to rabbit VX2 liver tumors: feasibility
studyRadiology249845854200810.1148/radiol.2491072027
|
|
18.
|
KH LeeE LiapiVP VenturaM BuijsJA VossenM
ValiJF GeschwindEvaluation of different calibrated spherical
polyvinyl alcohol microspheres in transcatheter arterial
chemoembolization: VX2 tumor model in rabbit liverJ Vasc Interv
Radiol1910651069200810.1016/j.jvir.2008.02.023
|
|
19.
|
FA BurgenerMR ViolanteComparison of
hepatic VX2-carcinomas after intraarterial, intra-portal and
intraparenchymal tumor cell injection: an angiographic and computed
tomographic study in the rabbitInvest
Radiol14410414197910.1097/00004424-197909000-00005
|
|
20.
|
B IzumiS TashiroY MiyauchiAnticancer
effects of local administration of mitomycin C via the
hepaticartery or portal vein on implantation and growth of VX2
cancer injected into rabbit liverCancer
Res464167417019863089588
|
|
21.
|
DM BerkowitzL AlexanderNK Hollenberga
simple cell-suspension method for transplantation of VX2 carcinomaJ
Natl Cancer Inst5423323419751113303
|
|
22.
|
JJ PhillipsSL ChangHI VargasPS DickmanJA
ButlerJD LipcamonMR and CT imaging of ethanol-treated liver tumors
in an animal modelMagn Reson
Imaging9201204199110.1016/0730-725X(91)90011-A2034053
|
|
23.
|
Y IkedaT MatsumataE AdachiT NishizakiK
SugimachiEthanol injection therapy in RBT-1 carcinoma of the rat
liver evokes enhancement of metastasisSurg
Oncol54912199310.1002/jso.29305401058377510
|
|
24.
|
O ThorstensenB IsbergU SvahnH JorulfN
VenizelosG JaremkoExperimental tissue transplantation using a
biopsy instrument and radiologic methodsInvest
Radiol29469471199410.1097/00004424-199404000-000158034455
|
|
25.
|
G CabibboM EneaM AttanasioJ BruixA CraxìC
CammàA meta-analysis of survival rates of untreated patients in
randomized clinical trials of hepatocellular
carcinomaHepatology5112741283201010.1002/hep.2348520112254
|
|
26.
|
J HänslerD NeureiterM
WasserburgerPercutaneous US-guided radiofrequency ablation with
perfused needle applicators: improved survival with the VX2 tumor
model in rabbitsRadiology230169174200414645878
|
|
27.
|
CJ YoonJW ChungJH ParkYH YoonJW LeeSY
JeongH ChungTranscatheter arterial chemoembolization with
paclitaxel-lipiodol solution in rabbit VX2 liver
tumorRadiology229126131200310.1148/radiol.229102102912944599
|
|
28.
|
T NishizakiT MatsumataT KanematsuC
YasunagaK SugimachiSurgical manipulation of VX2 carcinoma in the
rabbit liver evokes enhancement of metastasisJ Surg
Res499297199010.1016/0022-4804(90)90116-J2359299
|
|
29.
|
S VirmaniTK RheeRK RyuComparison of
hypoxia-inducible factor-1alpha expression before and after
transcatheter arterial embolization in rabbit VX2 liver tumorsJ
Vasc Interv Radiol1914831489200810.1016/j.jvir.2008.06.017
|