Introduction
Gastric cancer is a highly prevalent disease with an
impact on morbidity and mortality. Despite the progress in cancer
therapeutics and chemotherapy development with the introduction of
new drugs, advanced gastric cancer continues to have an extremely
poor prognosis and limited treatment options (1). Thus, new therapeutic strategies are
urgently required. Tumor endothelium is a new target for
treatments. A number of recent studies have sparked interest in the
notion of exploiting chemotherapeutic drugs as angiogenesis
inhibitors. Maximum tolerated dose (MTD) chemotherapy, targeting
the tumor cells directly, sometimes causes undesirable side effects
normally associated with traditional cytotoxic chemotherapy
regimens. Continuous, low-dose metronomic (LDM) chemotherapy,
targeting the endothelial cells directly, is thought to have fewer
side effects as well as a lack of drug resistance (2). Also, metronomic chemotherapy is
changing the paradigm that more is better (3).
Capecitabine, an oral fluoropyrimidine designed to
selectively deliver 5-fluorouracil (5-FU) to tumor cells, is not
inferior to 5-FU and it has a more convenient and comfortable
administration method. Capecitabine has been widely used in
advanced gastric cancer, either in monotherapy or in combination
(4). Even though capecitabine
shows higher specificity to tumor cells, many late-phase gastric
cancer patients cannot tolerate its side effects of diarrhea,
nausea, lymphopenia and hand-foot syndrome; furthermore, drug
resistance often occurs in the final stages. We postulated that
low-dose capecitabine may have anti-angiogenic effects on gastric
cancer. In this study, we evaluated its efficacy in impeding tumor
growth and microvasculature in gastric cancer.
The effects of LDM chemotherapy can be improved by
concurrent administration of a drug targeting angiogenesis
(5,6). The rationale for this combination is
that these targeted drugs, capable of specific blockade of
activated endothelial cell survival mechanisms, selectively enhance
the damaging or cytotoxic effects of LDM chemotherapy on newly
formed blood vessels (7). Green
tea extract and its major component (-)-epigallocatechin-3-gallate
(EGCG) possess obvious antiproliferative, pro-apoptotic and
anti-angiogenic effects (8). EGCG
is an agent which exhibits anti-angiogenic activities through a
variety of mechanisms (9,10). Several studies have suggested that
EGCG may reduce the toxicity of certain anticancer drugs and can be
used in adjuvant settings for cancer treatment (8).
Therefore, we designed this study to investigate the
efficacy of LDM chemotherapy with capecitabine, alone or in
combination with EGCG, in a nude mouse model of BGC-823 human
gastric carcinoma tumors.
Materials and methods
Materials
The BGC-823 human gastric cancer cell line was
obtained from the Cancer Research Institution of China Medical
University (China). A group of female BALB/c nude mice (6–8 weeks
of age) weighing 18–20 g were purchased from the Animal Experiment
Center of Beijing, China. Mice were housed under pathogen-free
conditions and fed with animal chow and water ad libitum.
Capecitabine (N-pentyloxycarbonyl-5′-deoxy-5-fluorocytidine) was
obtained from Roche Pharmaceutical Company (Nutley, NJ, USA). EGCG
was obtained from Shanghai Winherb Medical Company, China. SYBR
Green reagents were purchased from Takara Bio (Japan). Human
monoclonal antibody for vascular endothelial growth factor (VEGF)
was purchased from Fuzhou Maixin Company (China). Mouse monoclonal
antibody for CD31 was purchased from Dako (Japan).
Cell culture
Human BGC-823 gastric cancer cells were cultured in
Roswell Park Memorial Institute medium (RPMI-1640) supplemented
with 10% fetal bovine serum plus ampicillin and streptomycin
routinely, and incubated in 5% CO2 at 37°C.
Design of animal experiments
All of the animal experiments were approved by the
Institutional Animal Care and Use Committee of China Medical
University. BGC-823 cells (2×106) were suspended in 0.2
ml of phosphate-buffered saline (PBS) and injected into the right
flank of each BALB/c nude mice. Approximately 2 weeks later
(average tumor size 100 mm3), the mice were randomized
into five groups (n=10) as follows: control [intraperitoneal (i.p.)
injection of 0.2 ml physiological saline daily]; MTD (500 mg/kg
capecitabine daily by gavage for 2 weeks, followed by a 1-week rest
period); LDM (received 200 mg/kg capecitabine daily by gavage);
EGCG (i.p. injection of 1.5 mg EGCG daily) and the combined therapy
with LDM capecitabine and EGCG at doses identical to the single
agent.
Tumor growth and side effects
The mice were closely monitored and body weight and
tumor size were recorded twice a week. Tumor volume (TV) was
estimated using the formula: TV (mm3) =
(width2 × length)/2. Blood samples were collected
through orbital sinus once a week to count white blood cells (WBC).
During the experiment period, side effects, such as weight loss,
change in behavior and feeding, reaction to stimulation and
ruffling of fur, were observed. When a mouse died, the number of
days of survival was recorded.
Real-time polymerase chain reaction (PCR)
analysis
The frozen BGC-823 xenografts were collected and
total RNA was extracted from each xenograft by TRIzol method
(Invitrogen, USA). RNA was reverse-transcribed with oligo (dT)
primers and mRNA expression was analyzed by quantitative real-time
reverse transcription-PCR using the following primers: VEGF sense
5′-GAGCCTTGCCTTGCTGCTCTAC and antisense 5′-CACCAGGGTCTCGATTGGATG.
The relative expression of each gene was assessed in comparison to
the housekeeping gene β-actin amplified with the following primers:
sense 5′-TGGCACCCAGCACAATGAA and antisense
5′-CTAAGTCATAGTCCGCCTAGAAGCA. cDNAs were amplified for 45 cycles
with a PCR Thermal Cycler (Takara Bio) containing the intercalating
agent SYBR Green in a two-step amplification scheme (95°C, 5 sec
and 60°C, 30 sec). Amplifications were normalized to β-actin and
the quantitation of gene expression was performed using the
ΔΔCt calculation, where Ct is the threshold cycle; the
amount of target, normalized to the endogenous control and relative
to the calibrator (vehicle-treated control tumors), is given as
2−ΔΔCt.
Immunohistochemical detection of VEGF and
CD31
Tumor tissues were fixed immediately in 10% buffered
formalin phosphate and embedded in paraffin. Sections (5 μm) were
cut for immunohistochemistry using the PV-9000 kit (Beijing
Zhongshan Goldenbridge Biotechnology Company, Beijing, China).
Rabbit anti-human monoclonal antibody for VEGF (dilution 1:50) was
purchased from Fuzhou Maixin Company (China). Rat anti-mouse
monoclonal antibody for CD31 (dilution 1:100) was purchased from
Dako (Japan). Antigens were retrieved after they were placed in a
pressure cooker at a full pressure for 160 sec in citrate buffer
(pH 6.0). All procedures were implemented according to the
manufacturer's instructions, respectively. For the negative
controls, sections were processed as above, but treated with 0.01
mol/l PBS instead of the primary antibodies.
Cytoplasmic staining was scored positive for VEGF.
The degree of positivity was evaluated by calculating the
percentage of immunoreactive cells on a minimum of 500 cells
(11).
Microvessel density (MVD) was assessed by
immunohistochemical analysis with antibodies to the endothelial
marker CD31 and determined according to the method of Liu et
al (12). Briefly, the
immunostained sections were initially screened at low
magnifications (x40 and x100) to identify hot spots, which are the
areas of highest neovascularization. Any yellow brown-stained
endothelial cell or endothelial cell cluster that was clearly
separate from adjacent microvessels, tumor cells and other
connective tissue elements was considered a single, countable
microvessel. Within the hot spot area, the stained microvessels
were counted in a single high-power (x200) field, and the average
vessel count in three hot spots was considered the value of MVD.
All counts were performed by three investigators in a blinded
manner. Microvessel counts were compared between the observers, and
the discrepant results were reassessed. The consensus was used as
the final score for analysis.
Statistical analysis
Survival data were evaluated by Kaplan-Meier
survival analysis and other data were analyzed by ANOVA, followed
by the Student-Newman-Keuls test. All statistical analyses were
performed using SPSS 17.0 software package. Data are expressed as
the means ± standard error. P-values <0.05 were considered
significant.
Results
Tumor-growth assessment
As shown in Fig. 1,
in mice treated with sterile saline (control group), tumors grew
promptly – all the animals had large tumors after 3 weeks. Compared
to the control, tumor growth in mice treated with MTD capecitabine
was remarkably delayed for the first 2 weeks, after which tumors
began to grow progressively while on therapy. Notably, when LDM
capecitabine was administered continuously, tumor growth was
delayed compared to the MTD group when assessed after 3 weeks of
treatment (P<0.05; Fig. 1).
EGCG retarded tumor growth to much the same extent as MTD
capecitabine (P>0.05; Fig. 1).
Combined therapy with metronomic capecitabine and EGCG resulted in
tumor-growth delays that were more marked and enduring than those
resulting from either of them when used alone (P<0.05; Fig. 1).
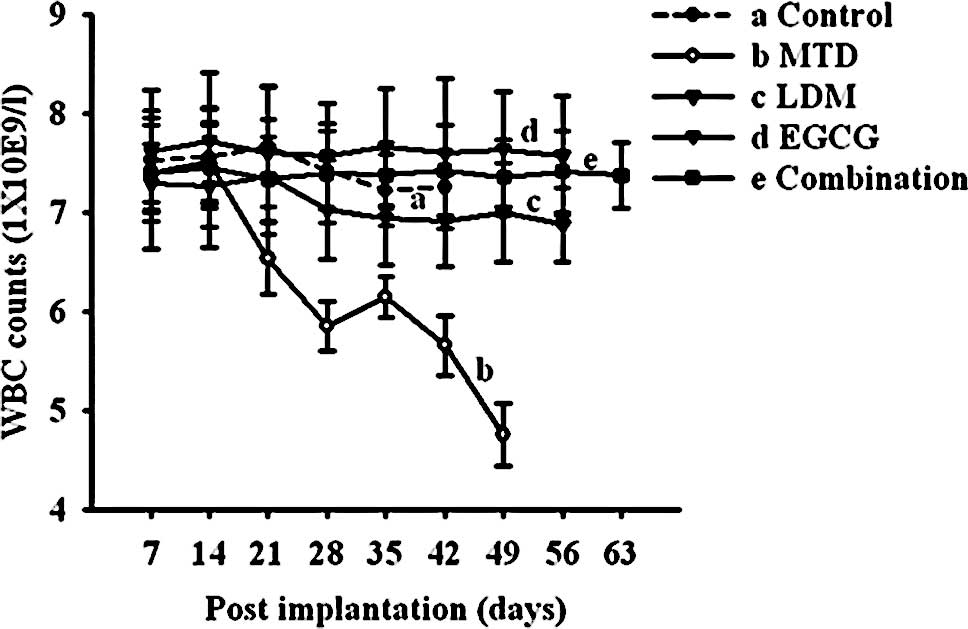
Evaluation of toxicity
Mice treated with the conventional MTD regimen
produced severe side effects, consisting of weight loss, leukopenia
and skin discoloration. As shown in Fig. 2, the five groups of mice had
similar WBC counts in the beginning, but 2 weeks after initiation
of antitumor therapy, a significant decrease in WBC counts was
observed in the MTD group (P<0.05; Fig. 2). The continuous LDM capecitabine
regimen, EGCG regimen and the combined therapy with LDM
capecitabine and EGCG were not associated with weight loss,
leucopenia or other signs of toxicity.
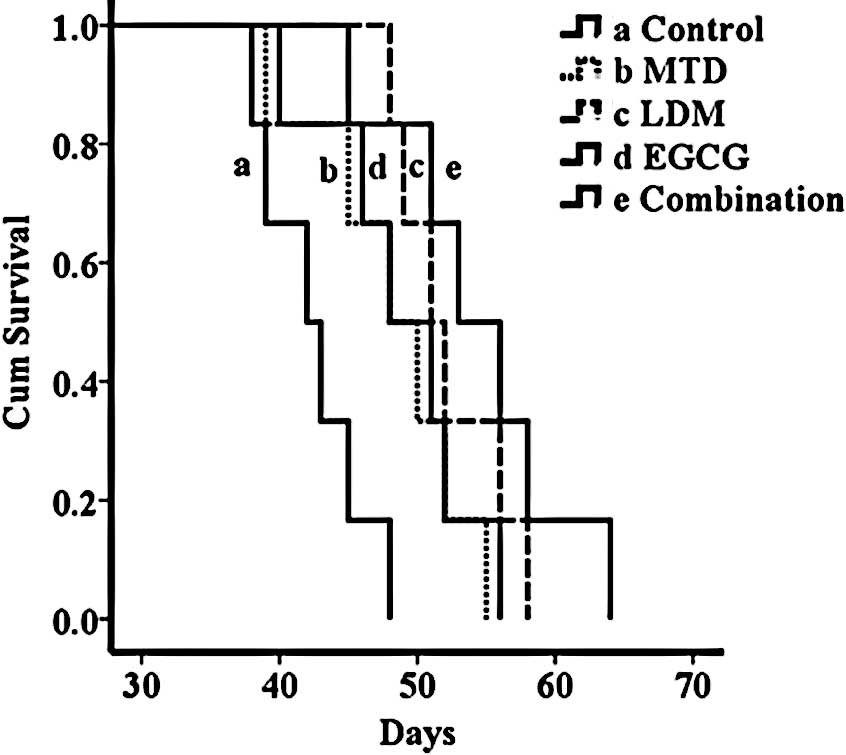
Survival analysis
Median survival time for mice in the control group
was 42 days. Treatment with MTD was able to improve median overall
survival to 48 days (P<0.05; Fig.
3). Mice receiving continuous LDM capecitabine presented a
median overall survival of 51 days, at no significant difference,
when compared to the conventional MTD regimen (P>0.05; Fig. 3). Mice treated with continuous LDM
capecitabine combined with EGCG showed almost the same survival
time as those treated with either of them when used alone
(P>0.05; Fig. 3).
Real-time PCR analysis
Fig. 4 shows the
modulation of human VEGF gene expression in the treated tumors
compared to the vehicle-treated control tumors. The VEGF expression
in the LDM capecitabine-treated group and combination group was
lower than that in the control group (both P<0.05; Fig. 4). The VEGF expression in the MTD
and EGCG groups also was lower than that in the control group,
however, without significant difference (P>0.05; Fig. 4).
Histopathologic analysis
A comparison of angiogenic indices revealed some
variance among different treatment groups (Table I). Tumors derived from mice in the
control group showed the highest microvessel counts, whereas those
from mice subjected to the MTD group had comparatively lower
microvessel counts (P<0.05; Table
I). The LDM or EGCG group had lower microvessel counts than the
MTD group (P<0.05; Table I).
However, the lowest counts in the combination group were measured
(P<0.05; Table I). There was
positive expression of VEGF in the cytoplasm of certain tumor
cells. Compared to the MTD group, VEGF expression was lower in the
continuous LDM capecitabine group (P<0.05; Table I). When EGCG was added to
continuous LDM capecitabine treatment, the indicators were much
lower (P<0.05; Table I). The
results indicated that metronomic capecitabine chemotherapy
inhibited tumor angiogenesis and its anti-angiogenic effect was
further improved when combined with EGCG.
 | Table I.Results of MVD and VEGF in
immunohistochemical staining. |
Table I.
Results of MVD and VEGF in
immunohistochemical staining.
| Group | MVD x̄ ± s
(n/x200) | VEGF x̄ ± s (%) |
|---|
| Control | 30.48±2.87 | 78.43±6.52 |
| MTD capecitabine | 20.13±1.76a | 54.38±3.16a |
| LDM capecitabine | 10.57±1.08b | 41.79±2.93b |
| EGCG | 10.64±1.31b | 50.02±4.75a |
| Combination | 4.01±1.49c | 16.41±2.52c |
Discussion
Our data, for the first time, showed that LDM
capecitabine, used alone or combined with EGCG, is effective in
pre-clinical settings of gastric cancer, as an anti-angiogenic and
antitumor schedule, modulating VEGF gene expression.
LDM chemotherapy may offer several advantages over
the MTD approach: i) it may significantly delay the onset of
acquired drug resistance; ii) it may facilitate long-term
coadministration of different chemotherapeutic drugs lowering
toxicity and adverse side effects; iii) more importantly, it seems
that despite the lower cumulative doses administered, it is
superior to MTD-based regimens for inhibiting tumor growth in
pre-clinical models and clinical trials (13–15).
Until recently, little was known about the anti-angiogenic effect
of capecitabine in gastric cancer. Our findings show that
metronomic capecitabine had significant effects on therapeutic
response and survival. LDM chemotherapy is less likely to acquire
resistance compared to conventional chemotherapy, since the target
of the therapy is presumed to be genetically stable and activated
endothelial cells rather than genetically unstable cancer cells
(16). In our study, tumor growth
was delayed at the prophase in mice receiving MTD capecitabine,
after which the tumors began to grow progressively, which could be
explained by drug resistance. However, when LDM capecitabine was
administered, the regimen produced a long period of growth delay in
tumor mass.
An attractive application of metronomic chemotherapy
is the ability to combine these regimens with biological agents, in
particular anti-angiogenic drugs, and the effects of these
schedules may be further enhanced (17,18).
Such combinations are particularly appealing as high local
concentrations of VEGF in the tumor environment promote multidrug
resistance in tumor endothelium (14,19,20).
In our study, metronomic capecitabine plus EGCG led to a
substantial tumor reduction over the respective monotherapies.
Moreover, we did not observe toxic effects at the doses
administered in the LDM and combinational groups, which remained
similar to those of control mice during the treatment period.
LDM was more effective at inhibiting tumor growth,
which may be attributed to its anti-angiogenic effects.
Angiogenesis is tightly regulated by pro-angiogenic and
anti-endothelial growth factors. Recent advances in the knowledge
of tumor angiogenesis have shed light on the pivotal role of VEGF
(21), and VEGF is an important
mediator of angiogenesis; it is a potent endothelial cell mitogen,
morphogen and vascular permeability-inducing agent (22). MVD is accepted as a standard
indicator of angiogenesis (23),
and VEGF expression is strictly correlated with MVD (24). In the present study, MTD
capecitabine and EGCG did not significantly change the expression
levels of VEGF mRNA; instead, the VEGF mRNA expression in the LDM
and combination groups decreased significantly. Indeed, MVD in
tumor xenografts was markedly decreased in metronomic schedules
when compared to the control group, and also to the MTD regimen,
which were in agreement with the findings of Bocci et al
(11) and Ji et al
(25). In addition, MVD in the
combination group decreased significantly compared to the LDM
group. VEGF protein expression in tumors was similar to that of
MVD. Our data indicated that LDM chemotherapy exhibited the
inhibitory effects of angiogenesis by decreasing MVD value and VEGF
expression. The effects were improved by concurrent administration
of EGCG.
Both LDM and MTD capecitabine increased the life
span of mice implanted with BGC-823 gastric cancer cells, with no
significant difference, when compared to each other. EGCG, given
alone or combined with LDM capecitabine, was also well tolerated
and prolonged the survival time of mice.
Our data indicate that LDM capecitabine and EGCG may
potentiate each other's antitumor and anti-angiogenesis activities.
The mechanism responsible for the interaction between LDM
capecitabine and EGCG remains unclear. On one hand, EGCG modulates
the expression of multidrug resistance proteins (26). Moreover, studies have shown the
inhibitory effect of EGCG on angiogenic growth and transcription
factors, VEGF and hypoxia-inducible factor-1 (HIF-1) (27). On the other hand, EGCG and green
tea are potent inhibitors of neutrophil-mediated angiogenesis in
vitro and in vivo (28). EGCG also inhibited VEGF-induced
endothelial cell proliferation, migration and tube formation
(29). In addition, EGCG has been
shown to modulate growth-factor signaling pathways and,
additionally, has effects on cell-cycle progression and tumor cell
invasion (30). The above effects
may contribute to the synergistic inhibition of tumor angiogenesis
and tumor growth. However, additional effort is required to fully
understand the exact mechanisms.
Taken together, LDM capecitabine chemotherapy alone
or its combination with EGCG inhibited angiogenesis, growth of
gastric cancer and improved survival with less toxicity in a nude
mouse model of BGC-823 gastric cancer. Clinical trials and further
pre-clinical studies will hopefully provide answers regarding the
use of continuous low-dose anti-angiogenic therapies for the
treatment of malignancies.
Acknowledgements
The study was supported by the
National Natural Science Foundation of China (nos. 30973503,
81071650 and 81050007), and the Research Fund for the Doctoral
Program of Higher Education (20092104110008).
References
|
1.
|
Albert A: New drugs in the treatment of
gastric tumors. Clin Transl Oncol. 10:256–261. 2008. View Article : Google Scholar
|
|
2.
|
Orlando L, Cardillo A, Rocca A, et al:
Prolonged clinical benefit with metronomic chemotherapy in patients
with metastatic breast cancer. Anticancer Drugs. 17:961–967. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Scharovsky OG, Mainetti LE and Rozados VR:
Metronomic chemotherapy is changing the paradigm that more is
better. Curr Oncol. 16:7–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tham CK, Choo SP and Poon DY: Capecitabine
with radiation is an effective adjuvant therapy in gastric cancers.
World J Gastroenterol. 16:3709–3715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bergers G and Hanahan D: Combining
antiangiogenic agents with metronomic chemotherapy enhances
efficacy against late-stage pancreatic islet carcinomas in mice.
Cold Spring Harb Symp Quant Biol. 67:293–300. 2002. View Article : Google Scholar
|
|
6.
|
Garcia AA, Hirte H, Fleming G, et al:
Phase II clinical trial of bevacizumab and low-dose metronomic oral
cyclophosphamide in recurrent ovarian cancer: a trial of the
California, Chicago, and Princess Margaret Hospital phase II
consortia. J Clin Oncol. 26:76–82. 2008. View Article : Google Scholar
|
|
7.
|
Klement G, Baruchel S, Rak J, et al:
Continuous low-dose therapy with vinblastine and VEGF receptor-2
antibody induces sustained tumor regression without overt toxicity.
J Clin Invest. 105:R15–R24. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Khan N, Afaq F, Saleem M, Ahmad N and
Mukhtar H: Targeting multiple signaling pathways by green tea
polyphenol (-)-epigallocatechin-3-gallate. Cancer Res.
66:2500–2505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Neuhaus T, Pabst S, Stier S, et al:
Inhibition of the vascular-endothelial growth factor-induced
intracellular signaling and mitogenesis of human endothelial cells
by epigallocatechin-3 gallate. Eur J Pharmacol. 483:223–227. 2004.
View Article : Google Scholar
|
|
10.
|
Fassina G, Vene R, Morini M, et al:
Mechanisms of inhibition of tumor angiogenesis and vascular tumor
growth by epigallocatechin-3-gallate. Clin Cancer Res.
10:4865–4873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bocci G, Falcone A, Fioravanti A, et al:
Antiangiogenic and anticolorectal cancer effects of metronomic
irinotecan chemotherapy alone and in combination with semaxinib. Br
J Cancer. 98:1619–1629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liu TG, Huang Y, Cui D, et al: Inhibitory
effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis
and growth of lung cancer in mice statistical analysis. BMC Cancer.
9:2502009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kamat AA, Kim TJ, Landen CN, et al:
Metronomic chemotherapy enhances the efficacy of antivascular
therapy in ovarian cancer. Cancer Res. 67:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Benelli R, Monteghirfo S, Balbi C, Barboro
P and Ferrari N: Novel antivascular efficacy of metronomic
docetaxel therapy in prostate cancer: hnRNP K as a player. Int J
Cancer. 124:2989–2996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bocci G, Nicolaou KC and Kerbel RS:
Protracted low-dose effects on human endothelial cell proliferation
and survival in vitro reveal a selective antiangiogenic window for
various chemotherapeutic drugs. Cancer Res. 62:6938–6943.
2002.PubMed/NCBI
|
|
17.
|
Zhang M, Tao W, Pan S, Sun X and Jiang H:
Low-dose metronomic chemotherapy of paclitaxel synergizes with
cetuximab to suppress human colon cancer xenografts. Anticancer
Drugs. 20:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kerbel RS: Inhibition of tumor
angiogenesis as a strategy to circumvent acquired resistance to
anticancer therapeutic agents. Bioessays. 13:31–36. 1991.
View Article : Google Scholar
|
|
20.
|
Castilla MA, Caramelo C, Gazapo RM, et al:
Role of vascular endothelial growth factor (VEGF) in endothelial
cell protection against cytotoxic agents. Life Sci. 67:1003–1013.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ma YP, Yang Y, Zhang S, et al: Efficient
inhibition of lung cancer in murine model by plasmid-encoding VEGF
short hairpin RNA in combination with low-dose DDP. J Exp Clin
Cancer Res. 29:562010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Machado DE, Berardo PT, Palmero CY, et al:
Higher expression of vascular endothelial growth factor (VEGF) and
its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a
rat model of peritoneal endometriosis is similar to cancer
diseases. J Exp Clin Cancer Res. 29:42010. View Article : Google Scholar
|
|
23.
|
Magnon C, Galaup A, Rouffiac V, et al:
Dynamic assessment of antiangiogenic therapy by monitoring both
tumoral vascularization and tissue degeneration. Gene Ther.
14:108–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Raspollini MR, Castiglione F, Garbini F,
et al: Correlation of epidermal growth factor receptor expression
with tumor microdensity vessels and with vascular endothelial
growth factor expression in ovarian carcinoma. Int J Surg Pathol.
13:135–142. 2005. View Article : Google Scholar
|
|
25.
|
Ji Y, Hayashi K, Amoh Y, et al: The
camptothecin derivative CPT-11 inhibits angiogenesis in a
dual-color imageable orthotopic metastatic nude mouse model of
human colon cancer. Anticancer Res. 27:713–718. 2007.
|
|
26.
|
Mei Y, Qian F, Wei D and Liu J: Reversal
of cancer multidrug resistance by green tea polyphenols. J Pharm
Pharmacol. 56:1307–1314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Domingo DS, Camouse MM, Hsia AH, et al:
Anti-angiogenic effects of epigallocatechin-3-gallate in human
skin. Int J Clin Exp Pathol. 3:705–709. 2010.PubMed/NCBI
|
|
28.
|
Donà M, Dell'Aica I, Calabrese F, et al:
Neutrophil restraint by green tea: inhibition of inflammation,
associated angiogenesis, and pulmonary fibrosis. J Immunol.
15:4335–4341. 2003.PubMed/NCBI
|
|
29.
|
Zhu BH, Zhan WH, Li ZR, et al:
(-)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by
reducing VEGF production and angiogenesis. World J Gastroenterol.
13:1162–1169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lang M, Henson R, Braconi C and Patel T:
Epigallocatechin-gallate modulates chemotherapy-induced apoptosis
in human cholangiocarcinoma cells. Liver Int. 29:670–677. 2009.
View Article : Google Scholar : PubMed/NCBI
|