Introduction
Pleural effusion is a common complication which most
commonly results from cardiac failure, pneumonia and malignant
neoplasms (1). However, it is
occasionally difficult to differentiate malignant pleural effusions
(MPEs) from benign effusions. The sensitivity of conventional
cytological examination is just 60% (2), while closed pleural biopsy is only
able to identify an additional 7% of the cytology-negative MPE
patients (3). Image-guided
percutaneous and thoracoscopic pleural biopsies provide a high
sensitivity (4), but they may not
be widely used in all facilities or be well-tolerated.
A number of tumor markers have been studied in
attempts to improve the accuracy of MPE diagnosis. Two previously
published meta-analyses (5,6)
investigated the diagnostic value of the pleural carcinoembryonic
antigen (CEA), carbohydrate antigens (CAs) 125, 15–3 and 19–9 and
CYFRA 21-1 in MPE but failed to identify a reliable tumor marker
with high sensitivity and specificity. Therefore, it is imperative
to identify a novel pleural marker to increase diagnostic
accuracy.
Vascular endothelial growth factor (VEGF), or
vascular permeability factor, is a glycoprotein that functions as a
mediator of angiogenesis. It is expressed by various types of
tumors (7) as well as certain
normal tissues, including the lung, kidney, adrenal gland, heart,
liver and stomach mucosa (8). VEGF
is pivotal in the formation of MPE, as it increases vascular
permeability and vascular leakage of fluid (9,10).
In addition, a high level of pleural VEGF has been found to be
correlated with malignancy (11)
and thus an increasing number of studies consider VEGF to be a
marker for the diagnosis of MPE (12–14).
However, conflicting results have been reported and the exact role
of VEGF remains unclear. Therefore, we performed the present
meta-analysis to establish the overall accuracy of pleural VEGF for
diagnosing MPE.
Materials and methods
Search strategy and study selection
To find relevant studies, we performed searches of
Pubmed (Medline), Embase, Web of Science and the Cochrane database
up to November 30, 2011, using the key words ‘pleural effusion’,
‘malignant pleural effusions’, ‘vascular endothelial growth
factor’, ‘sensitivity and specificity’ and ‘accuracy’. All searches
were limited to English language publications concerning human
studies. A manual search of the references of the retrieved
articles was conducted subsequently. Conference abstracts and
letters to the editor were excluded due to the limited data
provided. A study was included in the present meta-analysis if it
provided the sensitivity and specificity of pleural VEGF for the
diagnosis of MPE. Two authors (Y.-C. Shen and M.-Q. Liu)
independently screened the articles for inclusion. Disagreements
between the reviewers were resolved by consensus.
Data extraction and quality
assessment
The final articles included were assessed
independently by two reviewers (Y.-C. Shen and M.-Q. Liu). Data
retrieved from the studies included author, publication year,
patient source, test method, cut-off value, sensitivity,
specificity and methodological quality. To assess the trial
methodology, the articles were reviewed independently by two
authors (Y.-C. Shen and M.-Q. Liu) and assigned a quality score
using the STARD (standards for reporting diagnostic accuracy, a
guideline that aims to improve the quality of the reporting of
diagnostic studies, maximum score 25) (15) and the QUADAS (quality assessment
for studies of diagnostic accuracy, an evidence-based quality
assessment tool to be used in systematic reviews of diagnostic
accuracy studies, maximum score 14) tools (16).
Statistical analyses
The standard methods recommended for the diagnostic
accuracy of meta-analyses were used in the present study (17). The following indices of test
accuracy were computed for each study: sensitivity, specificity,
positive likelihood ratio (PLR), negative likelihood ratio (NLR)
and diagnostic odds ratio (DOR). The diagnostic threshold
identified for each study was used to plot a summary receiver
operating characteristic (SROC) curve (18). The average sensitivity, specificity
and other related indices of the studies were calculated using a
random-effects model (19).
Spearman’s rank correlation was performed as a test for threshold
effect. The χ2 and Fisher’s exact tests were used to
detect statistically significant heterogeneity across the studies.
If there were enough studies, subgroup analyses would be performed
to explore potential between-study heterogeneity (20). All analyses were performed using
two statistical software programs (Stata, version 11; Stata
Corporation, College Station, TX, USA and Meta-DiSc for Windows; XI
Cochrane Colloquium, Barcelona, Spain). All statistical tests were
two-sided and P<0.05 was considered to indicate a statistically
significant result.
Results
Quality reports and study
characteristics
Following independent review, 181 publications
concerning VEGF and pleural effusions were considered to be
eligible for inclusion in the analysis. Of these publications, 144
were excluded for being beyond the scope of the present study, one
was excluded due to the lack of a control group (9), two letters to the editor were
excluded due to the limited data they contained (21,22),
four publications were excluded as they recruited <10 patients
in one of study groups (10,11,14,23)
and 20 were excluded as they did not allow data extraction or
calculation of the sensitivity and specificity (12,13,24–41).
The remaining 10 studies, based on 514 patients with MPE and 511
without MPE, were available for the meta-analysis (42–51).
The diagnostic characteristics of these studies and their STARD and
QUADAS scores are outlined in Table
I. Of the 10 articles included, 8 had STARD scores ≥13 and 9
had QUADAS scores ≥10.
 | Table I.Summary of the studies included in the
meta-analysis. |
Table I.
Summary of the studies included in the
meta-analysis.
| | | | | | | | Quality scores
|
|---|
| Author/year
(ref.) | Country | Method | Cut-off | TP | FP | FN | TN | STARD | QUADAS |
|---|
| Fiorelli et
al, 2011 (51) | Italy | ELISA | 652 pg/ml | 31 | 5 | 18 | 25 | 15 | 11 |
| Chen et al,
2010 (50) | China | PCR | NA | 76 | 4 | 16 | 32 | 14 | 10 |
| Zhou et al,
2009 (49) | China | ELISA | 1.6 ng/ml | 44 | 25 | 18 | 39 | 17 | 12 |
| Duysinx et
al, 2008 (48) | Belgium | ELISA | 382 pg/ml | 44 | 18 | 20 | 21 | 17 | 11 |
| Cheng et al,
2008 (47) | China | PCR | NA | 11 | 9 | 3 | 5 | 12 | 9 |
| Xue et al,
2007 (46) | China | ELISA | 945.7 pg/ml | 34 | 7 | 8 | 38 | 17 | 12 |
| Shu et al,
2007 (45) | China | ELISA | 959.25 pg/ml | 15 | 2 | 17 | 47 | 20 | 13 |
| Sack et al,
2005 (44) | Germany | ELISA | NA | 77 | 50 | 19 | 68 | 16 | 11 |
| Momi et al,
2002 (43) | Japan | ELISA | 2000 pg/ml | 38 | 14 | 0 | 75 | 15 | 11 |
| Yeo et al,
1993 (42) | USA | IFA | 10 pm | 18 | 7 | 7 | 20 | 11 | 10 |
Diagnostic accuracy
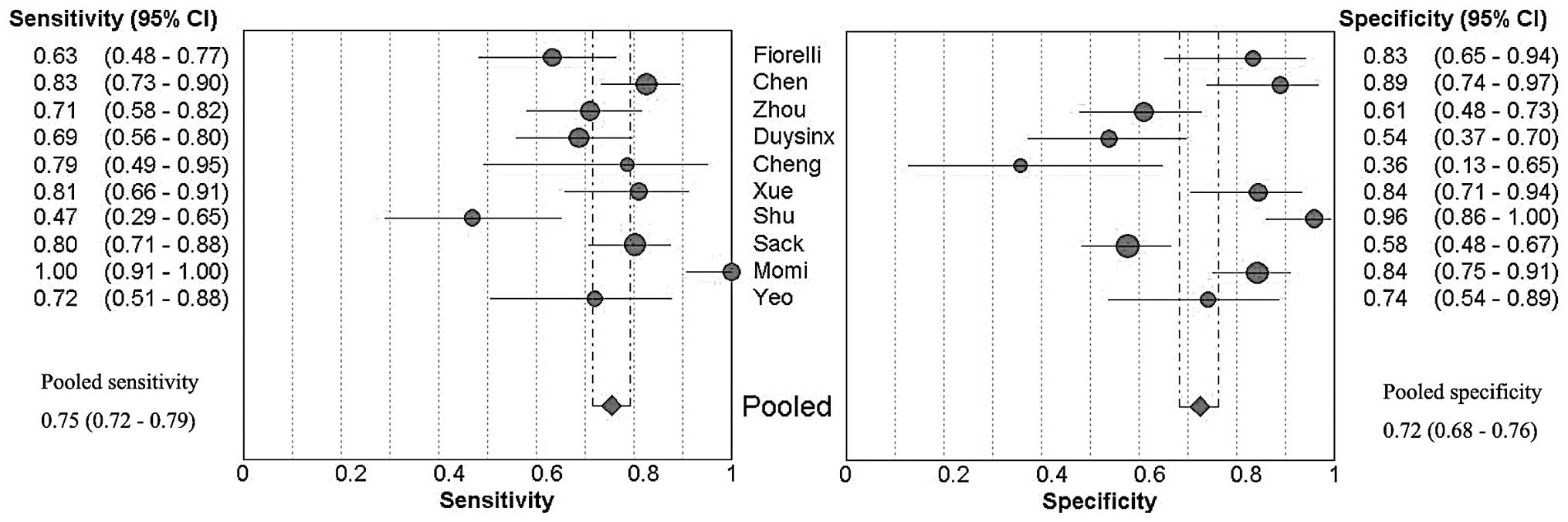
Forest plots of the sensitivity and specificity of
these 10 studies concerning pleural VEGF assays in the diagnosis of
MPE are shown in Fig. 1. The
average sample size of the studies included was 102 (range,
28–214). The sensitivity and specificity ranged from 0.47 to 1.00
[mean, 0.75; 95% confidence interval (CI), 0.72–0.79] and from 0.36
to 0.96 (mean, 0.72; 95% CI, 0.68–0.76), respectively. The PLR was
2.94 (95% CI, 1.97–4.41), the NLR was 0.38 (95% CI, 0.27–0.51) and
the DOR was 9.05 (95% CI, 4.60–17.80). χ2 values of
sensitivity, specificity, PLR, NLR and DOR were 44.02, 67.36,
57.84, 30.72 and 34.21, respectively, with all P-values <0.001,
suggesting a marked heterogeneity among the studies.
Fig. 2 shows the
SROC curve plotting the true-positive against the false-positive
rates of the individual studies. As a global measure of test
efficacy we used the Q-value, which is the intersection point of
the SROC curve with a diagonal line from the left upper corner to
the right lower corner of the ROC space and corresponds to the
highest common value of sensitivity and specificity for the test.
This point does not indicate the only or even the best combination
of sensitivity and specificity for a particular clinical setting,
but represents an overall measure of the discriminatory power of a
test. In the present meta-analysis, the maximum joint sensitivity
and specificity of our study was 0.75 (the Q-value). The area under
the curve (AUC) was 0.82, indicating that the level of overall
accuracy was not high.
Discussion
Our meta-analysis evaluates the diagnostic role of
pleural VEGF in MPE and our data demonstrate that determining
pleural VEGF results in a moderate sensitivity of 0.75 (95% CI,
0.72–0.79) and a specificity of 0.72 (95% CI, 0.68–0.76). It
appears that VEGF determination may be most applicable in screening
for MPE, although the relatively low specificity of VEGF may not be
sufficient to confirm the diagnosis of MPE. This trade-off has
significant clinical implications.
The SROC curve presents a global summary of test
performance and shows the trade-off between sensitivity and
specificity. The results of the analysis based on the SROC curve
revealed that the maximum joint sensitivity and specificity was
0.75, while the AUC was 0.82, suggesting that the level of overall
accuracy was not as high as expected. DOR, the ratio of the odds of
positive test results in patients with the disease relative to
those in patients without the disease, is a single indicator of
test accuracy that combines the data from sensitivity and
specificity into a single number (52). The value of a DOR ranges from 0 to
infinity, with higher values indicating a superior discriminatory
test performance (higher accuracy). A DOR of 1.0 indicates that a
test does not discriminate between patients with the disorder and
those without it. In our meta-analysis, the mean DOR was 9.05,
indicating that VEGF assays appeared to aid the diagnosis of MPE.
Since the SROC curve and the DOR are not easy to interpret and use
in clinical practice, while likelihood ratios are considered to be
more clinically meaningful, we also presented PLR and NLR as
measures of diagnostic accuracy. A PLR value of 2.94 suggests that
patients with MPE have an approximately 3-fold higher chance of
being VEGF assay-positive compared with patients without MPE, but
this is not high enough for clinical practice. On the other hand,
NLR was found to be 0.38 in the present meta-analysis. This means
that, if the VEGF assay result was negative, the probability that
the patient has MPE is 39%, which is not low enough to rule out
MPE.
Although the present study was performed with a
comprehensive search strategy and data extraction, our
meta-analysis has several limitations. First, we excluded
conference abstracts and letters to the editor. This may lead to
publication bias, which may also be introduced by inflation of
diagnostic accuracy estimates, since studies that report positive
findings are more likely to be accepted for publication. In
addition, due to the limited numbers of the studies included, we
did not use the STARD and QUADAS scores to perform the
meta-regression analysis to assess the effect of study quality on
the relative DOR of VEGF in the diagnosis of MPE. For the same
reason, we were unable to explore whether study design, including
blinded, cross-sectional, consecutive/random and prospective
designs, affects the diagnostic accuracy.
The results of the present meta-analysis suggest
that VEGF may, to a certain extent, play a role in the diagnosis of
MPE, while its diagnostic value is not satisfactory. The
combination of VEGF with other markers or examinations in pleural
effusion may aid the establishment of the diagnosis of MPE. For
instance, the combination of VEGF mRNA and endostatin mRNA has been
reported to result in a high-diagnostic performance, with a
sensitivity of 95.7% and an accuracy of 93.8%, respectively
(50). The combined use of
cytological examinations and VEGF has been found to increase the
detection rate of malignancy with respect to cytological
examination (51). Although the
traditional method for the diagnosis of MPE remains cytological
and/or histological examination, pathologists do not recommend
making a diagnosis based on cytological samples alone due to the
high risk of diagnostic error. In addition, invasive thoracoscopy
may not be available in all hospitals, so the VEGF test is not only
a useful adjunct to conventional diagnostic tools in diagnosing
malignancy, but also guides the inclusion of patients who may
benefit from further invasive procedures.
In summary, pleural VEGF determination plays a role
in the diagnosis of MPE, while the results of VEGF assays should be
interpreted in parallel with clinical findings and the results of
conventional tests.
Acknowledgements
This study was supported by grants
#30971327 and 31171103 from the National Natural Science Foundation
of China and #00-722 and 06-834 from the China Medical Board of New
York to Dr Fu-Qiang Wen.
References
|
1.
|
McGrath EE and Anderson PB: Diagnosis of
pleural effusion: a systematic approach. Am J Crit Care.
20:119–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bennett R and Maskell N: Management of
malignant pleural effusions. Curr Opin Pulm Med. 11:296–300.
2005.PubMed/NCBI
|
|
3.
|
Prakash UB and Reiman HM: Comparison of
needle biopsy with cytologic analysis for the evaluation of pleural
effusion: analysis of 414 cases. Mayo Clin Proc. 60:158–164. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lombardi G, Zustovich F, Nicoletto MO,
Donach M, Artioli G and Pastorelli D: Diagnosis and treatment of
malignant pleural effusion: a systematic literature review and new
approaches. Am J Clin Oncol. 33:420–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shi HZ, Liang QL, Jiang J, Qin XJ and Yang
HB: Diagnostic value of carcinoembryonic antigen in malignant
pleural effusion: a meta-analysis. Respirology. 13:518–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang
J and Yang HB: Diagnostic accuracy of tumour markers for malignant
pleural effusion: a meta-analysis. Thorax. 63:35–41. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Senger DR, Van de Water L, Brown LF, et
al: Vascular permeability factor (VPF, VEGF) in tumor biology.
Cancer Metastasis Rev. 12:303–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Berse B, Brown LF, Van de Water L, Dvorak
HF and Senger DR: Vascular permeability factor (vascular
endothelial growth factor) gene is expressed differentially in
normal tissues, macrophages, and tumors. Mol Biol Cell. 3:211–220.
1992. View Article : Google Scholar
|
|
9.
|
Zebrowski BK, Yano S, Liu W, Shaheen RM,
Hicklin DJ, Putnam JB Jr and Ellis LM: Vascular endothelial growth
factor levels and induction of permeability in malignant pleural
effusions. Clin Cancer Res. 5:3364–3368. 1999.PubMed/NCBI
|
|
10.
|
Kraft A, Weindel K, Ochs A, et al:
Vascular endothelial growth factor in the sera and effusions of
patients with malignant and nonmalignant disease. Cancer.
85:178–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ishimoto O, Saijo Y, Narumi K, et al: High
level of vascular endothelial growth factor in hemorrhagic pleural
effusion of cancer. Oncology. 63:70–75. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lim SC, Jung SI, Kim YC and Park KO:
Vascular endothelial growth factor in malignant and tuberculous
pleural effusions. J Korean Med Sci. 15:279–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hamed EA, El-Noweihi AM, Mohamed AZ and
Mahmoud A: Vasoactive mediators (VEGF and TNF-alpha) in patients
with malignant and tuberculous pleural effusions. Respirology.
9:81–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kishiro I, Kato S, Fuse D, Yoshida T,
Machida S and Kaneko N: Clinical significance of vascular
endothelial growth factor in patients with primary lung cancer.
Respirology. 7:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bossuyt PM, Reitsma JB, Bruns DE, et al:
Towards complete and accurate reporting of studies of diagnostic
accuracy: the STARD initiative. Standards for Reporting of
Diagnostic Accuracy. Clin Chem. 49:1–6. 2003. View Article : Google Scholar
|
|
16.
|
Whiting P, Rutjes AW, Reitsma JB, Bossuyt
PM and Kleijnen J: The development of QUADAS: a tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3:252003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Jones CM, Ashrafian H, Skapinakis P, Arora
S, Darzi A, Dimopoulos K and Athanasiou T: Diagnostic accuracy
meta-analysis: a review of the basic principles of interpretation
and application. Int J Cardiol. 140:138–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Moses LE, Shapiro D and Littenberg B:
Combining independent studies of a diagnostic test into a summary
ROC curve: data-analytic approaches and some additional
considerations. Stat Med. 12:1293–1316. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lijmer JG, Bossuyt PM and Heisterkamp SH:
Exploring sources of heterogeneity in systematic reviews of
diagnostic tests. Stat Med. 21:1525–1537. 2002.PubMed/NCBI
|
|
21.
|
Koniari I, Koletti B and Apostolakis E:
Vascular endothelial growth factor with tumour growth factor-beta,
endostatin, proteinases or cytokines might be useful for
differential diagnosis of pleural effusions. Interact Cardiovasc
Thorac Surg. 12:424–425. 2011. View Article : Google Scholar
|
|
22.
|
Kiropoulos TS, Kostikas K and
Gourgoulianis KI: Vascular endothelial growth factor levels in
pleural fluid and serum of patients with tuberculous pleural
effusions. Chest. 128:4682005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Economidou F, Antoniou KM, Soufla G, et
al: Role of VEGF-stromal cell-derived factor-1alpha/CXCL12 axis in
pleural effusion of lung cancer. J Recept Signal Transduct Res.
30:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kotyza J, Havel D, Vrzalová J, Kulda V and
Pesek M: Diagnostic and prognostic significance of inflammatory
markers in lung cancer-associated pleural effusions. Int J Biol
Markers. 25:12–20. 2010.PubMed/NCBI
|
|
25.
|
Bunatova K, Obermajer N, Kotyza J, Pesek M
and Kos J: Levels of cathepsins S and H in pleural fluids of
inflammatory and neoplastic origin. Int J Biol Markers. 24:47–51.
2009.PubMed/NCBI
|
|
26.
|
Economidou F, Antoniou KM, Tzanakis N,
Sfiridaki K, Siafakas NM and Schiza SE: Angiogenic molecule Tie-2
and VEGF in the pathogenesis of pleural effusions. Respir Med.
102:774–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Atanackovic D, Cao Y, Kim JW, et al: The
local cytokine and chemokine milieu within malignant effusions.
Tumour Biol. 29:93–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tomimoto H, Yano S, Muguruma H, Kakiuchi S
and Sone S: Levels of soluble vascular endothelial growth factor
receptor 1 are elevated in the exudative pleural effusions. J Med
Invest. 54:146–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Daniil ZD, Zintzaras E, Kiropoulos T,
Papaioannou AI, Koutsokera A, Kastanis A and Gourgoulianis KI:
Discrimination of exudative pleural effusions based on multiple
biological parameters. Eur Respir J. 30:957–964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yeh HH, Lai WW, Chen HH, Liu HS and Su WC:
Autocrine IL-6-induced Stat3 activation contributes to the
pathogenesis of lung adenocarcinoma and malignant pleural effusion.
Oncogene. 25:4300–4309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kalomenidis I, Kollintza A, Sigala I,
Papapetropoulos A, Papiris S, Light RW and Roussos C:
Angiopoietin-2 levels are elevated in exudative pleural effusions.
Chest. 129:1259–1266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Ruiz E, Alemán C, Alegre J, et al:
Angiogenic factors and angiogenesis inhibitors in exudative pleural
effusions. Lung. 183:185–195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Jin HY, Lee KS, Jin SM and Lee YC:
Vascular endothelial growth factor correlates with matrix
metalloproteinase-9 in the pleural effusion. Respir Med.
98:115–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Davidson B, Vintman L, Zcharia E, et al:
Heparanase and basic fibroblast growth factor are co-expressed in
malignant mesothelioma. Clin Exp Metastasis. 21:469–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
van Hensbergen Y, Broxterman HJ,
Hanemaaijer R, et al: Soluble aminopeptidase N/CD13 in malignant
and nonmalignant effusions and intratumoral fluid. Clin Cancer Res.
8:3747–3754. 2002.PubMed/NCBI
|
|
36.
|
J Strizzi L, Catalano A, Vianale G, et al:
Vascular endothelial growth factor is an autocrine growth factor in
human malignant mesothelioma. J Pathol. 193:468–475.
2001.PubMed/NCBI
|
|
37.
|
Verheul HM, Hoekman K, Jorna AS, Smit EF
and Pinedo HM: Targeting vascular endothelial growth factor
blockade: ascites and pleural effusion formation. Oncologist.
5(Suppl 1): 45–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cheng D, Lee YC, Rogers JT, Perkett EA,
Moyers JP, Rodriguez RM and Light RW: Vascular endothelial growth
factor level correlates with transforming growth factor-beta
isoform levels in pleural effusions. Chest. 118:1747–1753. 2000.
View Article : Google Scholar
|
|
39.
|
Yanagawa H, Takeuchi E, Suzuki Y, Ohmoto
Y, Bando H and Sone S: Vascular endothelial growth factor in
malignant pleural effusion associated with lung cancer. Cancer
Immunol Immunother. 48:396–400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Thickett DR, Armstrong L and Millar AB:
Vascular endothelial growth factor (VEGF) in inflammatory and
malignant pleural effusions. Thorax. 54:707–710. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Cheng D, Rodriguez RM, Perkett EA, Rogers
J, Bienvenu G, Lappalainen U and Light RW: Vascular endothelial
growth factor in pleural fluid. Chest. 116:760–765. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Yeo KT, Wang HH, Nagy JA, et al: Vascular
permeability factor (vascular endothelial growth factor) in guinea
pig and human tumor and inflammatory effusions. Cancer Res.
53:2912–2918. 1993.PubMed/NCBI
|
|
43.
|
Momi H, Matsuyama W, Inoue K, Kawabata M,
Arimura K, Fukunaga H and Osame M: Vascular endothelial growth
factor and proinflammatory cytokines in pleural effusions. Respir
Med. 96:817–822. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Sack U, Hoffmann M, Zhao XJ, et al:
Vascular endothelial growth factor in pleural effusions of
different origin. Eur Respir J. 25:600–604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Shu J, Sun G, Liu H and Liu J: Clinical
utility of vascular endothelial growth factor in diagnosing
malignant pleural effusions. Acta Oncol. 46:1004–1011. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Xue K, Xiong S and Xiong W: Clinical value
of vascular endothelial growth factor combined with
interferon-gamma in diagnosing malignant pleural effusion and
tuberculous pleural effusion. J Huazhong Univ Sci Technolog Med
Sci. 27:495–497. 2007. View Article : Google Scholar
|
|
47.
|
Cheng M, Chen Y, Yu X, Tian Z and Wei H:
Diagnostic utility of LunX mRNA in peripheral blood and pleural
fluid in patients with primary non-small cell lung cancer. BMC
Cancer. 8:1562008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Duysinx BC, Corhay JL, Hubin L, Nguyen D,
Henket M and Louis R: Diagnostic value of interleukine-6,
transforming growth factor-beta 1 and vascular endothelial growth
factor in malignant pleural effusions. Respir Med. 102:1708–1714.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Zhou WB, Bai M and Jin Y: Diagnostic value
of vascular endothelial growth factor and endostatin in malignant
pleural effusions. Int J Tuberc Lung Dis. 13:381–386.
2009.PubMed/NCBI
|
|
50.
|
Chen Y, Liang B, Zhao YJ, Wang SC, Fan YB
and Wu GP: Transcription expression and clinical significance of
vascular endothelial growth factor mRNA and endostatin mRNA in
pleural effusions of patients with lung cancer. Diagn Cytopathol.
Oct. 26–2010, (E-pub ahead of print). View
Article : Google Scholar
|
|
51.
|
Fiorelli A, Vicidomini G, Di Domenico M,
et al: Vascular endothelial growth factor in pleural fluid for
differential diagnosis of benign and malignant origin and its
clinical applications. Interact Cardiovasc Thorac Surg. 12:420–424.
2011. View Article : Google Scholar
|
|
52.
|
Glas AS, Lijmer JG, Prins MH, Bonsel GJ
and Bossuyt PM: The diagnostic odds ratio: a single indicator of
test performance. J Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|