Introduction
Esophageal carcinoma is an age-related neoplasm with
a 5-year overall survival rate of less than 35% (1,2).
Males more than 40 years of age are at the highest risk of
esophageal carcinoma (3). Results
of studies have revealed that age-related changes affect the
molecular crosstalk between the stromal and epithelial cells,
generating a more permissive environment for tumor growth and
metastasis (4). Senescence may be
one of the mechanism which is responsible for this age-related
change in the microenvironment. The definition of senescence is a
process that keeps the stable form of cell cycle arrest at the
G1 phase (5), as first
reported by Hayflick (6). At the
earlier stage of the process, senescence acts as an
antiproliferative factor, which prevents tumorigenesis and leads to
cell cycle arrest. However, if oncogenic mutations are present,
these senescent cells may become immortal and initiate the
development of additional genetic ‘hits’ in tumorigenesis (7–9).
Cell cycle regulators within the retinoblastoma (Rb) and p53
pathways, including the cyclin-dependent kinase (CDK) and CDK
inhibitor (CDKI) proteins, are the gatekeepers which maintain the
senescence program. p14ARF, p15INK4b and
p16INK4a have been identified as CDKIs and act as
senescence maintenance factors.
The INK4a/ARF locus (9p21) is crucial in the
pathogenesis of several types of malignant disorders and encodes
two unrelated cell cycle regulators, p14ARF and
p16INK4a, which have been regarded as significant
senescence markers in clinical diagnosis (10). The p16INK4a gene encodes
a p16 protein that binds competitively to CDK4 and, during
G1 phase, inhibits the interaction of CDK4 and cyclin
D1 to stimulate passage through the cell cycle (11–13).
The p16 protein is often highly expressed in senescent cells in
culture and is inactivated in a variety of human cancers.
p15INK4b is located centromeric to the p16/p14 gene
locus p14ARF, which is a major tumor suppressor and
causes cell cycle arrest through transforming growth factor β
(14).
Previous data revealed, through staining, that
p14ARF, p15INK4b and p16INK4a are
abundantly expressed in premalignant lung cancers and have
essentially no expression in malignant lung cancers, which
indicates that senescence is inactivated in the proliferated lung
epithelial cells of lung cancers (15). The same results have been observed
in pancreatic, hepatic and breast cancers. Tumorigenesis is defined
as an outcome of the accumulation of abnormal stroma facilitated by
peripheral senescent fibroblasts and the inactivation of the
senescence of proliferating epithelial cells. However, more recent
evidence has revealed the high expression of the senescence markers
p14ARF, p15INK4b, p16INK4a and
DCR2 in prostate cancer epithelial cells, indicating that
senescence continued to be activated in these proliferating
epithelial cells.
A balance between proliferation and senescence,
instead of the inactivity of senescence only, appears to decide the
fate of epithelial cells, converging on the probability of
epithelial tumorigenesis.
Since the true mechanism involved in the process
from senescence to tumor formation in epithelial cells remains
elusive (16–19), in the present study we compared the
expression of the senescence markers p14ARF,
p15INK4b and p16INK4a and the proliferation
markers bcl-2, ki-67 and skp2 in tissue blocks from normal
esophageal epithelium, esophageal intraepithelial dysplasia (EID)
and esophageal squamous cell carcinoma (ESCC). The purpose of the
present study was to describe the predictors of biological activity
in esophageal carcinoma and to provide evidence that may assist in
clinical diagnosis.
Materials and methods
Sample selection
The preparation of the tissue slides was performed
as previously described (20).
Tissue blocks (n=91) were created from samples obtained from 80
patients from the People's Hospital of Sichuan (Sichuan, China).
The samples included 20 cases of normal esophageal tissue, 11 cases
of EID, 49 cases of low-grade ESCC and 11 cases of high-grade ESCC
Specimens were obtained from patients (aged 42–74 years, mean age
57) who had not received either chemotherapy or irradiation prior
to surgery. The basic information of the ESCC patients is
summarized in Table I. All samples
were diagnosed in duplicate by pathologists from Sichuan University
(Sichuan, China) who observed the samples under the microscope and
individually scored each slide. The use of human tissue in this
study was approved by the Institutional Review Board.
 | Table I.Clinicopathological features of the
ESCC patients. |
Table I.
Clinicopathological features of the
ESCC patients.
| Feature | n (%) |
|---|
| Gender | |
| Male | 46 (77) |
| Female | 14 (23) |
| Degree of
differentiation | |
| Well | 17 (28) |
| Poor | 43 (72) |
| Lymph node
metastasis | |
| Negative | 28 (47) |
| Positive | 32 (53) |
| TNM stage | |
| I | 6 (10) |
| IIA | 22 (37) |
| IIB | 14 (23) |
| III | 18 (30) |
Immunohistochemical staining
The streptavidin-peroxidase immunohistostaining
method was performed as described previously (20). Briefly, samples were fixed in 10%
formalin buffer and embedded in paraffin. Tissue sections (4 μm
thick) were steamed in universal decloaker (Biocare Medical, Walnut
Creek, CA, USA) for antigen retrieval, followed by 19 min
protein-blocking (Biocare Medical). All slides were first incubated
against p14ARF, p15INK4b and
p16INK4a (1:300, for 1 h at room temperature; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and then treated with
anti-rabbit secondary antibody (Biocare Medical) and horseradish
peroxidase for 15 min each. The tissues were stained for 3 min with
high sensitivity 3,3′-diaminobenzidine tetrahydrochloride,
counterstained with hematoxylin, dehydrated and then mounted
(21,22).
On the basis of the expression patterns in the
esophageal epithelial cells, the expression of the
p14ARF, p15INK4b, p16INK4a, bcl-2,
ki-67 and skp2 proteins was considered positive when any positive
staining was observed in the epithelial cells. Immunostained tissue
slides were semi-quantitatively scored by two independent
pathologists (Z.J. and Y.F.) blinded to the clinical information.
Quantification was performed using a four-score grading system.
Statistical analysis
All statistical analyses were performed using the
SPSS 10.0 statistical software program (SPSS, Chicago, IL, USA).
The χ2 and Fisher's exact tests were used to assess the
correlation between the expression of these biological markers and
the clinical parameters of the patients. P<0.05 was considered
to indicate a statistically significant result.
Results
As shown in Fig. 1,
p14ARF, p15INK4b, p16INK4a and
bcl-2 expression was observed in the cytoplasm of esophageal
epithelial cells with no evidence of nuclear staining, while skp2
and ki-67 showed predominant nuclear staining at different portion
of epithelial.
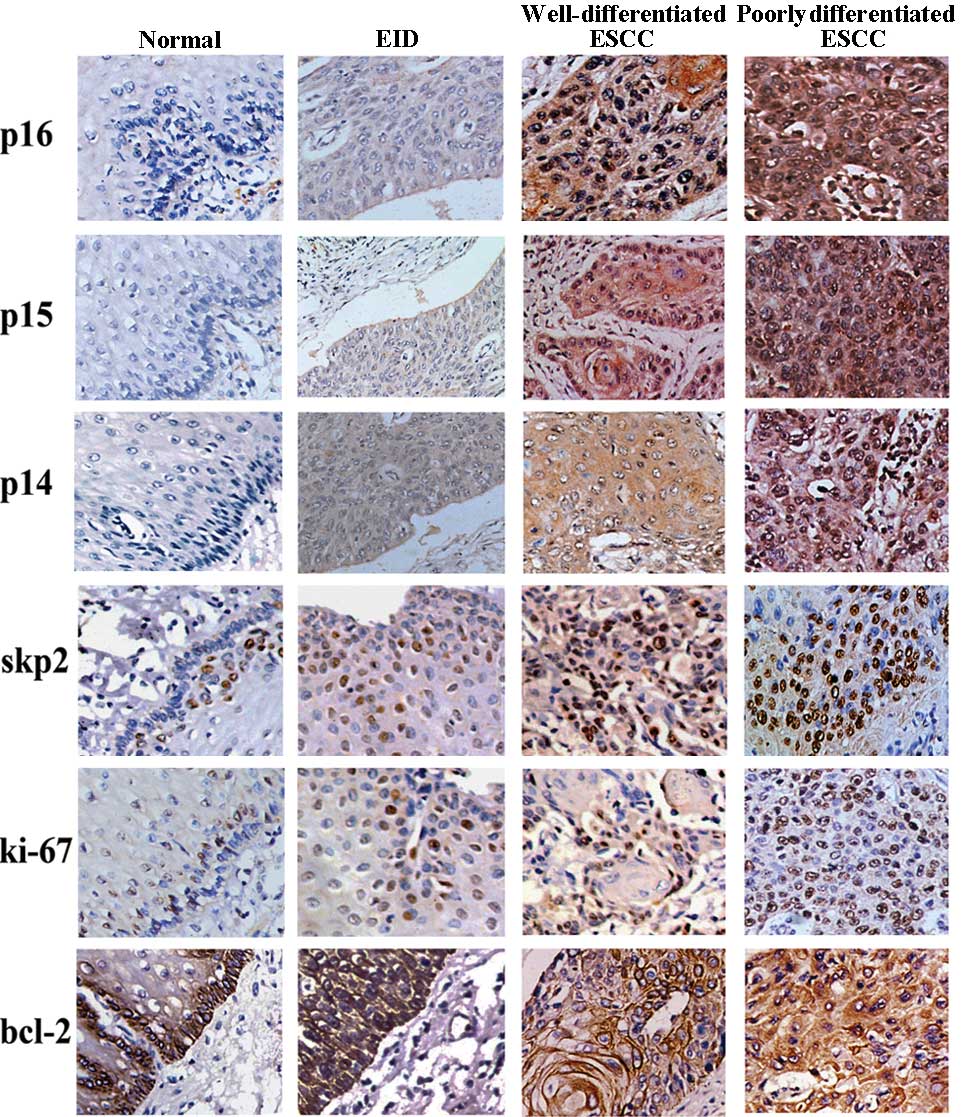 | Figure 1.Expression of different markers in
esophageal tissues (×40): Normal tissue, from top to bottom: (A)
p16, negative; (B) p15, negative; (C) p14, negative; (D) skp2,
negative; (E) ki-67, negative; (F) bcl-2, positive. EID, from top
to bottom: (A) p16, positive; (B) p15, positive; (C) p14, negative;
(D) skp2, positive, (E) ki-67, positive; (F) bcl-2, positive.
Well-differentiated ESCC, from top to bottom: (A) p16, positive;
(B) p15, positive; (C) p14, positive; (D) skp2, positive, (E)
ki-67, positive; (F) bcl-2, positive. Poorly differentiated ESCC,
from top to bottom: (A) p16, positive; (B) p15, positive; (C) p14,
positive; (D) skp2, positive, (E) ki-67, positive; (F) bcl-2,
positive. ESCC, esophageal squamous cell carcinoma; EID, esophageal
intraepithelial dysplasia. |
As shown in Table
II, overall, p14ARF, p15INK4b and
p16INK4a showed almost complete negative expression in
the normal esophageal epithelium, while marked positive expression
was observed in the EID tissues and different pathological lesions
of the ESCC tissues. More specifically, the p14ARF
expression was negative in most of the normal esophageal tissues
(85%), but diffuse positive staining was observed in the EID (82%)
and ESCC (100%) tissues, including 9 cases of EID and 60 cases of
ESCC. The p16INK4a staining was similar to that of
p15INK4b, with only 2 cases of normal esophageal tissue
having weakly positive and most of the EID and ESCC tissues showing
marked staining (73 and 88%, respectively). p15INK4b
showed positive staining in 73% of the EID and 92% of the ESCC
specimens, while no positive staining was observed in the normal
esophageal tissue specimens.
 | Table II.Comparison of the immunohistochemical
staining results for p14ARF, p15INK4b and
p16INK4a in the ESCC tissue specimens and corresponding
normal esophageal tissue specimens. |
Table II.
Comparison of the immunohistochemical
staining results for p14ARF, p15INK4b and
p16INK4a in the ESCC tissue specimens and corresponding
normal esophageal tissue specimens.
| p14ARF
| p15INK4b
| p16INK4a
|
|---|
| Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Normal (20) | 17 | 3 | <0.001 | 20 | 0 | <0.001 | 18 | 2 | <0.001 |
| EID (11) | 2 | 9 | | 3 | 8 | | 3 | 8 | |
| ESCC (60) | 0 | 60 | | 5 | 55 | | 7 | 53 | |
A total of 46 male and 14 female ESCC patients
participated in this study, 53% of whom had lymph node metastasis.
The TNM stages of these patients are listed in Table I. As shown in Table III, a statistically significant
correlation was observed between p16INK4a expression and
the degree of differentiation. The poorly differentiated ESCC
showed stronger p16INK4a staining compared with the
well-differentiated ESCC (P<0.05). p16INK4a
expression was observed in 95% of the poorly differentiated ESCC
and only 71% of the well-differentiated ESCC tissues. The positive
expression of p15INK4b was observed in 95% cases of
poorly differentiated ESCC and 82% cases of well-differentiated
ESCC. The positive staining of p14ARF was found in all
the ESCC cases. Although there is no statistical evidence to verify
that cases with lymph mode metastasis had a more marked
immunostaining of p14ARF, p15INK4b and
p16INK4a, the positive staining ratios are high in these
specimens. There was no statistical correlation between the
expression of p14ARF, p15INK4b and
p16INK4a between the genders and among the TNM
stages.
 | Table III.Immunohistochemical expression of
p14ARF, p15INK4b and p16INK4a and
their correlation with the clinicopathological parameters of the
ESCC patients. |
Table III.
Immunohistochemical expression of
p14ARF, p15INK4b and p16INK4a and
their correlation with the clinicopathological parameters of the
ESCC patients.
| p14ARF
| p15INK4b
| p16INK4a
|
|---|
| Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Gender | | | | | | | | | |
| Male | 0 | 46 | - | 2 | 44 | 0.141 | 4 | 42 | 0.410 |
| Female | 0 | 14 | | 3 | 11 | | 3 | 11 | |
|
Differentiation | | | | | | | | | |
| Well | 0 | 17 | - | 3 | 14 | 0.261 | 5 | 12 | 0.025 |
| Poor | 0 | 43 | | 2 | 41 | | 2 | 41 | |
| Lymph node
metastasis | | | | | | | | | |
| No | 0 | 28 | - | 4 | 24 | 0.275 | 4 | 24 | 0.851 |
| Yes | 0 | 32 | | 1 | 31 | | 3 | 29 | |
| TNM stage | | | | | | | | | |
| I | 0 | 6 | - | 2 | 4 | 0.088 | 0 | 6 | 1.000 |
| IIA | 0 | 22 | | 2 | 20 | | 3 | 19 | |
| IIB | 0 | 14 | | 1 | 13 | | 2 | 12 | |
| III | 0 | 18 | | 0 | 18 | | 2 | 16 | |
As shown in Table
IV, there was positive staining for the proliferation marker
skp2 in 64% of the EID and 72% of the ESCC cases but only 10% of
the normal esophageal tissues. The analysis of ki-67 revealed a
markedly high level of expression in the EID (91%) and ESCC (100%)
cases, while there was a low incidence of positive staining in the
basal epithelial cells of the normal tissue (20%). The bcl-2
immunostaining revealed that 36 ESCC cases (60%) and only 2 (10%)
of the normal esophageal tissues had positive bcl-2 expression. As
shown in Table V, cells that
showed a positive expression of bcl-2 were found in 15 (88%) of the
well-differentiated and 21 (49%) of the poorly differentiated ESCC
cases. The variant expression of bcl-2 may be due to the
heterogeneity of the cells.
 | Table IV.Comparison of the immunohistochemical
staining results for bcl-2, skp2 and ki-67 in the ESCC tissue
specimens and corresponding normal esophageal tissue specimens. |
Table IV.
Comparison of the immunohistochemical
staining results for bcl-2, skp2 and ki-67 in the ESCC tissue
specimens and corresponding normal esophageal tissue specimens.
| bcl-2
| skp2
| ki-67
|
|---|
| Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Normal (20) | 18 | 2 | <0.001 | 18 | 2 | <0.001 | 16 | 4 | <0.001 |
| EID (11) | 0 | 11 | | 4 | 7 | | 1 | 10 | |
| ESCC (60) | 24 | 36 | | 17 | 43 | | 0 | 60 | |
 | Table V.Immunohistochemical expression of
bcl-2, ki-67 and skp2 and their correlation with the
clinicopathological parameters of patients. |
Table V.
Immunohistochemical expression of
bcl-2, ki-67 and skp2 and their correlation with the
clinicopathological parameters of patients.
| bcl-2
| ki-67
| skp2
|
|---|
| Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Gender | | | | | | | | | |
| Male | 19 | 27 | 0.709 | 0 | 46 | - | 13 | 33 | 1.000 |
| Female | 5 | 9 | | 0 | 14 | | 4 | 10 | |
|
Differentiation | | | | | | | | | |
| Well | 2 | 15 | 0.005 | 0 | 17 | - | 9 | 8 | 0.028 |
| Poor | 22 | 21 | | 0 | 43 | | 8 | 35 | |
| Lymph node
metastasis | | | | | | | | | |
| No | 16 | 12 | 0.011 | 0 | 28 | - | 10 | 18 | 0.235 |
| Yes | 8 | 24 | | 0 | 32 | | 7 | 25 | |
| TNM stage | | | | | | | | | |
| I | 3 | 3 | 0.472 | 0 | 6 | - | 4 | 2 | 0.223 |
| IIA | 6 | 16 | | 0 | 22 | | 6 | 16 | |
| IIB | 7 | 7 | | 0 | 14 | | 3 | 11 | |
| III | 8 | 10 | | 0 | 18 | | 4 | 14 | |
Discussion
The incidence of esophageal carcinoma rises
exponentially with age, beginning from the fourth decade of life
and peaking at the age of 75 years. Evidence suggests that cellular
senescence is involved in causing organismal aging. Senescence, the
process by which cells permanently withdraw from the cell cycle in
response to diverse stress, may also contribute to this age-related
disease (23). The senescence
marker p16 was found to be upregulated with age in the progenitor
cells of the mouse brain, bone marrow and pancreas. However,
cellular senescence has two roles in cells. On one hand, senescence
is associated with aging, which significantly increases the
transformation of epithelial tumors. On the other hand, it is
acknowledged that senescence is crucial in the prevention of
cancer, which is achieved by inducing reversible proliferative
arrest mediated by ARF/p53 and irreversible proliferative arrest
mediated by the concomitant actions of INK4a/Rb and the p53 pathway
(4,24). In this process, the main barrier to
the overgrowth of cancer is the derepression of the INK4a/ARF locus
(25,26). Overall, cellular senescence has two
roles: cancer protection in the young and age promotion in the old.
To the best of our knowledge, epithelial cells are the origin of
most carcinomas, resulting from either the mutation of an oncogene
in an epithelial cell or, in a paracrine manner, by an adjacent
senescent fibroblast cell, converging on the promotion of
epithelial tumorigenesis. However, whether senescence occurs in
epithelial cells during transformation is unknown.
To date, evidence suggests that p14ARF,
p15INK4b and p16INK4a are widely
downregulated in several solid tumors (27), including hepatic (28), breast, urinary bladder, pancreatic
and esophageal carcinomas and gliomas (29). The INK4a/ARF locus encodes two
significant tumor suppressors, p16INK4 and ARF, which
share the same exons but encode different reading frames.
p16INK4a is an inhibitor of CDK4 and CDK6 and acts by
imposing a G1 cell cycle arrest. The INK4a/ARF locus has
been regarded as a controller of cancer evolution which is
expressed at low levels in most tissues in young organisms but
becomes derepressed with age. The inactivation of senescence
markers, due to homozygous deletion or hypermethylation of the
genes which encode them, may be a significant mechanism in the
dysfunction of the Rb and p53 growth regulation pathways during
ESCC development (30). However,
it has been reported that the senescence pathway remains intact in
a large number of prostate cancer and cervical squamous carcinoma
cases (31) even if the ki-67
index indicates increased proliferation in these cancers. Zhang
et al found that the senescence markers p14ARF,
p15INK4b, p16INK4a and DCR2 were expressed
more frequently in prostate carcinomas than in benign tissues
(32) and Meng et al
identified activated senescence markers in colon cancer cells
(33). Moreover, Schwarze
identified an alteration in the p16/pRb pathway in the majority of
primary prostate cancers in vitro (34). In our ESCC samples, the expression
of p14ARF, p15INK4b and p16INK4a
increased during ESCC progression and was also associated with
greater age, poor differentiation and, to some degree, a high tumor
stage. That is, although cancer cells generally lose their ability
to undergo senescence and apoptosis, certain tumor cells trigger
senescence in response to severe DNA damage or other stimuli
(16,35). Therefore, senescence may play a
role in cancer development.
skp2 is a member of the Skp1-Cullin-F-box protein
(SCF) complex and considered to be a proto-oncogene, as its
overexpression causes increased proliferation and metastasis, at
least in part through increased p27 proteolysis (24). Wang et al found that
phosphorylation at Ser72 is crucial for the ability of the skp2
protein to promote cell proliferation and tumorigenesis, through
several complementary mechanisms (36). skp2 also induces cells to undergo a
mitotic division cycle by degrading p27 in early G1
(37). The results of our
immunostaining assays revealed that skp2 is activated at the
dysplasia stage of esophageal tumorigenesis and maintains a high
level of expression in most ESCCs (38). The uninhibited proliferation
eventually leads to the development of ESCC.
The nuclear antigen ki-67, which is markedly
expressed in the S and M phases of the cell cycle (39–41),
was used to estimating cell growth. bcl-2 is a proto-oncogene which
has been identified as a biologically significant inhibitor of
apoptosis, whose overexpression leads to cell proliferation
(42–44). In our data, a high expression of
ki-67 was associated with the high expression of skp2 and the low
expression of bcl-2, which acts as a marker of proliferation
(27,45). We detected the expression of bcl-2
in EID and ESCC tissues and half of the ESCC cases showed a
paradoxical loss of bcl-2, which indicated a poor prognosis. The
high expression of bcl-2 in EID showed its anti-apoptotic function
by blocking p53-mediated G1 arrest (46). The paradoxical loss of bcl-2 in
ESCC may not be explained as a result of chemo-radiotherapy, since
none of the cases in our database were treated prior to surgery.
However, the same results were obtained in cervical and endothelial
carcinoma, indicating that other mechanisms may be involved.
In general, the role of senescence in tumorigenesis
attracts a great deal of attention, particularly concerning the
functions of p14ARF, p15INK4b and
p16INK4a. According to previous studies, tumorigenesis
in old age not only reflects the accumulation of oncogenic
mutations but also stromal alteration (47). Campisi (48) addresses these issues as good
citizen and bad neighbors. The epithelial cells in squamous
carcinomas always show senescence and apoptosis. Senescence serves
as a powerful barrier for tumorigenesis in epithelial cells.
However, in ESCC, the high expression of p14ARF,
p15INK4b and p16INK4a provides evidence for
the activation of senescence in the epithelial rather than stromal
cells. Further studies are required to explain this phenomenon.
However, the expression of markers of senescence and proliferation,
including p14ARF, p15INK4b,
p16INK4a, bcl-2, skp2 and ki-67 in ESCC and
promalignancies and a loss of their expression in normal tissue and
neoplasm may augment routine histological diagnostic methods for
difficult cases.
References
|
1.
|
Pisani P, Parkin DM and Ferlay J:
Estimates of the worldwide mortality from eighteen major cancers in
1985. Implications for prevention and projections of future burden.
Int J Cancer. 55:891–903. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sarbia M, Verreet P, Bittinger F,
Dutkowski P, Heep H, Willers R and Gabbert HE: Basaloid squamous
cell carcinoma of the esophagus: diagnosis and prognosis. Cancer.
79:1871–1878. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hu J, Li R, Sun L and Ni Y: Influence of
esophageal carcinoma operations on gastroesophageal reflux. Ann
Thorac Surg. 78:298–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shan W, Yang G and Liu J: The inflammatory
network: bridging senescent stroma and epithelial tumorigenesis.
Front Biosci. 14:4044–4057. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Stein GH and Dulic V: Origins of G1 arrest
in senescent human fibroblasts. Bioessays. 17:537–543. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hayflick L: Theories of biological aging.
Exp Gerontol. 20:145–159. 1985. View Article : Google Scholar
|
|
7.
|
Oller AR, Rastogi P, Morgenthaler S and
Thilly WG: A statistical model to estimate variance in long
term-low dose mutation assays: testing of the model in a human
lymphoblastoid mutation ssay. Mutat Res. 216:149–161. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jackson AL and Loeb LA: The mutation rate
and cancer. Genetics. 148:1483–1490. 1998.PubMed/NCBI
|
|
9.
|
Bissell MJ and Labarge MA: Context, tissue
plasticity, and cancer: are tumor stem cells also regulated by the
microenvironment. Cancer Cell. 7:17–23. 2005.PubMed/NCBI
|
|
10.
|
Rosu-Myles M and Wolff L: p15Ink4b: dual
function in myelopoiesis and inactivation in myeloid disease. Blood
Cells Mol Dis. 40:406–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang Z, Rosen DG, Yao JL, Huang J and Liu
J: Expression of p14ARF, p15INK4b, p16INK4a, and DCR2 increases
during prostate cancer progression. Mod Pathol. 19:1339–1343. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zindy F, Quelle DE, Roussel MF and Sherr
CJ: Expression of the p16INK4a tumor suppressor versus other INK4
family members during mouse development and aging. Oncogene.
15:203–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shapiro GI, Edwards CD, Ewen ME and
Rollins BJ: p16INK4A participates in a G1 arrest checkpoint in
response to DNA damage. Mol Cell Biol. 18:378–387. 1998.PubMed/NCBI
|
|
14.
|
Stone S, Dayananth P, Jiang P,
Weaver-Feldhaus JM, Tavtigian SV, Cannon-Albright L and Kamb A:
Genomic structure, expression and mutational analysis of the P15
(MTS2) gene. Oncogene. 11:987–991. 1995.PubMed/NCBI
|
|
15.
|
Fukai K, Yokosuka O, Imazeki F, Tada M,
Mikata R, Miyazaki M, Ochiai T and Saisho H: Methylation status of
p14ARF, p15INK4b, and p16INK4a genes in human hepatocellular
carcinoma. Liver Int. 25:1209–1216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rinehart CA and Torti VR: Aging and
cancer: the role of stromal interactions with epithelial cells. Mol
Carcinog. 18:187–192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Beck JC, Hosick HL and Watkins BA: Growth
of epithelium from a preneoplastic mammary outgrowth in response to
mammary adipose tissue. In Vitro Cell Dev Biol. 25:409–418. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hayashi N and Cunha GR: Mesenchyme-induced
changes in the neoplastic characteristics of the Dunning prostatic
adenocarcinoma. Cancer Res. 51:4924–4930. 1991.PubMed/NCBI
|
|
20.
|
Li TY, Xu LY, Wu ZY, Liao LD, Shen JH, Xu
XE, Du ZP, Zhao Q and Li EM: Reduced nuclear and ectopic
cytoplasmic expression of lysyl oxidase-like 2 is associated with
lymph node metastasis and poor prognosis in esophageal squamous
cell carcinoma. Hum Pathol. Dec 26–2011, (Epub ahead of print).
|
|
21.
|
Yang GZ, Li L, Ding HY and Zhou JS:
Cyclooxygenase-2 is over-expressed in Chinese esophageal squamous
cell carcinoma, and correlated with NF-kappaB: an
immunohistochemical study. Exp Mol Pathol. 79:214–218. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Grace VM, Shalini JV, lekha TT, Devaraj SN
and Devaraj H: Co-overexpression of p53 and bcl-2 proteins in
HPV-induced squamous cell carcinoma of the uterine cervix. Gynecol
Oncol. 91:51–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
DePinho RA: The age of cancer. Nature.
408:248–254. 2000. View
Article : Google Scholar
|
|
24.
|
Zheng WQ, Zheng JM, Ma R, Meng FF and Ni
CR: Relationship between levels of Skp2 and P27 in breast
carcinomas and possible role of Skp2 as targeted therapy. Steroids.
70:770–774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Gil J and Peters G: Regulation of the
INK4b-ARF-INK4a tumour suppressor locus: all for one or one for
all. Nat Rev Mol Cell Biol. 7:667–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kim WY and Sharpless NE: The regulation of
INK4/ARF in cancer and aging. Cell. 127:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Collado M, Gil J, Efeyan A, Guerra C,
Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM,
Barbacid M, et al: Tumour biology: senescence in premalignant
tumours. Nature. 436:6422005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jin M, Piao Z, Kim NG, Park C, Shin EC,
Park JH, Jung HJ, Kim CG and Kim H: p16 is a major inactivation
target in hepato-cellular carcinoma. Cancer. 89:60–68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Forbes S, Clements J, Dawson E, Bamford S,
Webb T, Dogan A, Flanagan A, Teague J, Wooster R, Futreal PA and
Stratton MR: COSMIC 2005. Br J Cancer. 94:318–322. 2006. View Article : Google Scholar
|
|
30.
|
Esteller M, Corn PG, Baylin SB and Herman
JG: A gene hyper-methylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
31.
|
Sano T, Masuda N, Oyama T and Nakajima T:
Overexpression of p16 and p14ARF is associated with human
papillomavirus infection in cervical squamous cell carcinoma and
dysplasia. Pathol Int. 52:375–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Li TY, Xu LY, Wu ZY, Liao LD, Shen JH, Xu
XE, Du ZP, Zhao Q and Li EM: Reduced nuclear and ectopic
cytoplasmic expression of lysyl oxidase-like 2 is associated with
lymph node metastasis and poor prognosis in esophageal squamous
cell carcinoma. Hum Pathol. Dec 26–2011.(Epub ahead of print).
|
|
33.
|
Meng RD, McDonald ER 3rd, Sheikh MS,
Fornace AJ Jr and El-Deiry WS: The TRAIL decoy receptor TRUNDD
(DcR2, TRAIL-R4) is induced by adenovirus-p53 overexpression and
can delay TRAIL-, p53-, and KILLER/DR5-dependent colon cancer
apoptosis. Mol Ther. 1:130–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Schwarze SR, Shi Y, Fu VX, Watson PA and
Jarrard DF: Role of cyclin-dependent kinase inhibitors in the
growth arrest at senescence in human prostate epithelial and
uroepithelial cells. Oncogene. 20:8184–8192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Roberson RS, Kussick SJ, Vallieres E, Chen
SY and Wu DY: Escape from therapy-induced accelerated cellular
senescence in p53-null lung cancer cells and in human lung cancers.
Cancer Res. 65:2795–2803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Wang Z, Gao D, Fukushima H, Inuzuka H, Liu
P, Wan L, Sarkar FH and Wei W: Skp2: A novel potential therapeutic
target for prostate cancer. Biochim Biophys Acta. 1825:11–7.
2012.PubMed/NCBI
|
|
37.
|
Mani A and Gelmann EP: The
ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol.
23:4776–4789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Dowen SE, Scott A, Mukherjee G and Stanley
MA: Overexpression of Skp2 in carcinoma of the cervix does not
correlate inversely with p27 expression. Int J Cancer. 105:326–330.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
40.
|
Gerdes J: Ki-67 and other proliferation
markers useful for immunohistological diagnostic and prognostic
evaluations in human malignancies. Semin Cancer Biol. 1:199–206.
1990.PubMed/NCBI
|
|
41.
|
Gerdes J, Li L, Schlueter C, Duchrow M,
Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E and Flad HD:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873. 1991.
|
|
42.
|
Reed JC: Bcl-2 and the regulation of
programmed cell death. J Cell Biol. 124:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Singh BB, Chandler FW Jr, Whitaker SB and
Forbes-Nelson AE: Immunohistochemical evaluation of bcl-2
oncoprotein in oral dysplasia and carcinoma. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 85:692–698. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Vaux DL, Cory S and Adams JM: Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Ecker K and Hengst L: Skp2: caught in the
Akt. Nat Cell Biol. 11:377–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Guillouf C, Grana X, Selvakumaran M, De
Luca A, Giordano A, Hoffman B and Liebermann DA: Dissection of the
genetic programs of p53-mediated G1 growth arrest and apoptosis:
blocking p53-induced apoptosis unmasks G1 arrest. Blood.
85:2691–2698. 1995.PubMed/NCBI
|
|
47.
|
Bishop JM: Cancer: the rise of the genetic
paradigm. Genes Dev. 9:1309–1315. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Campisi J: Senescent cells, tumor
suppression, and organismal aging: good citizens, bad neighbours.
Cell. 120:513–522. 2005. View Article : Google Scholar : PubMed/NCBI
|















