Introduction
Pleural effusion is a frequent complication in
patients with cardiac failure, pneumonia, tuberculosis and
neoplasms (1). Malignancy is one
of the most significant causes of pleural effusion and more than
90% of malignant pleural effusions (MPEs) are caused by metastatic
diseases (2). It is necessary to
elucidate their etiologies, yet to differentiate MPE from benign
pleural effusion remains a clinical challenge (3). Initial diagnostic methods include
cytological, histological, biochemical and thoracocentesis
examinations. However, the overall sensitivity of cytological
examination is only 60% (3),
thoracoscopic pleural biopsy or image-guided percutaneous pleural
biopsy provides a relative high sensitivity, but may not be
available in all hospitals or well-tolerated (4).
A series of tumor markers have been well-studied for
their ability to improve the diagnosis of MPE. Three published
studies have investigated the diagnostic value of the pleural
vascular endothelial growth factor, carcinoembryonic antigen (CEA),
carbohydrate antigens (CA) 125, 15–3 and 19–9, and CYFRA 21–1 in
MPE but have failed to identify a reliable tumor marker with both
high sensitivity and high specificity. The authors did not
recommend using one tumor marker alone for the diagnosis of MPE
(5–7). Therefore it is imperative to find a
new diagnostic tool to facilitate diagnostic accuracy.
Telomerase is a specialized reverse transcriptase
that adds TTAGGG repeats to the telomeric ends of chromosomal DNA
to maintain the telomeric length (8). The expression of telomerase activity
has been shown to be correlated with telomeric length (9). Telomerase is active in many types of
human cancers but is not detectable in most normal somatic cells
(10,11). Therefore telomerase activity may be
a universal and specific marker for diagnosing a wide variety of
cancers (12). Several studies
have shown that the measurement of telomerase activity may be a
useful and noninvasive method to detect malignancy in body fluid,
particularly when used in combination with conventional cytological
examination (13,14). A number of studies on the potential
diagnostic role of telomerase activity assay in MPE have been
published and have reported varying results. The present
meta-analysis aimed to establish the overall accuracy of the
telomerase activity assay in the diagnosis of MPE.
Materials and methods
Meta-analysis
The present meta-analysis was performed according to
the guidelines of the Preferred Reporting Items for Systematic
Reviews and Meta-Analysis (PRISMA) statement and with methods
recommended by the Cochrane Diagnostic Test Accuracy Working Group
(15,16).
Search strategy and study selection
To identify studies that have evaluated the evidence
of using telomerase activity in order to diagnose MPE, we performed
a search of the Pubmed (Medline), Embase and Cochrane databases up
to March 15, 2012, using the key words ‘pleural effusion’,
‘malignant pleural effusions’ and ‘telomerase activity’. Although
no language restrictions were imposed on the search criteria, only
English-and Chinese-language publications concerning human studies
were included in the present meta-analysis. In addition, a manual
search of the reference lists of eligible papers was also
conducted.
Conference abstracts were excluded due to the
limited data provided. A study was included in the present
meta-analysis if it provided data on both sensitivity and
specificity of pleural telomerase activity for the diagnosis of
MPE. Studies with fewer than 10 patients were excluded to avoid
selection bias. Two authors (Y-C Shen and Z-N Chen) independently
screened the articles for inclusion. Disagreements between authors
were resolved by consensus.
Data extraction and quality
assessment
The final set of articles was assessed independently
by two reviewers (Y-C Shen and Z-N Chen). The data retrieved from
the reports included author, publication year, patient source, test
method, sensitivity and specificity data, and methodological
quality. When the same patients were reported in several studies,
only the most informative article was included to avoid duplication
of information. The methodological quality of studies was evaluated
by a QUADAS tool (Quality Assessment for Studies of Diagnostic
Accuracy, an evidence based quality assessment tool to be used in
systematic reviews of diagnostic accuracy studies, maximum score
14) (17).
Statistical analyses
The standard methods recommended for the
meta-analyses of diagnostic accuracy studies were used in the
present study (18). The following
measures of test accuracy were calculated for each study:
sensitivity, specificity, positive likelihood ratio (PLR), negative
likelihood ratio (NLR) and diagnostic odds ratio (DOR), together
with their 95% confidence interval (CI). The present meta-analysis
was based on a summary receiver operating characteristic (SROC)
curve, and the sensitivity and specificity for the single test
threshold identified for each study was used to plot an SROC curve
(19). A random-effects
meta-analysis was performed in order to account for the differences
between study variability for each study. The Spearman's rank
correlation was performed as a test for threshold effect.
Chi-squared and Fisher's exact tests were used to detect
statistically significant heterogeneity across studies. Since
publication bias is of concern for meta-analyses of diagnostic
studies, we tested for the potential presence of this bias using
Deeks funnel plots (20). All
analyses were performed using two statistical software programs
(Meta-DiSc for Windows; XI Cochrane Colloquium, Barcelona, Spain
and Stata, version 11; Stata Corporation, College Station, TX,
USA). All statistical tests were two-sided and P<0.05 was
considered to indicate a statistically significant result.
Results
Study exclusion criteria
After independent review, eight studies with 678
samples on the use of telomerase activity in patients with MPE were
considered eligible for inclusion in the present meta-analysis
(21–28). The major reasons for excluding the
other studies were as follows: non-diagnostic studies or studies
cannot reconstruct the diagnostic 2 by 2 table; limited samples, or
mixed with other serous effusions and duplicated studies. The
clinical summary of these studies, along with the QUADAS scores,
are outlined in Table I.
 | Table IClinical summary of the included
studies. |
Table I
Clinical summary of the included
studies.
| Sample size
| | | | | | | |
|---|
| Study, year
(ref.) | MPE | Non-MPE | Standard | Method | TP | FP | FN | TN | QUADAS |
|---|
| 1. Yang et al,
1998 (21) | 92 | 52 |
Histology/Cytology | PCR | 84 | 3 | 8 | 49 | 12 |
| 2. Yang et al,
2001 (22) | 30 | 35 | Histology | PCR-ELISA | 27 | 2 | 3 | 33 | 11 |
| 3. Dikmen et
al, 2003 (23) | 63 | 46 |
Histology/Cytology | PCR | 52 | 9 | 11 | 37 | 10 |
| 4. Lee et al,
2005 (24) | 31 | 63 |
Histology/Cytology | PCR-ELISA | 10 | 5 | 21 | 58 | 11 |
| 5. Maneechotesuwan
et al, 2006 (25) | 29 | 16 |
Histology/Cytology | PCR | 10 | 8 | 19 | 8 | 10 |
| 6. Li et al,
2008 (26) | 31 | 32 |
Histology/Cytology | PCR-ELISA | 27 | 3 | 4 | 29 | 9 |
| 7. Mousavi et
al, 2009 (27) | 19 | 9 |
Histology/Cytology | PCR-ELISA | 19 | 1 | 0 | 8 | 9 |
| 8. Li et al,
2010 (28) | 80 | 50 |
Histology/Cytology | PCR-ELISA | 57 | 7 | 23 | 43 | 10 |
Study characteristics and quality
report
There were five English and three Chinese articles.
The average sample size in the eight studies was 85 (range from 28
to 144) and the samples included 375 patients with MPE and 303
non-MPE. Telomerase activity was measured by a PCR-based or
PCR-ELISA method. The current gold standards, cytological and
histological examinations, are highly reliable methods to identify
MPE. The quality of the eight studies was generally high with six
studies having QUADAS scores ≥10.
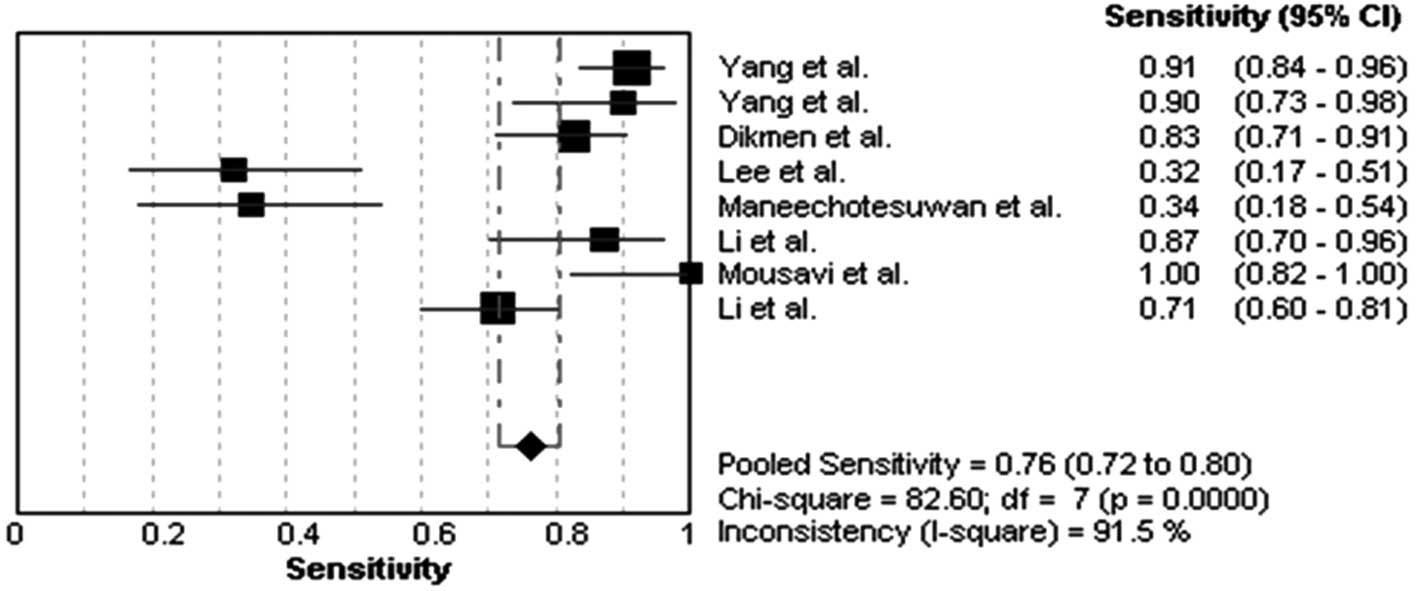
Diagnostic accuracy for MPE
The heterogeneity analysis revealed
I2-values of 91.5% for sensitivity and 67.1% for
specificity. The existence of significant heterogeneity occurred in
the eight studies, thus the random effects model approach was
selected for the present meta-analysis. The forest plots of the
sensitivity and specificity for eight telomerase activity assays in
diagnosing MPE are shown in Figs.
1 and 2, respectively. The
pooled sensitivity was 0.76 (95% CI, 0.72–0.80), specificity was
0.87 (95% CI, 0.83–0.91). The PLR was 5.19 (95% CI, 2.36–11.42),
the NLR was 0.25 (95% CI, 0.11–0.53) and the DOR was 23.18 (95% CI,
6.11–87.83).
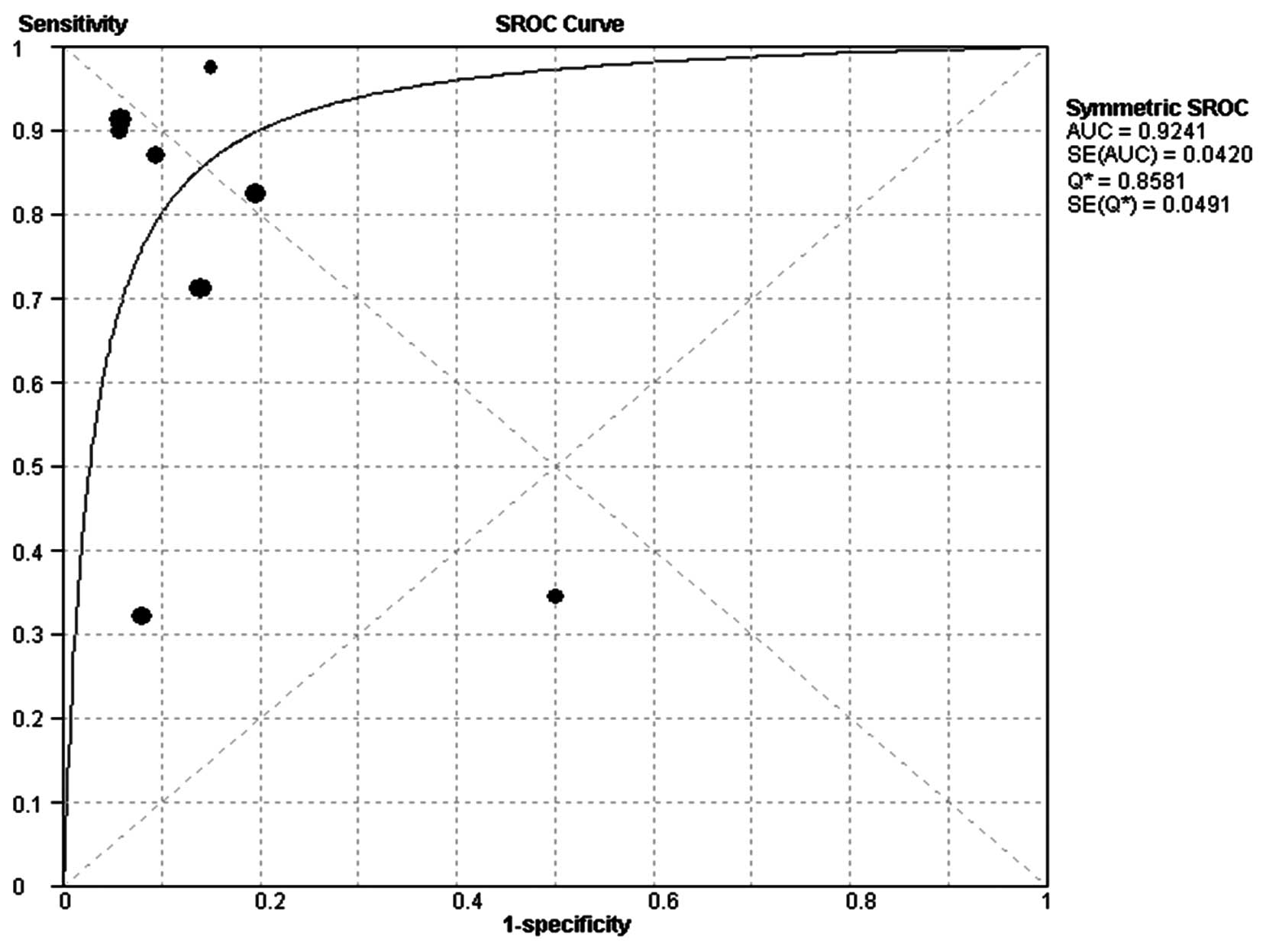
The SROC curve plotting the true-positive against
the false-positive rates of individual studies is shown in Fig. 3. The area under curve (AUC) was
0.92, indicating that the level of overall accuracy was high.
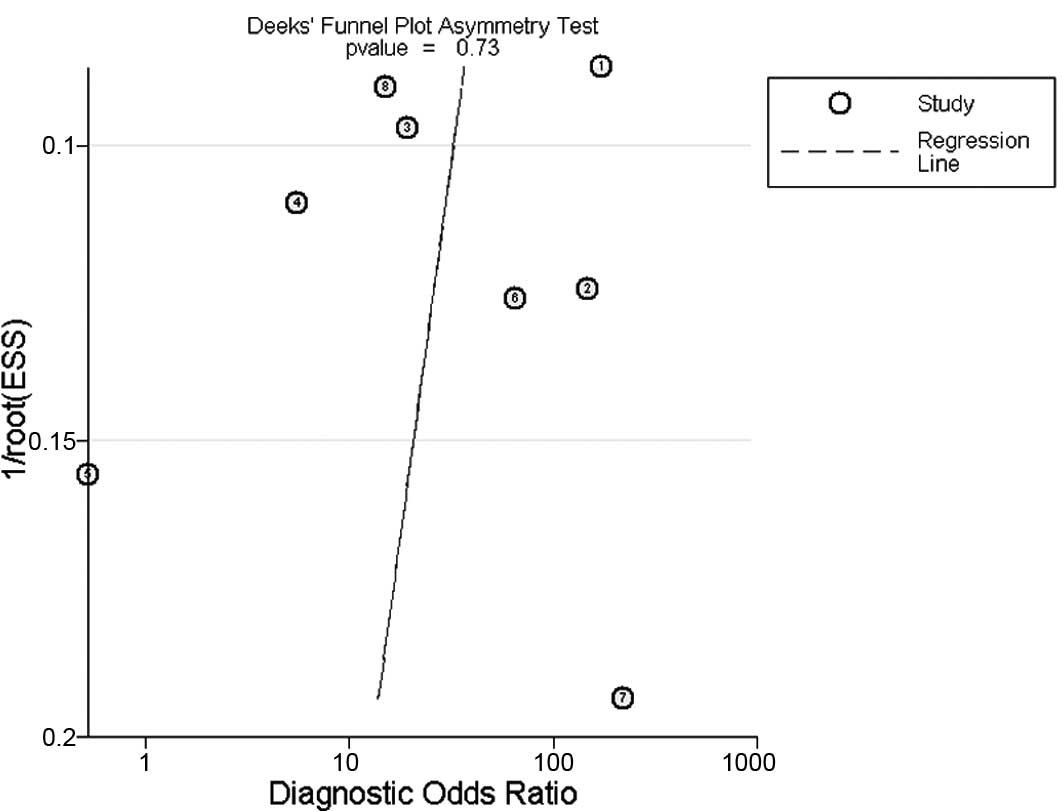
Publication bias
The Deeks funnel plot asymmetry test was used to
evaluate potential publication bias; the statistically
insignificant value (P=0.73) for the slope coefficient suggests
symmetry in the data and a low likelihood of publication bias
(Fig. 4)
In addition, we did not perform a meta-regression
analysis with QUADAS scores to assess the effect of study quality
on relative DOR of telomerase activity in the diagnosis of MPE due
to the limited numbers of studies included. For the same reason, we
could not explore whether or not the study design including
blinded, cross-sectional, consecutive/random and prospective design
affected diagnostic accuracy.
Discussion
The present meta-analysis evaluated the overall
diagnostic role of pleural telomerase activity in MPE. To the best
of our knowledge, this is the first meta-analysis to assess the
diagnostic role of telomerase activity in MPE. We found a summary
AUC of 0.92, a summary estimate of 0.76 for sensitivity and 0.87
for specificity. It appears that telomerase activity examination
plays a valuable role in the diagnosis of MPE. It may be more favor
in confirming MPE.
The SROC curve has been recommended to represent the
performance of a diagnostic test, and shows the trade-off between
sensitivity and specificity based on data from a meta-analysis
(29,30). The AUC and an index Q*
are recognized as potentially useful summaries of the curve. We
used the Q-value, intersection point of the SROC curve with a
diagonal line from the left upper corner to the right lower corner
of the ROC space, which corresponds to the highest common value of
sensitivity and specificity for the test and represents an overall
measure of the discriminatory power of a test. In the present
study, the Q-value was 0.86, demonstrating that the maximum joint
sensitivity and specificity was 0.86. The AUC also measures the
overall accuracy of diagnostic studies. If the AUC is 1, then the
telomerase activity test differentiates perfectly between MPE and
non-MPE subjects. An AUC of greater than 0.9 indicates high
diagnostic accuracy. In our meta-analysis, the AUC was 0.92,
suggesting the level of overall accuracy was high.
The DOR is a single indicator of test accuracy that
combines the data from sensitivity and specificity into a single
number (31). It is the ratio of
the odds of positive test results in the diseased, relative to the
odds of positive test results in the non-diseased. The value of a
DOR ranges from 0 to infinity, with higher values indicating an
improved discriminatory test performance. In the present
meta-analysis, the mean DOR was 23.18, suggesting that the
telomerase activity assays appeared to be useful in the diagnosis
of MPE. Since the SROC curve and DOR are not easy to interpret and
use in clinical practice, likelihood ratios are considered more
clinically meaningful (32). The
PLR was 5.19, indicating that patients with MPE have an approximate
5-fold higher chance of being telomerase activity assay-positive
compared with patients without MPE. However, the PLR was not high
enough for making a definitive clinical decision. The NLR was found
to be 0.25, which means if the telomerase activity assay result was
negative, the probability that this patient has MPE is 25%, which
is not low enough to exclude MPE.
Our meta-analysis suggests that telomerase activity
determination plays a valuable role in diagnosing MPE, especially
in the confirmation of MPE. The reported sensitivities varied among
studies, ranging from 0.32 to 1.00. As Lee (24) suggested that the ELISA-based
telomerase activity assay is relatively insensitive, it is
unsuitable as a routine screening tool for MPE. False-positive
telomerase activity due to lymphocytic contamination may weaken the
diagnostic value for MPE in a tuberculosis-endemic area (24). The combination with other markers
for pleural effusion would aid in increasing the sensitivity. For
instance, the combination of telomerase activity with CEA or
CYFRA21-1 was found to provide a high sensitivity of 0.93 and 0.90,
respectively (26,28). The further development of the
method of telomerase activity measurement may increase diagnostic
accuracy for MPE. Hansson et al (33) reported that telomerase activity
measurement in situ based on cytospins from fresh
cytological material correlated well to final diagnoses and could
differentiate between MPE and non-MPE cases. It was suggested that
this method may improve the diagnostic accuracy for MPE (33,34).
Cytological and/or histological examinations remain the traditional
method for diagnosing MPE, however, pathologists do not recommend a
diagnosis solely based on cytological samples due to the high risk
of diagnostic error. In addition, the invasive thoracoscopy may not
be available in all hospitals (3,4).
Thus, the importance of the telomerase activity test is not only to
provide high specificity with which to confirm the diagnosis of
MPE, but also to guide the inclusion of patients who may benefit
from further invasive procedures.
The present meta-analysis had several limitations.
Although we strengthened the present meta-analysis comprehensive
search strategy and data extraction, only eight studies with 678
samples were included. The evidence generated from the limited
studies and samples may limit the interpretation of the
meta-analysis results. Secondly, we excluded conference abstracts
and non-English or non-Chinese publications, which may have led to
publication bias (35), which may
also be introduced by inflation of diagnostic accuracy estimates
since studies that report positive findings are more likely to be
accepted for publication. However, there was no publication bias
reported in the present meta-analysis due to the limited number of
studies included. We did not use QUADAS scores to perform the
meta-regression analysis or explore whether or not the study design
affected diagnostic accuracy.
Based on the evidence compiled in this
meta-analysis, telomerase activity measurement plays a role in the
diagnosis of MPE, and it is likely to be a useful diagnostic tool
for confirming MPE. The results of telomerase activity assays
should be interpreted in parallel with clinical findings and the
results of conventional tests. Studies with larger sample sizes and
improved telomerase activity examination methods are still required
to determine the diagnostic performance of the telomerase activity
assay for MPE.
Acknowledgements
This work is supported by grants
30971327 and 31171103 from the National Natural Science Foundation
of China and grants 00-722 and 06-834 from the China Medical Board
of New York to Dr Fu-Qiang Wen.
References
|
1.
|
Light RW: Pleural effusions. Med Clin
North Am. 95:1055–1070. 2011. View Article : Google Scholar
|
|
2.
|
Bennett R and Maskell N: Management of
malignant pleural effusions. Curr Opin Pulm Med. 11:296–300.
2005.PubMed/NCBI
|
|
3.
|
McGrath EE and Anderson PB: Diagnosis of
pleural effusion: a systematic approach. Am J Crit Care.
20:119–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lombardi G, Zustovich F, Nicoletto MO,
Donach M, Artioli G and Pastorelli D: Diagnosis and treatment of
malignant pleural effusion: a systematic literature review and new
approaches. Am J Clin Oncol. 33:420–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shen YC, Liu MQ, Wan C, Chen L, Wang T and
Wen FQ: Diagnostic accuracy of vascular endothelial growth factor
for malignant pleural effusion. Exp Ther Med. March 14–2012.(Epub
ahead of print).
|
|
6.
|
Shi HZ, Liang QL, Jiang J, Qin XJ and Yang
HB: Diagnostic value of carcinoembryonic antigen in malignant
pleural effusion: a meta-analysis. Respirology. 13:518–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang
J and Yang HB: Diagnostic accuracy of tumour markers for malignant
pleural effusion: a meta-analysis. Thorax. 63:35–41. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zvereva MI, Shcherbakova DM and Dontsova
OA: Telomerase: structure, functions, and activity regulation.
Biochemistry (Mosc). 75:1563–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rhyu MS: Telomeres, telomerase, and
immortality. J Natl Cancer Inst. 87:884–894. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chen CH and Chen RJ: Prevalence of
telomerase activity in human cancer. J Formos Med Assoc.
110:275–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ouellette MM, Wright WE and Shay JW:
Targeting telomerase-expressing cancer cells. J Cell Mol Med.
15:1433–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Heaphy CM and Meeker AK: The potential
utility of telomere-related markers for cancer diagnosis. J Cell
Mol Med. 15:1227–1238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tangkijvanich P, Tresukosol D,
Sampatanukul P, et al: Telomerase assay for differentiating between
malignancy-related and nonmalignant ascites. Clin Cancer Res.
5:2470–2475. 1999.PubMed/NCBI
|
|
14.
|
Braunschweig R, Yan P, Guilleret I, et al:
Detection of malignant effusions: comparison of a telomerase assay
and cytologic examination. Diagn Cytopathol. 24:174–180. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar
|
|
16.
|
Leeflang MM, Deeks JJ, Gatsonis C and
Bossuyt PM; Cochrane Diagnostic Test Accuracy Working Group:
Systematic reviews of diagnostic test accuracy. Ann Intern Med.
149:889–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Whiting PF, Weswood ME, Rutjes AW, Reitsma
JB, Bossuyt PN and Kleijnen J: Evaluation of QUADAS, a tool for the
quality assessment of diagnostic accuracy studies. BMC Med Res
Methodol. 6:92006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Devillé WL, Buntinx F, Bouter LM, et al:
Conducting systematic reviews of diagnostic studies: didactic
guidelines. BMC Med Res Methodol. 2:92002.PubMed/NCBI
|
|
19.
|
Moses LE, Shapiro D and Littenberg B:
Combining independent studies of a diagnostic test into a summary
ROC curve: data-analytic approaches and some additional
considerations. Stat Med. 12:1293–1316. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yang CT, Lee MH, Lan RS and Chen JK:
Telomerase activity in pleural effusions: diagnostic significance.
J Clin Oncol. 16:567–573. 1998.PubMed/NCBI
|
|
22.
|
Yang Z and Xie C: Comparison of the
diagnostic value of telomerase activity with CEA level in benign
and malignant pleural effusions. Zhonghua Jie He He Hu Xi Za Zhi.
24:201–203. 2001.(In Chinese).
|
|
23.
|
Dikmen G, Dikmen E, Kara M, Sahin E, Doğan
P and Ozdemir N: Diagnostic implications of telomerase activity in
pleural effusions. Eur Respir J. 22:422–426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee WY: Limitations of detection of
malignancy in pleural effusions using ELISA-based TRAP assay:
comparison with cytological examination. Cytopathology. 16:227–232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Maneechotesuwan K, Lertworawiwat A,
Tscheikuna J and Wamanuttajinda V: Comparison of telomerase
activity between malignant and tuberculous pleural effusions. J Med
Assoc Thai. 89(Suppl 5): S46–54. 2006.PubMed/NCBI
|
|
26.
|
Li GB, Wang M, Guo S, Lü Y and Wang W:
Value of combined pleural effusion testing of telomerase and CEA
for differential diagnosis of malignant and nonmalignant pleural
effusion. Chin J Cancer Prev Treat. 15:299–300. 318:2008.
|
|
27.
|
Mousavi SA, Bahari R and Karimi MA:
Comparison of telomerase activity in malignant and benign pleural
effusions. Tanaffos. 8:17–23. 2009.
|
|
28.
|
Li H, Fu J, Xiu Y and Zhou Q: Diagnostic
significance of combining telomerase activity with CYFRA21-1 level
in differentiating malignant pleural effusion caused by lung cancer
from benign pleural effusion. Zhongguo Fei Ai Za Zhi. 13:652–654.
2010.(In Chinese).
|
|
29.
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Jones CM and Athanasiou T: Summary
receiver operating characteristic curve analysis techniques in the
evaluation of diagnostic tests. Ann Thorac Surg. 79:16–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Glas AS, Lijmer JG, Prins MH, Bonsel GJ
and Bossuyt PM: The diagnostic odds ratio: a single indicator of
test performance. J Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Stengel D, Bauwens K, Sehouli J,
Ekkernkamp A and Porzsolt F: A likelihood ratio approach to
meta-analysis of diagnostic studies. J Med Screen. 10:47–51. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Hansson M, Zendehrokh N, Ohyashiki J, et
al: Telomerase activity in effusions: a comparison between telomere
repeat amplification protocol in situ and conventional telomere
repeat amplification protocol assay. Arch Pathol Lab Med.
132:1896–1902. 2008.
|
|
34.
|
Dejmek A, Yahata N, Ohyashiki K, et al: In
situ telomerase activity in pleural effusions: a promising marker
for malignancy. Diagn Cytopathol. 24:11–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Song F, Khan KS, Dinnes J and Sutton AJ:
Asymmetric funnel plots and publication bias in meta-analyses of
diagnostic accuracy. Int J Epidemiol. 31:88–95. 2002. View Article : Google Scholar : PubMed/NCBI
|