Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
connective-tissue disease, predominantly affecting females aged
between their late teens and early 40s, has a wide range of
clinical features (1). Although
SLE is prevalent worldwide, the Chinese population has a relatively
high prevalence rate of 9–92 cases per 100,000 individuals
(1,2). Prior to the addition of Benlysta as
the most recent agent in 2011, there were few drugs for SLE
treatment approved by the US Food and Drug Administration (FDA),
including glucocorticoids, antimalarials and aspirin (3). Glucocorticoids are the main
therapeutic strategy and the most effective anti-inflammatory drugs
available for the treatment of a number of chronic autoimmune
diseases, including SLE (4).
However, when exhibiting poor responses, even to high-doses of
systemic steroids, some SLE patients lose disease control,
requiring other immunosuppressive therapies. Preventing steroid
resistance and maintaining disease control are significant
challenges to overcome in treating SLE patients.
The overexpression of P-glycoprotein (P-gp) in
peripheral lymphocytes, which may lead to the exclusion of
glucocorticoids, has emerged as one of the mechanisms involved in
poor responses to steroid treatment in SLE patients (5). P-gp is a 170-kDa product of the
multidrug resistance 1 (MDR-1) gene, a member of the ATP binding
cassette transporter superfamily. P-gp molecules function as drug
efflux pumps that transport numerous drugs out of cells, including
antibiotics and cytotoxins, as well as several drugs commonly used
for treating autoimmune diseases. This means that high expression
levels of active P-gp in peripheral lymphocytes result in poor
disease control by steroid therapy (6). Therefore, in the present study, the
P-gp expression and activity of peripheral blood lymphocytes
obtained from SLE patients who had previously undergone long-term
steroid treatment was examined. Furthermore, the effects of P-gp
inhibitors on lymphocytes from steroid-resistant SLE patients in
vitro were investigated.
Materials and methods
Subjects
The Ethics Committee of Remin Hospital of Wuhan
University approved this study and informed consent was obtained
from all the healthy subjects and patients. The study included 60
SLE patients and 30 healthy subjects. All the SLE patients met the
diagnostic criteria for SLE established by the American Rheumatism
Association in 1997 and had received systemic steroids as the only
systemic immunosuppressive therapy for ≥6 months (7). The disease activity of the SLE
patients was assessed using the SLE Disease Activity Index-2000
(SLEDAI-2000), and all of the patients had the active form of the
disease (SLEDAI-2000>4) (8–10).
The SLE patients were further subclassified into an active SLE
group, whose SLEDAI was ≤12, and a severely active SLE group, whose
SLEDAI was >12 (10,11). The SLEDAI index is a global score
index developed for the assessment of SLE disease activity, the
range of which is between 0 and 105 (8,9). The
index has been demonstrated to be reliable, to have construct
validity and to be sensitive to change (8,9).
Table I shows the demographic
characteristics of the SLE patients and healthy controls.
 | Table IClinical characteristics of the study
patients. |
Table I
Clinical characteristics of the study
patients.
| Characteristic | Healthy control
(n=30) | SLE patients
(n=60) |
|---|
| Age, mean ± SD,
years | 30.5±9.4 | 29.3±10.5 |
| Gender
(female/male) | 24/6 | 54/6 |
| SLEDAI score, median
(range) | - | 14 (3–37) |
| SLE involvement, no.
of patients | | |
| Lupus
nephritis | - | 44 |
| Blood
abnormalities | - | 30 |
| Vasculitis | - | 23 |
| Arthritis | - | 22 |
| Myositis | - | 9 |
| Serositis | - | 7 |
| CNS lupus | - | 1 |
| Prednisolone (or
equivalent) treatment | | |
| Dosage, median
(range), mg/day | - | 22 (5–100) |
| Duration, median
(range), months | - | 26 (1–228) |
Isolation of peripheral blood
lymphocytes
Peripheral blood lymphocytes were isolated by
density-gradient centrifugation. Heparinized venous peripheral
blood was obtained from the SLE patients and healthy controls. The
blood was diluted by adding an equal volume of 0.9% NaCl. A total
of 6 ml of diluted blood was carefully layered over 3 ml of
lymphocyte separation medium (MP Biomedicals LLC. Solon, OH, USA)
and was centrifuged at 800 x g for 20 min at room temperature in a
swing-out rotor. After centrifugation, the mononuclear cells formed
a distinct band at the sample interface. The harvested fraction was
diluted with buffered RPMI-1640 medium (Invitrogen, Gaithersburg,
MD, USA) to reduce the density of the solution, and the cells were
pelleted by centrifugation for 10 min at 250 x g. Platelets were
removed by layering the cells suspended in buffered RPMI-1640
medium and centrifuging them for 15 min at 350 x g. The pellets
were used as mononuclear cells.
P-gp expression assay
P-gp expression levels in lymphocytes were analyzed
using standard flow cytometry procedures as described previously
(12). Lymphocytes
(1×106) were incubated with 5 μl PE-conjugated anti-P-gp
(CD243) monoclonal antibody (eBioscience, Inc., San Diego, CA, USA)
or 5 μl PE-conjugated matched-isotype control antibody (IgG2a,
eBioscience) for 30 min at 4°C, washed twice in PBS and
subsequently analyzed on a FACScan (Becton-Dickinson, Mountain
View, CA, USA). At least 10,000 cells were counted and lymphocytes
were analyzed and separated according to their forward and side
scatter characteristics. Results are expressed as the percentage of
positive cells.
Rhodamine 123 (Rh123) efflux assay
P-gp activity was measured using the Rh123 efflux
assay as described previously (12). The fluorescent dye Rh123
(Sigma-Aldrich, St. Louis, MO, USA) was added to 2 ml lymphocytes
(1x106/ml) at a final concentration of 10 μg/ml and
cells were incubated at 37°C for 30 min. Subsequently, the
Rh123-loaded cells were washed twice with cold PBS and resuspended
in PBS at the original volume. The Rh123-loaded cells were then
separated into two aliquots, which were incubated for 30 min at
37°C in the absence or presence of verapamil (Sigma), a P-gp
inhibitor (final concentration, 10 μM). Lymphocytes were kept on
ice until they were analyzed using a FACScan (Becton-Dickinson).
P-gp activity was determined for each subpopulation of cells by the
percentage of the mean Rh123 fluorescence intensity (MFI) in the
absence or presence of verapamil, i.e., P-gp activity (%) = [(MFI
of cells with verapamil - MFI of cells without verapamil)/MFI of
cells with verapamil] x 100.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from peripheral blood
lymphocytes (5×106 cells) of SLE patients and of healthy
controls using TRIzol reagent (Invitrogen, Eugene, OR, USA)
according to the manufacturer’s instructions and was quantified by
measuring the absorbance at 260 nm. cDNA was synthesized from each
total RNA using a Moloney murine leukemia virus reverse
transcriptase first strand kit (Invitrogen, Shanghai, China). An
aliquot of the RT product was then processed for DNA amplification
by PCR using the primers: 5′-CCC ATC ATT GCA ATA GCA GG-3′ (sense)
and 5′-GTT CAA ACT TCT GCT CCT GA-3′ (antisense) for P-gp/MDR-1;
5′-AGC GAG CAT CCC CCA AAG TT-3′ (sense) and 5′-GGG CAC GAA GGC TCA
TCA TT-3′ (antisense) for β-actin. The reaction mixture (20 μl)
contained 3 μl template cDNA, 2 μl of primers (50 pmol each of
upstream and downstream primers), 5 μl RNase-free water and 10 μl
2X Es Taq MasterMix (including Es Taq DNA polymerase, 2X Es Taq PCR
Buffer, 3 mM MgCl2 and 400 μM dNTP mix; CWBIO, Beijing,
China). Thermal cycling conditions were as follows: denaturation at
94°C for 20 sec, annealing at 55°C for 30 sec, extension at 72°C
for 30 sec for 25 cycles and a final extension at 72°C for 2 min.
PCR products were separated on 2% agarose gels containing Goldview
dye (SBS Genetech Co., Ltd., Shanghai, China). A 100-bp DNA Ladder
(CWBIO) was electrophoresed on the same gel to determine product
size. The gel was photographed and the amount of MDR1 and β-actin
from each sample was analyzed by scanning densitometry. To
normalize the results, the amount of P-gp/MDR1 was divided by the
amount of β-actin.
Treatment of lymphocytes with P-gp
inhibitors in vitro
The lymphocytes were purified from three SLE
patients with high levels of P-gp expression. Fluorescent dye Rh123
was added to 2 ml lymphocytes (1×106/ml) at a final
concentration of 10 μg/ml, and cells were incubated at 37°C for 30
min. Subsequently, the Rh123-loaded cells were washed twice with
cold PBS and resuspended in PBS at the original volume. The
Rh123-loaded cells were then separated into four aliquots, which
were incubated for 30 min at 37°C in the absence or presence of 100
μM cyclophosphamide (Sigma), 100 μM mycophenolic acid (Sigma) or
100 μM emodin (Sino-FDA, Beijing, China). The cells were washed
with cold PBS and resuspended in PBS, then kept on ice until they
were analyzed using a FACScan.
Statistical analysis
Data were shown as the median or mean ± SD.
Differences between groups were determined using the non-parametric
Mann-Whitney U test or Student’s t-test. Correlations between two
variables were analyzed using Spearman’s rank correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS 13.0 or
GraphPad Prism version 5 (GraphPad Software, San Diego, CA,
USA).
Results
Expression and activity of P-gp protein
in peripheral blood lymphocytes from SLE patients
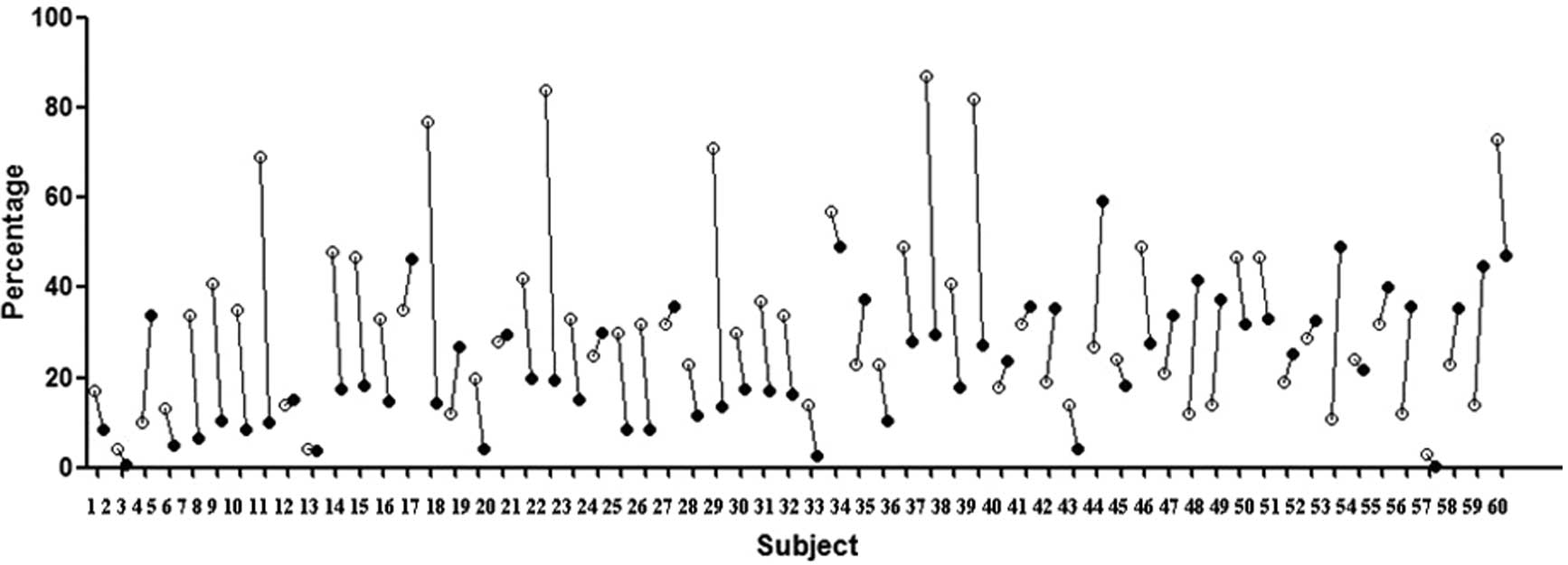
A total of 60 SLE patients and 30 healthy volunteers
were screened. We tested P-gp expression in lymphocytes using an
anti-human MDR1 antibody and tested P-gp activity using an Rh123
efflux assay and the results were expressed as the percentage of
positive cells or Rh123-efflux percentage, respectively. The
majority of SLE patients expressed the P-gp protein in peripheral
blood lymphocytes and the efflux-function of this P-gp was active
in the majority of patients (Fig.
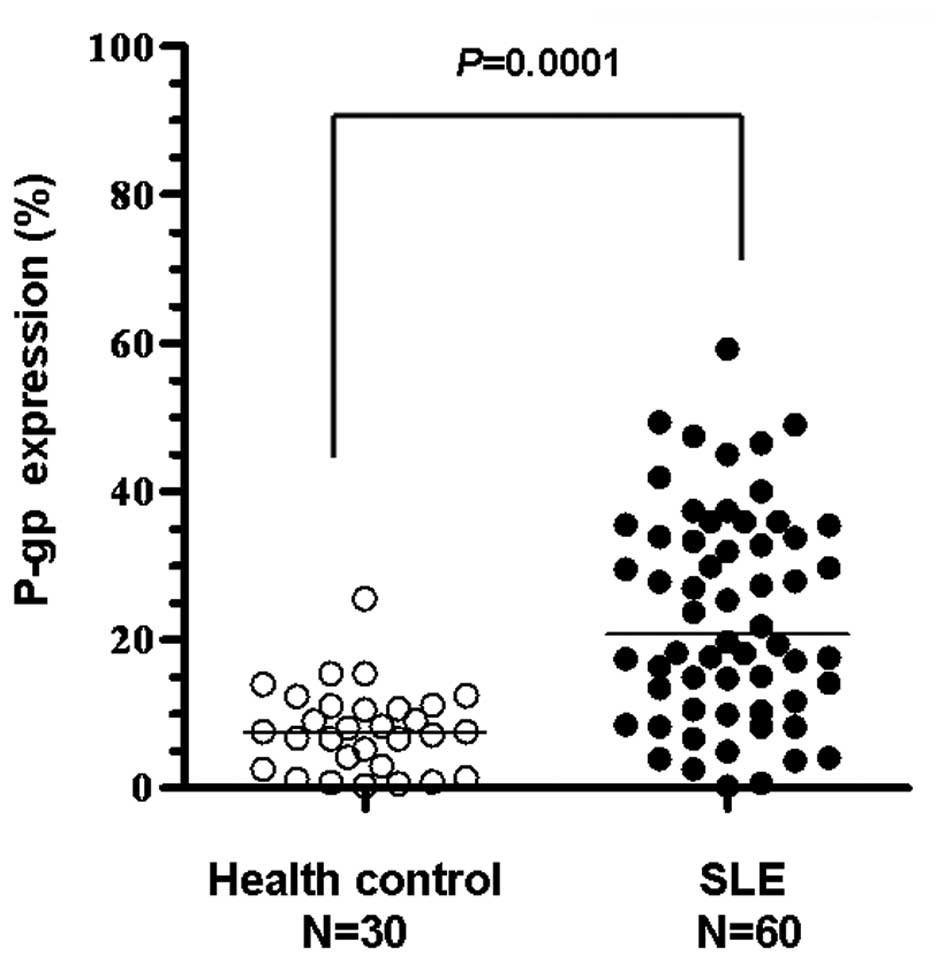
1). The expression levels of P-gp in the lymphocytes of healthy
volunteers were minimal (Fig. 2)
and P-gp expression in SLE patients with a long history of steroid
use was significantly higher compared with the healthy controls
(P=0.0001; Fig. 2).
Differences in the activity and
expression of P-gp in peripheral blood lymphocytes between patients
with active and severely active SLE
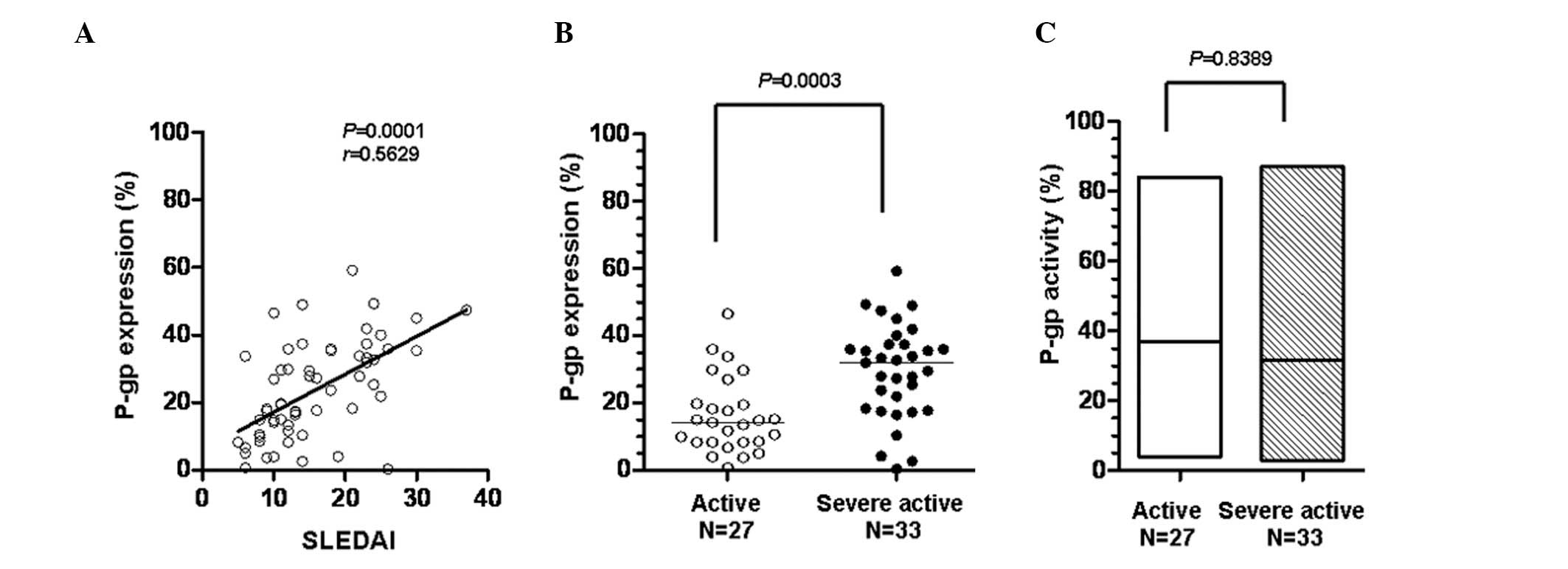
The SLE disease activity was estimated using the
SLEDAI scoring system. To explore whether activity and expression
of P-gp affect disease control by systemic steroids, the
correlation of the SLEDAI scores with the P-gp expression levels of
the 60 SLE patients was analyzed. The SLEDAI scores demonstrated a
statistically significant positive correlation with the expression
level of P-gp (P= 0.0001; Fig.
3A). The criterion for defining active SLE was SLEDAI≤12,
otherwise SLE was defined as severely active. Fig. 3B shows that P-gp expression in the
severely active SLE group was significantly higher than that in the
active SLE group (P=0.0003), although P-gp activity was not
significantly different between the two groups (P= 0.8389; Fig. 3C). These results suggest that high
levels of P-gp expression in the peripheral lymphocytes of
steroid-treated SLE patients may be the cause of poor disease
control in SLE patients who have received long-term steroid
treatment.
Effects of intensive intravenous
cyclophosphamide (CTX) administration on the expression and
function of P-gp in peripheral bood lymphocytes in vivo
We aimed to determine whether P-gp expression in
peripheral lymphocytes was inhibited by other intensive
immunosuppressive therapies in vivo. We selected 10 SLE
patients whose disease was highly active after >6 months of
steroid therapy and placed them on an intravenous CTX regimen for
the first time. Table II shows the
demographic characteristics and therapeutic regimen of these
patients, while Fig. 4 shows P-gp
mRNA expression in the peripheral lymphocytes prior to and after
CTX treatment. Of the 10 patients, 9 demonstrated no clear changes
in P-gp protein expression and function. A marked reduction in P-gp
expression was observed in only 1 patient (23.7 to 10.4%; Table II). None of the patients exhibited
significantly different P-gp mRNA expression in their peripheral
lymphocytes following CTX therapy (Fig. 4). In contrast to expectations, the
results indicated that intensive intravenous CTX administration was
unable to influence the P-gp expression in the peripheral
lymphocytes of SLE patients in vivo.
 | Table IIEffects of intensive intravenous
cyclophosphamide administration on the expression and activiy of
P-glycoprotein in peripheral bood lymphocytes in vivo. |
Table II
Effects of intensive intravenous
cyclophosphamide administration on the expression and activiy of
P-glycoprotein in peripheral bood lymphocytes in vivo.
| Patients | Gender | Age (years) | Intravenous CTX
dosage (g) | SLEDAI
| P-gp expression (%)
| P-gp activity (%)
|
|---|
| Before therapy | After therapy | Before therapy | After therapy | Before therapy | After therapy |
|---|
| 1 | Female | 19 | 2 | 37 | 37 | 51.2 | 47.4 | 73 | 69 |
| 2 | Female | 22 | 2 | 16 | 18 | 37.9 | 35.9 | 32 | 45 |
| 3 | Female | 17 | 2 | 23 | 23 | 41.9 | 37.4 | 14 | 33 |
| 4 | Female | 29 | 2 | 25 | 21 | 21 | 18.3 | 47 | 54 |
| 5 | Female | 32 | 2 | 30 | 11 | 35.5 | 29.4 | 84 | 72 |
| 6 | Female | 35 | 2 | 18 | 14 | 23.7 | 10.4 | 23 | 37 |
| 7 | Male | 20 | 2 | 23 | 23 | 35.9 | 32.8 | 34 | 44 |
| 8 | Female | 22 | 2 | 23 | 18 | 27 | 24.6 | 41 | 49 |
| 9 | Female | 24 | 2 | 19 | 19 | 27.9 | 23.9 | 57 | 44 |
| 10 | Female | 27 | 2 | 14 | 13 | 19.5 | 16.4 | 41 | 36 |
Effects of P-gp inhibitors on the
efflux-function of P-gp in the peripheral lymphocytes of SLE
patients in vitro
In light of our previous findings, we designed an
in vitro experiment to elucidate whether there are P-gp
inhibitors, such as CTX, mycophenolic acid (MPA) and emodin, which
affect the efflux-function of P-gp in the lymphocytes of SLE
patients (13–15) Three patients with active SLE and
high expression levels of active P-gp in their peripheral
lymphocytes were selected. The Rh123-efflux assay indicated that
1.5- to 2-fold increases of the mean fluorescence intensity (MFI)
occurred in cells treated with 100 μM CTX or 100 μM emodin, but no
shift of the MFI peak was observed in cells treated with 100 μM MPA
(Fig. 5). These results suggest
that CTX and emodin has the potential to increase steroid
accumulation in the lymphocytes of glucocorticoid-resistant SLE
patients by inhibiting P-gp efflux activity.
Discussion
SLE is an autoimmune disease characterized by an
excess of autoantibodies produced by activated B cells and
auto-reactive T cells. Glucocorticoids are key drugs for treating
patients with active SLE. Insensitivity or resistance to systemic
glucocorticoids in the treatment of active SLE patients has been
reported and studied for a number of years. Several distinct
mechanisms contributing to inhibit glucocorticoid activity in SLE
patients have been identified, and overexpressed P-gp in peripheral
lymphocytes was demonstrated to be one of these mechanisms
(5,16–20).
P-gp is a 170-kDa product of the MDR-1 gene, and functions as an
energy-dependent trans-membrane efflux pump. P-gp is usually
expressed in a wide variety of healthy tissues and cells, including
the epithelial cells of the intestine, hepatocytes, the adrenal
glands, renal proximal tubules and the endothelium of blood-brain
and maternal-fetal barriers, as well as lymphocytes (6,21).
The physiological role of P-gp has been defined as the
detoxification and transport of metabolites due to its function as
a one-way energy-dependent pump (6). Highly expressed P-gp in lymphocytes
has been demonstrated to be involved in mechanisms of
glucocorticoid insensitivity or resistance in several immune
diseases besides SLE, including asthma, inflammatory bowel disease,
immune thrombocytopenia and rheumatoid arthritis (RA) (16,22–26).
However, little was known about P-gp in SLE patients. Assuming that
the overexpression of active P-gp molecules caused the efflux of
steroids from the intracellular space and thus prevented disease
control in SLE patients by steroid therapy, we tested P-gp activity
and expression in lymphocytes from SLE patients. Active and highly
expressed P-gp was observed in the majority of SLE patients. Our
findings indicate that for SLE patients with a long history of
steroid use, P-gp expression level was positively correlated with
disease activity and that the P-gp expression level in SLE patients
influenced the level of disease control by long-term steroid
administration.
Subsequently, we aimed to determine whether the
expression or function of P-gp in the peripheral lymphocytes of SLE
patients could be altered by a CTX regimen in vivo.
Following treatment with 2.0 g intense CTX, only one patient
demonstrated a notable decrease in the expression of P-gp protein
although the patient’s P-gp mRNA levels did not change
significantly as a result of CTX therapy. Our results also
demonstrated that the immunosuppressive agent CTX was unable to
suppress or promote P-gp expression. Three P-gp inhibitors, CTX,
MPA (a metabolic product of mycophenolate mofetil) and emodin were
then used in an attempt to block the efflux activity of P-gp
(13–15), and CTX and emodin demonstrated
characteristics of a competitor of the P-gp efflux activity.
In conclusion, we have demonstrated that the
overexpression of P-gp in peripheral lymphocytes causes a poorer
response to steroid therapy in the long-term and results in poor
disease control in SLE patients. Emodin, an active ingredient
derived from the herb, Chinese rhubarb (Rheum palmatum),
possesses a promising effect for overcoming P-gp-mediated acquired
steroid resistance.
Abbreviations:
|
SLE
|
systemic lupus erythematosus
|
|
MDR-1
|
multidrug resistance-1
|
|
P-gp
|
P-glycoprotein
|
|
SLEDAI
|
SLE Disease Activity Index
|
|
MFI
|
mean fluorescence intensity
|
|
Rh123
|
Rhodamine 123
|
|
CTX
|
cyclophosphamide
|
|
MPA
|
mycophenolic acid
|
|
Emodin
|
1,3,8-trihydroxy-6-methylanthraquinone
|
Acknowledgements
The authors would like to thank Dr Yan
Wang (Institute of Hydrobiology, Chinese Academy of Science, Wuhan)
for technical help on the flow cytometry and Dr Vincent J Hearing
(National Institutes of Health, Bethesda, MD, USA) for revision of
the English manuscript. This study was partially supported by
grants from the National Natural Science Foundation of China (NSFC
grant no. 8107138).
References
|
1
|
D’Cruz DP, Khamashta MA and Hughes GR:
Systemic lupus erythematosus. Lancet. 369:587–596. 2007.
|
|
2
|
Parker BJ and Bruce IN: High dose
methylprednisolone therapy for the treatment of severe systemic
lupus erythematosus. Lupus. 16:387–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wallace DJ: Advances in drug therapy for
systemic lupus erythematosus. BMC Med. 29:772010. View Article : Google Scholar
|
|
4
|
Barnes PJ: Mechanisms and resistance in
glucocorticoid control of inflammation. J Steroid Biochem Mol Biol.
120:76–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsujimura S, Saito K, Nakayamada S, Nakano
K and Tanaka Y: Clinical relevance of the expression of
P-glycoprotein on peripheral blood lymphocytes to steroid
resistance in patients with systemic lupus erythematosus. Arthritis
Rheum. 52:1676–1683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richaud-Patin Y, Soto-Vega E, Jakez-Ocampo
J and Llorente L: P-glycoprotein in autoimmune diseases. Autoimmun
Rev. 3:188–192. 2004. View Article : Google Scholar
|
|
7
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus (letter). Arthritis Rheum. 40:17251997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2000. J
Rheumatol. 29:288–291. 2002.PubMed/NCBI
|
|
9
|
Yee CS, Farewell VT, Isenberg DA, et al:
The use of Systemic Lupus Erythematosus Disease Activity Index-2000
to define active disease and minimal clinically meaningful change
based on data from a large cohort of systemic lupus erythematosus
patients. Rheumatology (Oxford). 50:982–988. 2011. View Article : Google Scholar
|
|
10
|
Feng X, Wu H, Grossman JM, et al:
Association of increased interferon-inducible gene expression with
disease activity and lupus nephritis in patients with systemic
lupus erythematosus. Arthritis Rheum. 54:2951–2962. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petri M, Kim MY, Kalunian KC, et al:
Combined oral contraceptives in women with systemic lupus
erythematosus. N Engl J Med. 353:2550–2558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Fuente H, Baranda L, Hernández MI,
et al: Lack of involvement of P-glycoprotein (P-gp) in pemphigus
patients with poor response to steroid therapy. J Dermatol Sci.
28:219–226. 2002.PubMed/NCBI
|
|
13
|
Grishanova AY, Melnikova EV, Kaledin VI,
Nikolin VP and Lyakhovich VV: Possible role of P-glycoprotein in
cyclophosphamide resistance of transplanted mouse RLS
lymphosarcoma. Bull Exp Biol Med. 139:611–614. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Figurski M, Shaw LM and Burckart
GJ: The impact of P-glycoprotein and Mrp2 on mycophenolic acid
levels in mice. Transpl Immunol. 19:192–196. 2008.(In English and
Russian).
|
|
15
|
Li J, Liu P, Mao H, Wanga A and Zhang X:
Emodin sensitizes paclitaxel-resistant human ovarian cancer cells
to paclitaxel-induced apoptosis in vitro. Oncol Rep.
21:1605–1610. 2009.PubMed/NCBI
|
|
16
|
Guiducci C, Gong M, Xu Z, Gill M, et al:
TLR recognition of self nucleic acids hampers glucocorticoid
activity in lupus. Nature. 465:937–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsujimura S, Saito K, Tokunaga M, et al:
Overcoming treatment unresponsiveness mediated by P-glycoprotein
overexpression on lymphocytes in refractory active systemic lupus
erythematosus. Mod Rheumatol. 15:28–32. 2005. View Article : Google Scholar
|
|
18
|
Foote A, Briganti EM, Kipen Y, et al:
Macrophage migration inhibitory factor in systemic lupus
erythematosus. J Rheumatol. 31:268–273. 2004.PubMed/NCBI
|
|
19
|
Hoi AY, Hickey MJ, Hall P, Yamana J, et
al: Macrophage migration inhibitory factor deficiency attenuates
macrophage recruitment, glomerulonephritis, and lethality in
MRL/lpr mice. J Immunol. 177:5687–5696. 2006. View Article : Google Scholar
|
|
20
|
Li X, Zhang FS, Zhang JH and Wang JY:
Negative relationship between expression of glucocorticoid receptor
alpha and disease activity: glucocorticoid treatment of patients
with systemic lupus erythematosus. J Rheumatol. 37:316–321. 2010.
View Article : Google Scholar
|
|
21
|
Callaghan R, Crowley E, Potter S and Kerr
ID: P-glycoprotein: so many ways to turn it on. J Clin Pharmacol.
48:365–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wasilewska A, Zoch-Zwierz W, Pietruczuk M
and Zalewski G: Expression of P-glycoprotein in lymphocytes from
children with nephrotic syndrome, depending on their steroid
response. Pediatr Nephrol. 21:1274–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barnes PJ and Adcock IM: Glucocorticoid
resistance in inflammatory diseases. Lancet. 373:1905–1917. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farrell RJ and Kelleher D: Glucocorticoid
resistance in inflammatory bowel disease. J Endocrinol.
178:339–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsujimura S, Saito K, Nakayamada S and
Tanaka Y: Etanercept overcomes P-glycoprotein-induced drug
resistance in lymphocytes of patients with intractable rheumatoid
arthritis. Mod Rheumatol. 20:139–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maillefert JF, Maynadie M, Tebib JG, et
al: Expression of the multidrug resistance glycoprotein 170 in the
peripheral blood lymphocytes of rheumatoid arthritis patients. The
percentage of lymphocytes expressing glycoprotein 170 is increased
in patients treated with prednisolone. Br J Rheumatol. 35:430–435.
1996. View Article : Google Scholar
|