Introduction
Many medicinal plants provide beneficial sources of
vitamins, dietary fiber and phytochemicals. A number of herbs are
widely used owing to their flavor and healthy constituents which,
not only regulate body homeostasis, but also prevent degenerative
diseases (1–3). Angelica sp. is a common
vegetable known for its vitamin content (4). Early studies have shown that the
vegetable contains vitamins C, B1 and B2, chlorophylls and diverse
minerals (4,5). The moist leaves exhibit exceptional
antioxidant activity and therefore the herb is widely used as a
functional food ingredient. Additionally, the herb has therapeutic
effects on hypertension, constipation, diuresis, arteriosclerosis
and most importantly cancer, which are attributable to its small
molecular constituents, such as flavonoids, saponin and coumarins
(5). Further research on the
chemical composition of oils has led to the identification of
dozens of small compounds (6). The
oils exhibit strong activity in suppressing PANC-1 pancreatic
cancer cell and Crl breast cancer cell growth (7). Angelica keiskei is broadly
employed in alternative medicine as a remedy to treat bowel
disturbance syndrome, arthritis and immune diseases (8). However, there is limited scientific
information regarding its effects on various degenerative
disorders. Findings of another study have shown that Angelica
keiskei has the ability to reduce inflammation in a chronic
ethanol-induced in vivo test. Upon treatment of ICR mice
with 10, 25 and 50 mg/kg extracts p.o., alcohol-induced
hepatotoxicity was improved, indicating that Angelica
keiskei indirectly safeguards the liver against oxidative
stress induced by free radicals (9). An earlier investigation by our group
revealed anti-asthmatic activities of an aqueous leaf extract in an
ovalbumin-induced animal model (10). Mice sensitized to ovalbumin were
orally administered the Angelica archangelica extract and
their lungs were analyzed via hematoxylin and eosin (H&E)
staining to measure the IL-4/13 cytokine content. The extract
exhibited strong anti-asthmatic activity via regulation of the
CD4+ cell population, IL-4/13 expression and
asthma-related molecular markers in the lungs (10).
The Korea Food and Drug Administration has
recommended submission of data from eye irritancy, skin irritancy
and phototoxicity tests to obtain approval and authorization of the
use of test compounds as functional cosmetic ingredients(s). Plant
extracts with pungent flavors appear to cause irritation when
exposed to the skin (4). Unwanted
mild or transient reactions to cosmetics are common in patients
with allergic contact dermatitis. Various adverse effects including
acute/chronic toxicity, irritation and sensitization, can be
assessed using in vivo, in vitro, semi in vivo
and ex vivo animal models, even in modified tests (11–13).
A specific component or constituent should not exert toxic effects
on the skin (particularly the eye in the case of cosmetics) and
should only be passed and approved in cases where no damage/changes
to the eye lens are observed in animals or clinical trials for the
development of cosmetics (14).
Although cosmetic ingredients rarely trigger serious damage, their
advantages in skin protection or tissue regeneration should be
emphasized.
In the current study, we performed the acute eye
lens mucosal irritation test with Angelica keiskei leaf
fractions using an in vivo animal model. Various parameters
were assessed by comparing the degree of acute toxicity induced to
confirm whether these fractions have potential for development in
cosmetic applications.
Materials and methods
Animals and care
New Zealand White rabbits (9 weeks old, male,
weighing 2.0–2.2 kg) were purchased from M&J Animal Supplies
Co. (Seoul, Korea) and fed a commercial diet (Purina, Seoul, Korea)
and water ad libitum. Animal protocols were performed in
accordance with the guidelines of the Committee of International
Association for the Study of Pain on Research and Ethical Issues
(15). The rabbits were allowed to
adjust to the laboratory surroundings for at least 1 week prior to
the experiments. The number of rabbits in each group was 3.
Sample preparation
Angelica keiskei leaf purchased from Myung-il
Farm Co. (Eumsung, Korea) was used throughout the experiments. The
slice-dried leaf was pulverized with a homogenizer (20,000 rpm for
15 min; Shinil, Seoul, Korea). Powder was extracted with distilled
water (powder:distilled water = 1:10; w/v) or ethanol (powder:50%
ethanol = 1:6; w/v) at 60°C for 12 h in a shaking incubator (JSR,
Gongju, Korea), followed by filtration (Filter paper No. 1,
Whatman, Schleicher & Schuell, Buckinghamshire, UK).
Lyophilization was carried out in a freeze-dryer (Bondiro, Il-Shin,
Seoul, Korea) to obtain the final samples (Fig. 1B and C). The voucher specimens of
the Angelica keiskei leaf and powder were deposited in the
Laboratory of Food Enzyme Biotechnology, Kyungpook National
University (#2010-Ak).
Draize eye irritancy test
Fractions (100 mg/ml of concentrated aqueous and
ethanol fractions) were dripped into the eyes of each New Zealand
White rabbit (n=5) with their eyes held open and clipped at the
lid. As a positive control, 10% of sodium dioctyl sulfosuccinate
was employed. Progressive damage/changes to the rabbit eye were
recorded each day for 7 days. Reactions to the fractions included
swelling of the eyelid, iris inflammation, ulceration and
hemorrhaging, as described in other studies, with slight
modifications (16,17).
Sample treatment
Following instillation of the aqueous and ethanol
fractions of the Angelica keiskei leaf, eye lens mucosa was
evaluated for local mucosal irritation. Saline was used as the
control. The conjunctival sac in the right eye of the rabbits was
treated with the sample (Fig. 1D and
E; concentrated to 0.1 ml), control (saline) or positive
control (sodium dioctyl sulfosuccinate). After applying once for 2
sec, washing with saline was performed as for the control group.
The undiluted sample was administered once under the eyelid, which
was slightly pulled away to form a space to allow easy
administration (aqueous or ethanol fraction, 0.1 ml each) into the
conjunctival sac. Following this, the cornea, iris and conjunctiva
were examined daily at the designated times (1, 2, 3, 4, 5, and 7
days) to evaluate acute toxicity in the eye lens mucosa.
Analysis of parameters
Lesions were observed in experiments whereby the
test substance was not applied to the left eye for comparison
purposes. On days 1, 2, 3, 4, 5, and 7 following the application of
the test fractions, we examined the following criteria of the naked
eye: corneal opacity, turbidity range, reaction of the iris and
conjunctiva, edema and emission discharge. Irritancy in the eye
lens mucosa was evaluated based on redness, irritancy, ocular
lesions or inflammation by trained examiners under the authority of
a professional from the Center of Laboratory Animal and Care,
Kyungpook National University.
Results and Discussion
In the course of screening natural resources such as
food and/or oriental herb plants for active components exerting
anti-inflammatory effects in cosmetic application, we observed that
aqueous and ethanol fractions of the Angelica keiskei leaf
exhibit a potent whitening effect at a concentration of 100 μg/ml
with no cytotoxicity in both in vitro and in vivo
assays (data not shown).
Recently, Angelica sp. has become a valuable
food source in Asia, as these edible plants provide health benefits
owing to their antioxidant activities, although some species have a
pungent flavor (4). The leaf is
consumed by vegetarians both in restaurants and at home and
contains various nutrients including vitamins, flavonoids, flavonol
and other polyphenol compounds (9,10).
For the approval of cosmetics produced using food or chemical
sources, the Korean Food and Drug Administration accepts only
safety data derived from the most widely used animal tests, such as
the Draize eye irritancy test, which involves placing drops of the
substance into rabbit eyes. The guidelines for the use of a test
compound in cosmetics as a mixture/ingredient involves the control
of specific skin toxicity (18).
The main constituents associated with toxicity are complicated by
the presence of various components such as amine, nitrous compounds
and other beneficial and/or disadvantageous substances.
Nevertheless, several reports on the antitumor, anti-diabetic and
anti-inflammatory activities of known or unknown extracts/fractions
of herbal or medicinal plants have been documented thus far
(2,3). In previous studies aimed at promoting
biological and other applicable purposes, we examined whether these
fractions/extracts have biological activity in ameliorating
degenerative disorders, including atopic dermatitis. Initially,
food ingredients are produced using raw grains, cereals, fruits,
vegetables and medicinal herbs and thereafter processed into
biomaterials using a range of methods. Following the application of
valuable supplementary techniques, such as supercritical
extraction, microbial fermentation, biotransformation or chemical
modification, biomaterials may be converted into a cosmetic,
cosmeceutical, neutraceutical or drug. For this reason, we
developed a screening system for antioxidant and anti-tyrosinase
agents from medicinal plant extracts and examined their
anti-asthmatic activities, as described previously (19,20).
Safety data were obtained following the
administration of aqueous and ethanol fractions of the Angelica
keiskei leaf (Fig. 1D and E;
100 mg/ml in a total volume of 100 μl) into rabbit eyes. When
saline was used as the control, we did not observe congestion
symptoms around the pupil and whites of the eye (data not shown).
The Draize eye irritancy test is strictly observational and does
not adequately reflect the degree of irritancy in humans, therefore
it is generally considered crude, imprecise and unreliable.
However, this remains the most convincing test in animal models. We
applied the following criteria for precise evaluation of toxic
symptoms: swelling, inflammation and ulceration on the eye lens.
Initially, following treatment with aqueous and ethanol fractions
or saline, eyelid and eye mucosa membranes were examined daily. In
terms of ocular lesions in the cornea, scores were measured as
described in Materials and methods. Lesions were scored using a
point system: 0 for no suppuration or haze, 1 when slightly opaque
compared to the normal difference in transparency, 2 when easily
observed as partly semi-transparent, 3 when not observed at the end
of the pupil and 4 when the cornea, but not the iris, was opaque
and turbid.
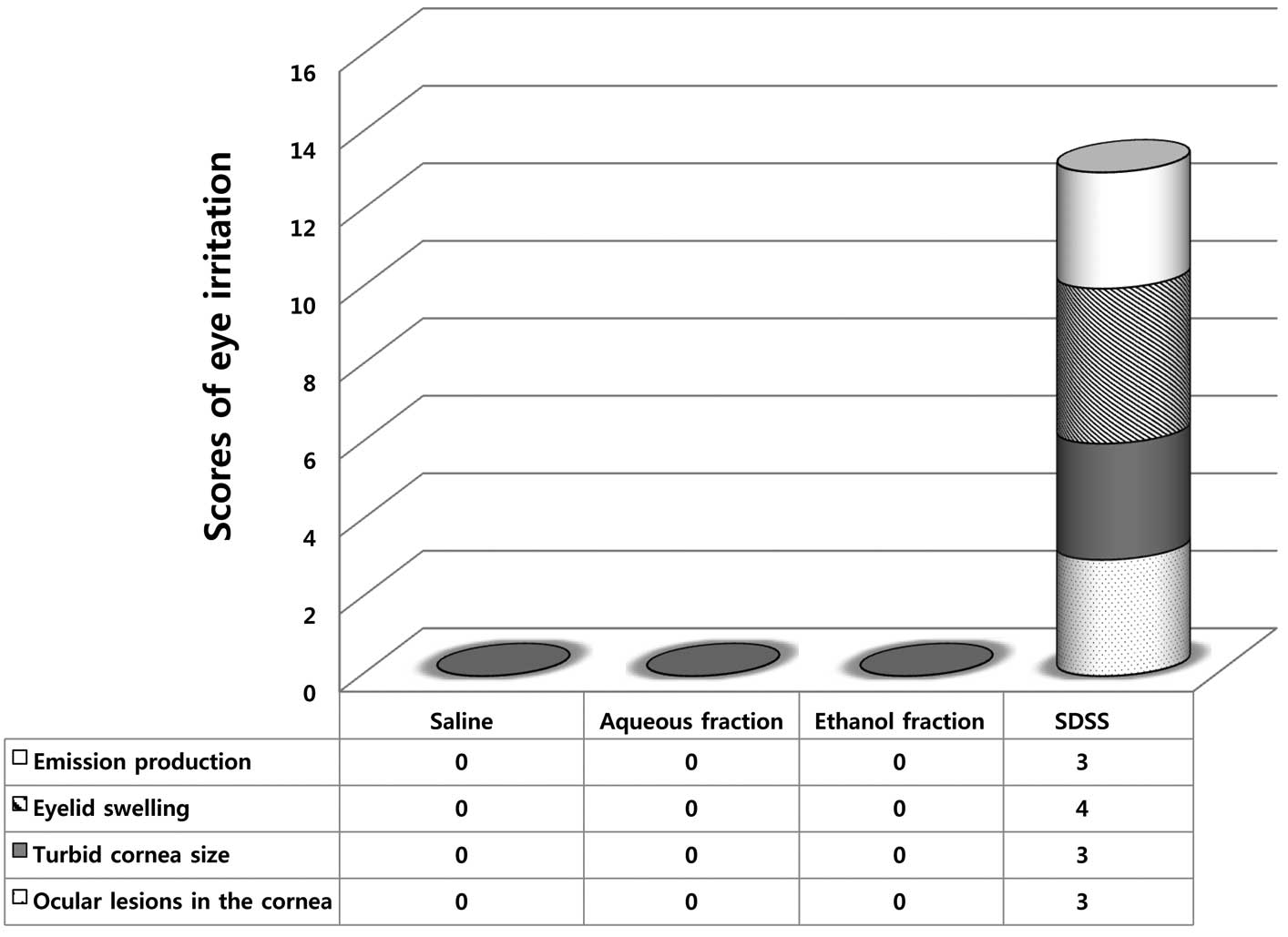
Aqueous and ethanol fractions of Angelica
keiskei leaf did not induce perforated ocular lesions in the
cornea (Fig. 2, dotted section of
column 4), similar to the patterns observed with saline (Fig. 2, column 1) with no additional
symptoms. In terms of turbid cornea size, scores were measured as
follows: 0 for no turbidity, 1 when the size of the cornea was 1/4
or less, 2 when greater than 1/4 but less than 1/2, 3 when greater
than 1/2 but less than 3/4 and 4 when greater than 3/4 up to a
value of 1 or more. Notably, cornea size and turbidity were not
affected by the fractions (Fig. 2,
columns 3 and 4). Additionally, the effects of the fractions on
eyelid swelling were examined. Eyelid swelling was scored as
follows: 0 for no swelling, 1 when normal or slightly swollen
(including nictitating membrane), 2 in cases of significant
swelling of the eyelid resulting in partial abduction, 3 when
swelling of the eyelid was approximately half of the wound and 4
when more than half of the eyelid was swollen. Using the point
scoring system, we confirmed that the eyelid was not affected in
terms of swelling upon treatment with the fractions (Fig. 2, columns 2 and 3). Production of
emissions was analyzed as follows: 0 for no emission, 1 when a
small amount of internal eyelash was observed, 2 for wet exhaust
and 3 when a large area around the eye and eyelid and/or eyelashes
had wet exhaust. As a result, no emission was observed in the eye
or eyelid/eyelashes following treatment with the fractions
(Fig. 2, columns 2 and 3).
Overall, we detected no changes or damage induced by
either the aqueous or ethanol fractions in contrast to sodium
dioctyl sulfosuccinate which caused severe toxic symptoms (Fig. 2, comparing column 4 with columns 2
and 3), as assessed by anatomical and clinical observations. The
criteria for determining whether or not other parameters are
associated with acute eye irritancy were assessed. Observations at
the set time intervals (day 1, 2, 3, 4 and 5) revealed that neither
the aqueous nor the ethanol fractions affected the ocular lesions
in the cornea (Fig. 3A, lanes 2
and 3), turbid cornea size (Fig.
3B, lanes 2 and 3), eyelid swelling (Fig. 3C, lanes 2 and 3) and emission
production (Fig. 3D, lanes 2 and
3), whereas sodium dioctyl sulfosuccinate induced perforated
damages (Fig. 3, lane 4 of A, B, C
and D). Additionally, we did not specify two recommended tests in
this report; however, skin irritancy and phototoxicity tests were
also negative, whereby wounds treated with the fractions showed
similar patterns of recovery as those treated with the control
(PBS), and UV exposure of 8-methoxypsoralen plus fraction
treatments did not induce tumors in mouse skin (data not shown). A
few positive toxicity cases have reportedly been identified from
other safety tests, and therefore, we cannot exclude the
possibility of toxicity based on acute, sub-acute or chronic safety
tests, such as the soap chamber test, repeat insult test or murine
local lymph node assay (21–23).
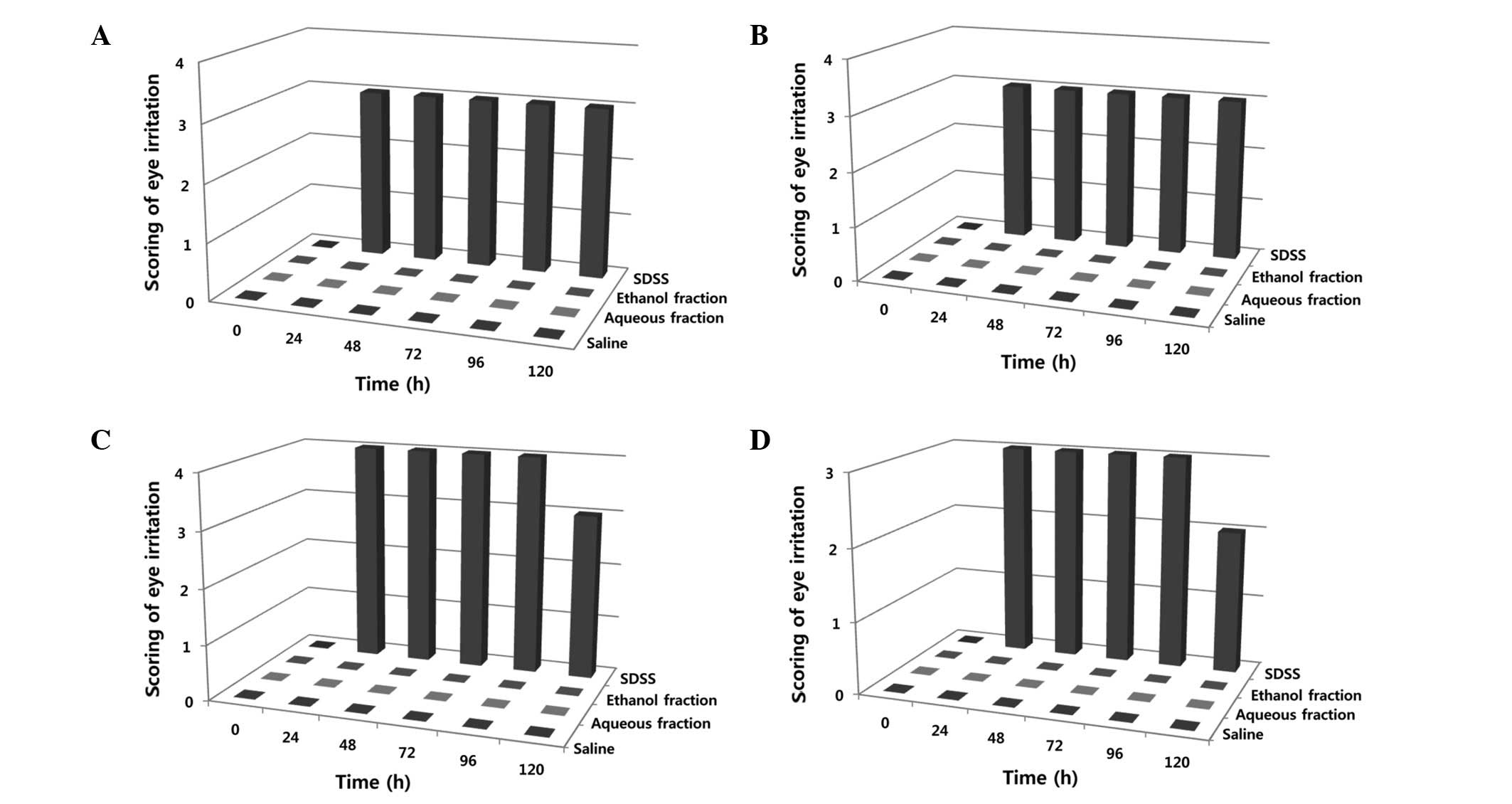 | Figure 3Observation of acute eye irritancy at
set time intervals. Scoring standards are similar to those
presented in Materials and methods. Observations were made at 6
time-points: 0, 24, 48, 72, 96, and 120 h. The scores of eye
irritation are shown at each time interval. (A) Ocular lesions in
the cornea, (B) turbid cornea size, (C) eyelid swelling and (D)
emission production. Data denote a classical result of five
independent observations. Saline, 1st lanes; aqueous fraction, 2nd
lanes; ethanol fraction, 3rd lanes; SDSS, 4th lanes. SDSS, sodium
dioctyl sulfosuccinate. |
In summary, Angelica keiskei leaf fractions
do not induce acute toxicity in the eye irritancy test;
specifically, no haze, swelling, redness or emissions in the eye
mucosa were observed. Angelica keiskei is therefore a
potential candidate for development in the cosmetic industry and/or
other applicable purposes. We await the purification and isolation
of the pure compound(s), which should aid in establishing those
that may be successfully applied for long-term use with no
hazardous effects, such as phytoestrogens and toxicants.
Acknowledgements
This study was supported by the
Technology Transfer Project, Kyungpook National University
Technopark, Daegu, Korea (S.-H.L). We thank Dr Jin-Chul Heo and
Dong-Yoon Nam for their technical assistance. This research was
also supported by Kyungpook National University Research Fund,
2012.
References
|
1
|
Sørensen JM: Herb-drug, food-drug,
nutrient-drug, and drug-drug interactions: mechanisms involved and
their medical implications. J Altern Complement Med. 8:293–308.
2002.PubMed/NCBI
|
|
2
|
Pan MH, Lai CS, Dushenkov S and Ho CT:
Modulation of inflammatory genes by natural dietary bioactive
compounds. J Agric Food Chem. 57:4467–4477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan MH, Ghai G and Ho CT: Food bioactives,
apoptosis, and cancer. Mol Nutr Food Res. 52:43–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarker SD and Nahar L: Natural medicine:
the genus Angelica. Curr Med Chem. 11:1479–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piersen CE: Phytoestrogens in botanical
dietary supplements: implications for cancer. Integr Cancer Ther.
2:120–138. 2003. View Article : Google Scholar
|
|
6
|
Lopes D and Strobl H: Kolodziejczyk P:
14-Methylpentadecano-15-lactone (muscolide): a new macrocyclic
lactone from the oil of Angelica keiskei L. Chem Biodivers.
1:1880–1887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sigurdsson S, Ogmundsdottir HM and
Gudbjarnason S: Antitumour activity of Angelica keiskei leaf
extract. In Vivo. 19:191–194. 2005.
|
|
8
|
Sigurdsson S, Ogmundsdottir HM and
Gudbjarnason S: The cytotoxic effect of two chemotypes of essential
oils from the fruits of Angelica keiskei L. Anticancer Res.
25:1877–1880. 2005.PubMed/NCBI
|
|
9
|
Yeh ML, Liu CF, Huang CF and Huang TC:
Hepatoprotective effect of Angelica keiskei in chronically
ethanol-treated mice. Pharmacology. 68:70–73. 2003.
|
|
10
|
Heo JC and Lee SH: Amelioration of
asthmatic-related symptoms by an aqueous extract of Angelica
archangelica L. J Life Sci. 18:1336–1341. 2008. View Article : Google Scholar
|
|
11
|
Tavaszi J, Budai P, Pálovics A and
Kismányoki A: An alternative test battery in detecting ocular
irritancy of agrochemicals. Commun Agric Appl Biol Sci. 73:891–895.
2008.PubMed/NCBI
|
|
12
|
Scott L, Eskes C, Hoffmann S, Adriaens E,
Alepée N, Bufo M, Clothier R, Facchini D, Faller C, Guest R, et al:
A proposed eye irritation testing strategy to reduce and replace in
vivo studies using bottom-up and top-down approaches. Toxicol In
Vitro. 24:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osborne R, Perkins MA and Roberts DA:
Development and intralaboratory evaluation of an in vitro human
cell-based test to aid ocular irritancy assessments. Fundam Appl
Toxicol. 28:139–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nigam PK: Adverse reactions to cosmetics
and methods of testing. Indian J Dermatol Venereol Leprol.
75:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Draize JH, Woodard G and Calvery HO:
Methods for the study of irritation and toxicity of substances
applied topically to the skin and mucous membranes. J Pharmacol Exp
Ther. 82:377–390. 1944.
|
|
17
|
Aoshima H, Saitoh Y, Ito S, Yamana S and
Miwa N: Safety evaluation of highly purified fullernenes: based on
screening of eye and skin damage. J Toxicol Sci. 34:555–562. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Draize JH: Dermal toxicity. Appraisal of
the Safety of Chemicals in Foods, Drugs and Cosmetics. Association
of Food and Drug Officials of the United States. Texas State
Departmemt of Health; Austin, Texas: pp. 46–59. 1959
|
|
19
|
Heo JC, Park JY, Kwon TK, Chung SK, Kim SU
and Lee SH: Development of high-throughput screening method for
anti-asthmatic agents by food-derived libraries. Korean J Food
Preserv. 12:267–274. 2005.
|
|
20
|
Heo JC, Woo SW, Kweon MA, Park JY, Lee HK,
Son M, Rho JR and Lee SH: Aqueous extract of a seed from
Helianthus annuus alleviates asthmatic symptoms in vivo. Int
J Mol Med. 21:57–61. 2008.
|
|
21
|
Korting HC, Herzinger T, Hartinger A,
Kerscher M, Angerpointner T and Maibach HI: Discrimination of the
irritancy potential of surfactants in vitro by two cytotoxicity
assays using normal human keratinocytes, HaCaT cells and 3T3 mouse
fibroblasts: correlation with in vivo data from a soap chamber
assay. J Dermatol Sci. 7:119–129. 1994. View Article : Google Scholar
|
|
22
|
Tardiff RG, Hubner RP and Graves CG:
Harmonization of thresholds for primary skin irritation from
results of human repeated insult patch tests and laboratory animal
skin irritation tests. J Appl Toxicol. 23:279–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basketter DA and Kimber I: Skin
irritation, false positives and the local lymph node assay: a
guideline issue? Regul Toxicol Pharmacol. 61:137–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|