Introduction
Early multiple organ failure (MOF) and subsequent
infection are serious complications of acute necrotizing
pancreatitis (ANP) and are the leading cause of mortality in ANP
patients, accounting for >80% of the total ANP mortality
(1). Damage to the intestinal
barrier function (IBF) and bacterial translocation (BT) are
currently considered the most likely sources of pathogens. The gut
also plays a role in priming neutrophils and the release of
inflammatory cytokines, which initiate and propagate nearly all the
detrimental consequences of severe inflammation and sepsis. Future
research approaches and potential therapeutic measures that may
restore and preserve gut barrier function are being explored
(2).
Tripterygium has clear anti-inflammatory and
immunomodulatory effects, and inhibits the excessive release of
inflammatory mediators at multiple levels (3). In the current study, Tripterygium
wilfordii Hook F multiglycosides (TWG) were used to treat a rat
model of ANP. During the treatment, the mechanism of TWG on the
treatment of intestinal mucosal barrier dysfunction caused by ANP
was investigated through observation of serum amylase levels,
lipase activity, plasma endotoxin levels, organ bacterial cultures,
plasma D-lactate concentrations, pathological changes of the
pancreas and intestinal mucosa and the survival rates of the
rats.
Materials and methods
Animals and pancreatitis model
SD rats (male, 10–12 weeks old, weighing 200–250 g)
were fasted but allowed to drink water freely for 16 h before the
experiment. They were allocated randomly to one of three groups:
the sham operation (SO), ANP and ANP+TWG groups. The SO group rats
(n=12), underwent laparotomy under general anesthesia, as described
for the ANP group, and sham intubation of the cholo-pancreatic duct
but without any drug injection. These rats were sacrificed 24 h
later. In the ANP group, laparotomy was performed through a midline
incision. After cannulation of the common biliopancreatic duct with
a 28-gauge, 0.5-inch microfine catheter, a microaneurysm clip was
placed on the bile duct below the liver and another around the
common biliopancreatic duct at its entry into the duodenum to avoid
reflux of enteric contents into the duct. Then, 2 ml/kg 3.5% sodium
taurocholate (Sigma, St. Louis, MO, USA) was slowly infused into
the common biliopancreatic duct at a volume of 2.0 ml/kg at 0.25
ml/min. The infusion pressure was kept <3.4–3.9 kPa, as measured
using a mercury manometer. When the infusion was complete, the two
microclips were removed and the abdomen was closed in two layers.
All procedures were performed using sterile techniques. The ANP+TWG
group underwent the induction of acute pancreatitis and treatment
with TWG. After pancreatitis was induced, as described for the ANP
group, the rats were injected intraperitoneally 60 min later with
TWG 50 mg/kg. Six animals in each group were randomly selected for
observation of survival. The other animals were sacrificed 24 h
after surgery.
Amylase and lipase measurement
Blood samples were drawn from the aorta 8 h after
the induction of ANP, and serum amylase/lipase levels were measured
using a standard clinical automated analyzer.
Plasma endotoxin
Following laparotomy under sterile conditions, blood
samples from the abdominal aorta were acquired and centrifuged to
isolate plasma. The endotoxin activities of the plasma were
determined using a Limulus amebocyte lysate (LAL) assay kit
(QCL-1000, catalog no. 50-647U; BioWhittaker, Shanghai, China)
according to the manufacturer’s instructions. Two different charges
of elastase preparations were tested.
Quantitative cultures and bacterial
identification
Following laparotomy under sterile conditions, blood
samples from the abdominal aorta and ascites were acquired and put
into sterile tubes. Mesenteric lymph nodes (MLNs), one portion of
the pancreas, livers and lungs were harvested, put into anaerobic
chambers, and processed for the culturing of aerobic and anaerobic
organisms using a standardized method. Each sample was weighed and
homogenized. Afterwards, the homogenates were diluted serially,
quantitatively plated in duplicate on phenylethyl alcohol and
MacConkey II agar, and then incubated aerobically at 37°C for 24 h.
Bacterial counts were expressed as colony-forming U/g tissue, and
counts of ≥1,000 colony-forming U/g were considered to represent a
positive culture. Gram-negative bacteria were identified using the
API-20E system (bioMerieux Vitek, Hazelwood, MO, USA).
Gram-positive bacteria were identified to the genus level using
standard microbiological methods.
D(-)-lactate determination
The plasma from systemic blood samples was obtained
and subjected to a deproteination and neutralization process by
acid/base precipitation using perchloric acid and potassium
hydroxide. The protein-free plasma was then assayed for
D(-)-lactate concentration by a previously described
enzymatic-spectrophotometric method with minor modification
(4).
Histopathologic analysis
A portion of the pancreas from the same anatomical
location in each rat, including the main pancreatic duct, was fixed
in 10% neutral-buffered formalin and embedded in paraffin. One
paraffin section stained with hematoxylin and eosin was examined
for each pancreas. Two pathologists, who were blinded to the
treatment protocol, scored the tissues with respect to edema,
acinar necrosis, inflammatory infiltrate, hemorrhage, fat necrosis
and perivascular inflammation in 20 fields. The scores for each
histological examination were summed, yielding a maximum score of
24, as defined by Schmidt et al(5).
Electron microscopy
The relationship between zymogen granules and
autophagic vacuoles was examined in the acinar cells of rats 5 h
after duct ligation using transmission electron microscopy. From
the freshly excised pancreas, small cubes <1 mm3 in
size were sliced and immediately fixed in Karnovsky’s fixative
(2.5% glutaraldehyde, 4% paraformaldehyde and 1 M Na cacodylate
buffer, pH 7.4). The samples were post-fixed in 1% osmium
tetroxide, dehydrated in graded concentrations of ethanol,
incubated in ascending concentrations of propylene oxide and
embedded in Spurr’s epoxy resin. Ultrathin sections were stained
with uranyl acetate and bismuth subnitrate and viewed under a
Hitachi transmission electron microscope (H-7000) at Fudan
University.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Translocation incidence was evaluated by
Fisher’s exact test. The significance of differences in total
histopathologic scores, serum amylase activities and cytokine
levels were assessed using one-way analysis of variance and Tukey’s
HSD as post hoc tests. Detailed histo-pathological scores (e.g.,
for edema and acinar necrosis) were assessed using the
Kruskal-Wallis test, and subgroup analyses were conducted using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant result. All statistical measurements were
performed using SPSS PC version 9.05 (SPSS, Inc., Chicago, IL,
USA).
Results
Survival rates of the ANP and ANP+TWG
groups
The majority of the animals in the ANP group died in
the first 3 days after the induction of pancreatitis: at the 3-day
interval, 5 of 6 rats had died, corresponding to a death rate of
83.3%. By contrast, TWG led to a significant reduction of the death
rate, with only 2 deaths in 6 rats (33.3%). Autopsy of the rats
that died during the experiment revealed extensive intra- and
extrapancreatic necrosis, pancreatic hemorrhage and moderate to
massive hemorrhagic ascites in animals who died within 48 h after
the induction of pancreatitis. At later time intervals, the upper
abdomen had changed to an inflammatory mass harboring multiple
abscess formations, which caused severe intestinal obstruction in
certain cases. In the lungs, exudative congestion was observed,
whereas the liver and kidneys appeared grossly normal.
Plasma amylase and lipase levels
Compared with baseline values, all rats with
pancreatitis showed increased lipase and amylase levels
(P<0.01). However, the amylase and lipase concentrations were
found to be decreased (P<0.01) in the ANP+TWG group (Table I).
 | Table I.Amylase, lipase and endotoxin levels
in different groups of rats. |
Table I.
Amylase, lipase and endotoxin levels
in different groups of rats.
| Group | Sample no. | Amylase (U/l) | Lipase (U/dl) | Endotoxin
(EU/ml) |
|---|
| SO | 6 | 5000±580 | 200±9 | 0.033±0.007 |
| ANP | 12 | 30000±900 | 800±220 | 0.067±0.012 |
| ANP+TWG | 12 | 10000±550a | 600±90a | 0.052±0.014a |
Plasma endotoxin
The plasma endotoxin level of the ANP group was
0.067±0.012 EU/ml, which was significantly higher than that of the
SO group (0.0330±0.007 EU/ml; P<0.01). However, the endotoxin
levels in the TWG treated group (0.052±0.014 EU/ml) were
significantly lower than those in the ANP group (P<0.01;
Table I).
Bacterial cultures
The results of bacterial culture were negative for
the SO group. The positive rate of the ANP group was 43.1%, in
which the highest positive rate (75%) was obtained for the ascites
bacterial culture (Table II).
However, with the exception of MLNs, the positive rate of the
ANP+TWG group was significantly lower than that of the ANP group
(P<0.05). The most common bacteria identified in the ANP and
ANP+TWG groups were Enterococcus sp. and Proteus sp.
(Table III).
 | Table II.Results of bacterial cultures (%). |
Table II.
Results of bacterial cultures (%).
| Group | Sample no. | Ascites | Blood | MLNs | Liver | Pancreas | Spleen |
|---|
| SO | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| ANP | 12 | 9 (75) | 2 (17) | 6 (50) | 8 | 8 (67) | 7 (59) |
| ANP+TWG | 12 | 4 (34) | 1 (9) | 2 (17) | 0 | 0 | 0 |
 | Table III.Bacterial classifications of the ANP
and ANP+TWG groups. |
Table III.
Bacterial classifications of the ANP
and ANP+TWG groups.
| Group | Enterococcus
sp. | Proteus
sp. | E. coli | Bacillus |
Pneumococcus |
|---|
| ANP | 9 | 7 | 5 | 1 | - |
| ANP+TWG | 3 | 1 | - | - | - |
Changes in plasma D(-)-lactate
The level of D(-)-lactate in the systemic blood of
the SO group was lower than that of the ANP group. Following TWG
treatment, the level of D(-)-lactate declined significantly
(Table IV).
 | Table IV.Comparison of plasma D(-)-lactate
concentrations in rats of different groups. |
Table IV.
Comparison of plasma D(-)-lactate
concentrations in rats of different groups.
| Group | Sample no. | D(-)-lactate
concentration (mg/l) |
|---|
| SO | 6 | 9.888±1.008 |
| ANP | 12 | 11.098±2.745a |
| ANP+TWG | 12 | 7.772±2.916b |
Changes in histopathology
Pancreas
The following features were observed in the pancreas
specimens: i) General observation: for the ANP group, extensive
bloody intra-abdominal ascites, widening of the ducts and
saponification spots around the pancreas and mesentery were
observed, the volume of the pancreas increased, and congestion and
edema occurred with hemorrhage and necrosis. The degree of
pathological change in the ANP+TWG group was milder than that in
the ANP group at the same time interval. ii) Light microscopy
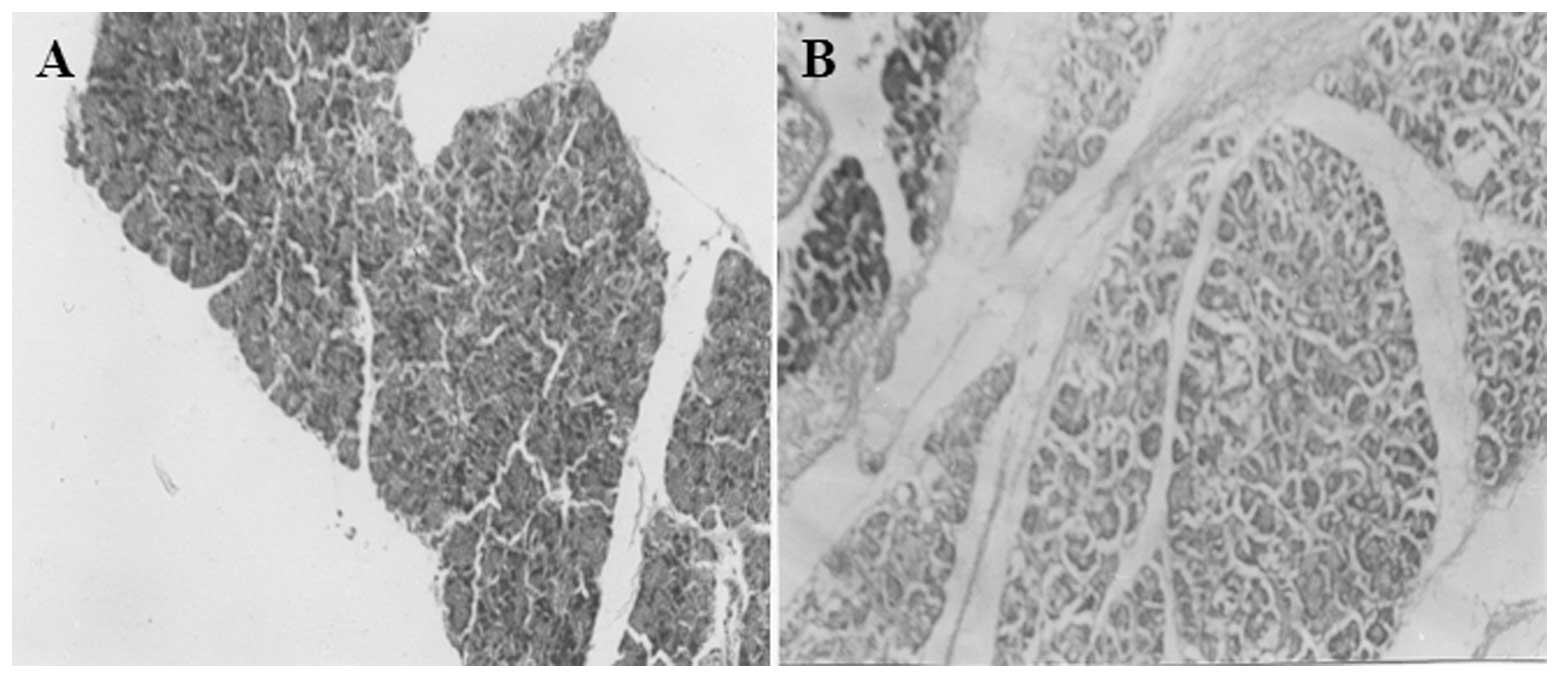
observation: in the ANP group (Fig.
1A), pancreatic interstitial hyperemia and edema, widening of
lobular intervals, including acinar intervals, vascular congestion,
interstitial hemorrhage and saponified or extensive acinar necrosis
occurred, accompanied by extensive monocyte and neutrophil
infiltration. Fat necrosis with saponification spots also occurred.
In the ANP+TWG group, acinar necrosis, hemorrhage and fat necrosis
were similar to those in the ANP group. However, the degree of
pancreatic edema and inflammatory cell infiltration in the ANP+TWG
group was reduced (Fig. 1B),
compared with that in the ANP group at the same time interval. iii)
Pathological evaluation: the levels of congestion, edema and
necrosis in the ANP group were significantly higher than those in
the SO group. In the ANP+TWG group, the pathological features were
clearly reduced (Table V).
 | Table V.Pathology evaluation of rats with
ANP. |
Table V.
Pathology evaluation of rats with
ANP.
| Group | Edema | Inflammation | Hemorrhage | Necrosis |
|---|
| SO | 0.17±0.02 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| ANP | 3.00±0.00 | 3.28±0.39 | 2.89±0.44 | 2.67±0.33 |
| ANP+TWG | 2.39±0.08a | 2.06±0.74a | 1.00±0.44a | 0.84±0.17a |
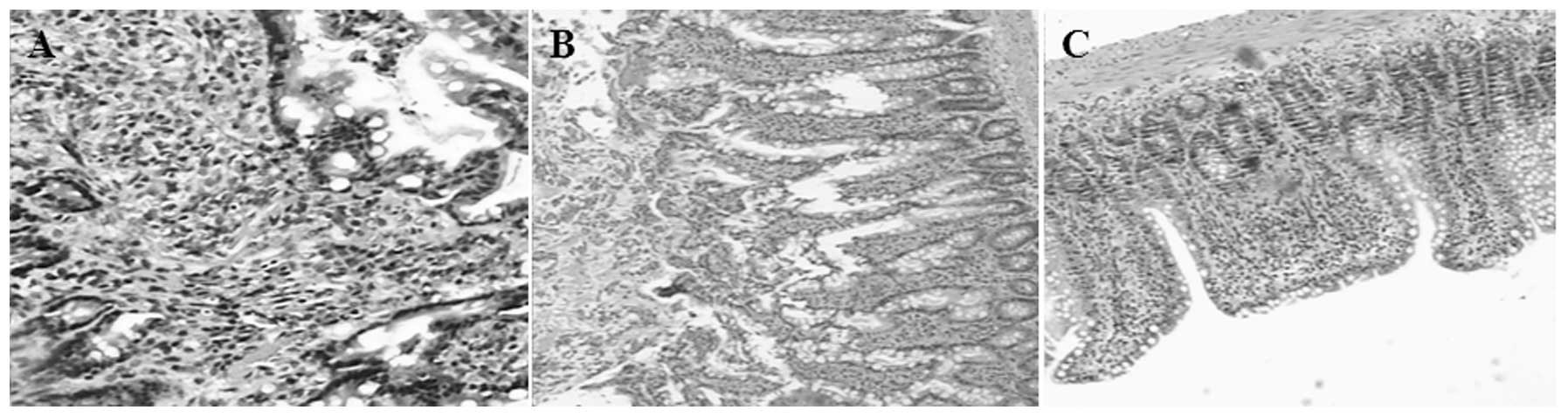
Ileal mucosa
The following featues were observed in the ileal
mucosa: i) Light microscopy observation: for the ANP group, there
was marked congestion, edema, lymphatic expansion and villous
thickening, together with monocyte infiltration and inflammation,
columnar epithelial cell necrosis and loss, some loss of villi and
damage in the mesenchymal layer of the ileal mucosa (Fig. 2A and B). The lesions in the ANP+TWG
group were clearly reduced in severity compared with those in the
ANP group. Active epithelium hyperplasia of the intestinal mucosa
was observed in the ANP+TWG group (Fig. 2C). ii) Electron microscopic
analyses: cells were compact, microvilli were arranged compactly
and neatly, and organelles maintained their integrity in the SO
group (Fig. 3A). By contrast, in
the ANP group, marked edema, loose particles and organelle damage
in the cells, disordered and necrotic microvilli and damaged mucosa
were visible (Fig. 3B). However,
slightly loose particles and almost compact organelles were
observed in the ANP+TWG group (Fig.
3C). iii) Villous height and mucosal thickness were clearly
reduced in the ANP group, but in the TWG treatment group, the
pathological changes observed in the ANP group were alleviated
significantly (Table VI).
 | Figure 3.Electron microscopy observation of rat
ileum. (A) In the SO group, cells and organelle retained their
integrity, and microvilli were arranged in a compact and orderly
manner (scale bar, 1 μm). (B) In the ANP group, cellular edema
occurred, granules in the cells were loose and some mucosal
fracture was observed (scale bar, 2 μm). (C) In the ANP+TWG group,
the granules in the cells were slightly loose but the organelles
retained their integrity (scale bar, 1 μm). SO, sham operation;
ANP, acute necrotizing pancreatitis; TWG, Tripterygium
wilfordii Hook F multiglycosides. |
 | Table VI.Comparison of villous height and
mucosal thickness in rats of different groups. |
Table VI.
Comparison of villous height and
mucosal thickness in rats of different groups.
| Group | Sample no. | Villous height
(μm) | Mucosal thickness
(μm) |
|---|
| SO | 6 | 57.25±3.580 | 90.653±8.026 |
| ANP | 12 |
44.278±6.081a |
70.833±10.217a |
| ANP+TWG | 12 |
55.597±6.902b |
87.736±11.794b |
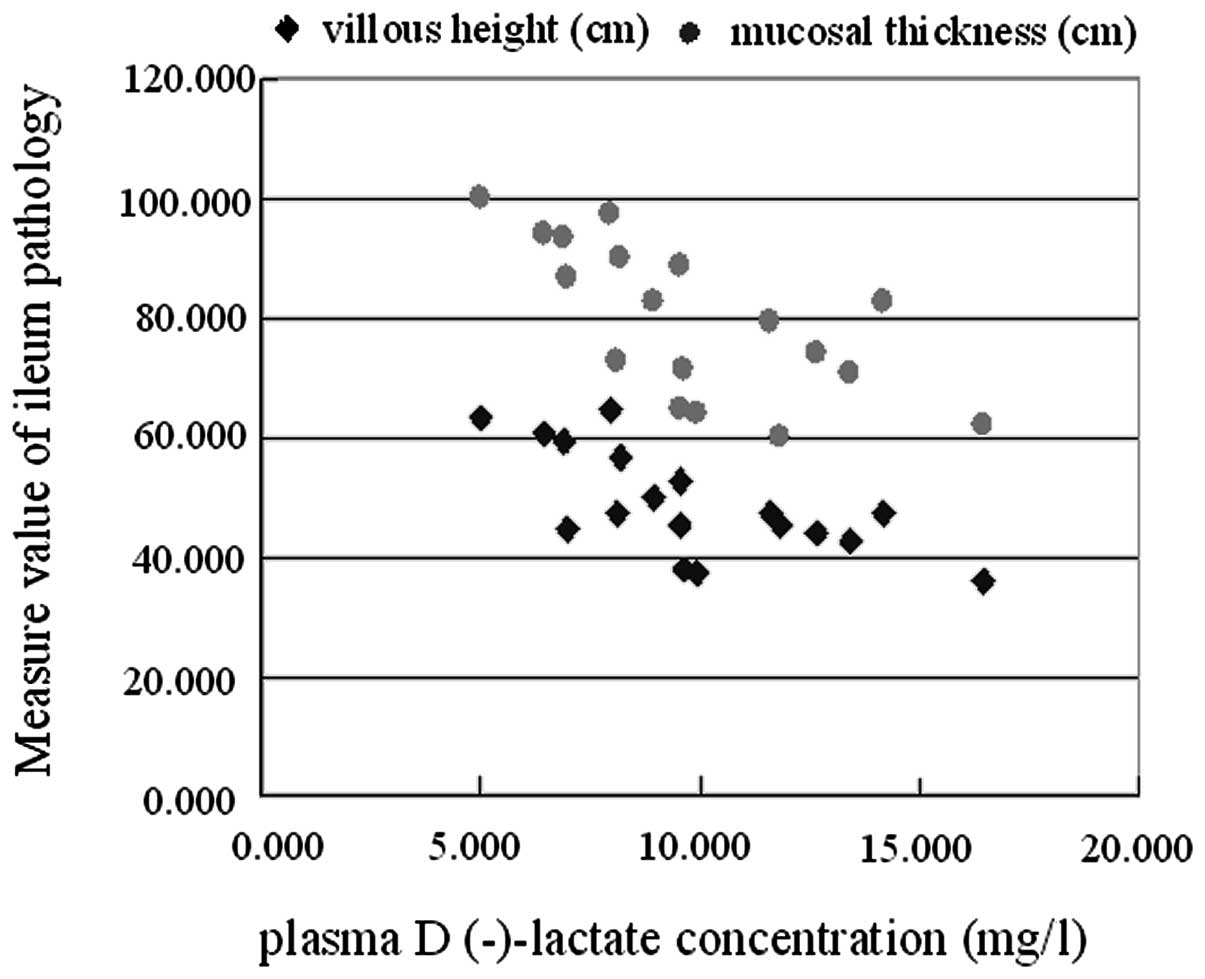
Correlation between plasma
D(-)-lactate concentration and villous height and mucosal
thickness
The correlations between plasma D(-)-lactate
concentration and villous height and mucosal thickness were
examined using regression analysis. Plasma D(-)-lactate
concentration and villous height showed a significant negative
correlation (r=−0.684, P<0.01), as did plasma D(-)-lactate
concentration and mucosal thickness (r=−0.677, P<0.01; Fig. 4).
Discussion
Changes in the permeability of the intestinal mucosa
may be manifested through alterations in gastrointestinal
pathology, intestinal microbes and metabolites, obstacles in IgA
synthesis and secretion and endotoxemia. Plasmid-labeled E.
coli have demonstrated that intestinal bacteria are able to
pass through the intestinal mucosa to reach the mesenteric lymph
nodes, and to reach other remote organs via the blood in ANP
(6).
Nettelbladt et al and Cicalese et
al(7,8) reported that labeled bacterial strains
may be detected at remote organs after feeding labeled bacteria to
ANP mice, and the occurrence of bacterial translocation (BT) was
100%. During ANP, the blood flow in the body is redistributed;
blood flow in the gastrointestinal tract is reduced, which results
in intestinal mucosal ischemia and hypoxia. Intestinal
microcirculation blood flow declines within 15 min of ANP. A study
by Lemaire et al(9) found
that following ischemia of the intestinal mucosa for 30–40 min,
epithelium cells stop the synthesis of protective glycoprotein and
absorb endotoxin before pathological damage occurs. During ANP,
large amounts of cytokines and inflammatory mediators are released.
Among them, TNF-α, IL-β, IL-8 and leukotriene may increase ischemia
and injury of the intestinal mucosa. The ‘two-hit’ theory suggests
that leukocytes which perfuse to various tissues are activated by
pro-inflammatory factors that are released by macrophages during
ANP; the activated leukocytes release a large number of protein
decomposition enzymes, oxygen free radicals and cytokines, causing
an excessive systemic immune inflammatory response and further
aggravating the injury of the intestinal mucosa, eventually forming
a vicious cycle of damage-increased permeability-further damage
(10). There is a significantly
positive correlation between gut hyper-permeability and the
severity of pancreatitis. An increase of intestinal permeability
indicates gut barrier dysfunction (11). Though these potential mechanisms,
ANP eventually leads to disturbances of the microcirculation,
ischemia and increases of vascular permeability, impairment of the
intestinal mucosal barrier, and translocation of intestinal
bacteria and endotoxins into the blood. At the same time, products
of bacterial metabolism, including D-lactic acid, are absorbed into
the blood, further increasing the systemic inflammatory
response.
D-lactic acid is the one of metabolism products of
inherent intestinal bacteria, including E. coli,
Lactobacillus and Klebsiella. Mammalian liver is lacking
in the enzymes that are able to break down D-lactic acid. D-lactic
acid passes into blood via the portal vein, and leads to a
concentration change. Therefore, plasma D-lactate concentrations in
the inferior vena cava may reflect the permeability of the
intestinal tract. Murry et al(12) found that the D-lactic acid
concentrations in patients with intestinal ischemia were
significantly increased among patients undergoing laparotomy.
Another report showed that the D-lactate concentration correlated
significantly with the intestinal mucosal injury score in critical
patients with intestinal ischemia, intestinal ischemia-reperfusion
and burns (13). It was also
synchronized with the level of endotoxin, and increased
significantly within 1–1.5 h after ANP. Therefore, D-lactic acid is
an early warning signal for intestinal mucosa dysfunction, and may
be detected simply to monitor intestinal dysfunction in critical
illness. Ammori et al used the kidney excretion ratio of
oral polyethylene glycol 3350 to polyethylene glycol 400 to
investigate the permeability of the intestine in ANP (14). The authors found that the ratio in
the patients who developed MOF and/or succumbed was much higher
than that in other critically ill patients, which suggests that the
early increase in intestinal permeability of patients with severe
acute pancreatitis is likely to have a significant impact on
pathophysiological changes.
In the current study, light microscopy in the ANP
group showed interstitial edema and a small amount of bleeding in
the ileum, expansion of lymph ducts and vessels, neutrophil
infiltration with lymphoid follicular hyperplasia, villous edema,
thickening, height reduction, arrangment disorder and necrosis and
thinning of the mucosa. Electron microscopy showed marked edema in
the cells, granular loosening, rupture of organelles, disordered
microvilli, large areas of necrosis and detachment, and damage in
areas of the mucosa. These morphological changes indicate the
mechanical damage of the intestinal barrier. The increase of plasma
D-lactate concentration was identified to be significantly
correlated with the thickness of the intestinal mucosa, which
demonstrates that the plasma concentration of D-lactate reflects
the functional damage of the intestinal mucosal barrier in the ANP
rats, which provides the conditions for BT. The SO group revealed
no such changes, which is consistent with earlier studies (15). Other experiments showed that the
endotoxin levels and positive rates of organ bacterial culture were
significantly increased in ANP rats. Bacterial culture results
revealed that the positive rate in the ANP group was 55.6%; the
highest positive rate was observed in ascites 75%. Strain
identification revealed that the bacteria most commonly involved
were Enterococcus species and Proteus mirabilis,
which demonstrates that endotoxemia and intestinal BT are the
result of early increases of intestinal permeability and intestinal
barrier damage.
Chinese medicine has been widely used in the
treatment of ANP. The clinical application of triptolide is common
in traditional Chinese medicine (16). Triptolide is an immunosuppressant
which has anti-inflammatory activity and inhibits cell-mediated and
humoral-mediated immune function. TWG is a compound that is
chemically purified from the root of Tripterygium by
removing the toxic root skin and other toxic components, which have
a high value in original Chinese medicine.
It has been identified that Tripterygium has
a dual role in immune regulation. Low doses increase the cytotoxic
activity of natural killer cells, correct distribution disorders of
T subset cells and regulate the immune response. In addition,
triptolide itself has a direct anti-inflammatory effect. It
inhibits the increase in vascular permeability in inflammation, the
chemotaxis of inflammatory cells, the production and release of
inflammatory mediators and platelet aggregation.
Tripterygium, due to its potent anti-inflammatory and immune
regulatory effects, is able to inhibit the production of cytokines,
including TNF-α, IL-6 and IL-8, and the phagocytic function of
phagocytes (17,18). The immunosuppressive, cartilage
protective and anti-inflammatory effects of TWG extracts are well
documented (19). Our previous
studies have shown that Tripterygium wilfordii is able to
reduce serum endotoxin, TNF-α and IL-1 levels in ANP rats (3). Wang et al(20) in another study found that a
combination of Salvia miltiorrhiza and Tripterygium
wilfordii was able to significantly reduce serum amylase,
inhibit excessive inflammatory cytokine production and reduce the
extent of tissue damage in the treatment of ANP.
The presence of large amount of ascites associated
with ANP results in intestinal paralysis and weakened
gastrointestinal motility, which undermines the resistance of the
intestine to bacteria invasion. During ANP, systemic immunity is
impaired. Intestinal bacteria and bacterial toxins are able to pass
through the mucosa into the submucosa, reach mesenteric lymph nodes
and eventually enter distant organs through the blood (2). The current study found that serum
endotoxin, serum amylase and lipase activities in the ANP+TWG group
were significantly lower than in the ANP group. The plasma
D-lactate concentration also decreased in the ANP+TWG group. The
positive rates of bacterial cultures from the parenteral organs,
blood and ascites in the ANP+TWG group were lower than in the ANP
group. Furthermore, the pathology score determined by optical
microscopy was improved in the ANP+TWG group. It was demonstrated
that TWG reduces the intestinal permeability to endotoxins in ANP,
maintains the structure integrity of mucosal cells and has a
therapeutic effect on intestinal barrier dysfunction and BT in rats
with ANP.
In conclusion, intestinal barrier dysfunction and
intestinal BT exist in ANP rats. TWG improves the pancreatic and
intestinal damage, strengthens the biological barriers of the
intestinal tract, reduces gut-derived bacteria and the incidence of
endotoxin translocation, and prevents the development of ANP.
References
|
1.
|
Hong W, Ma BJ and Cai D: New progress of
diagnosis and treatment of acute pancreatitis. J Hepatopancreatobil
Surg. 3:1652001.(In Chinese).
|
|
2.
|
Ammori BJ: Role of the gut in the course
of severe acute pancreatitis. Pancreas. 26:122–129. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jin C, Ni QX, Zhang QH, Xiang Y, Zhang N
and Zhang YL: An experimental study on the immunoregulatory effect
of Tripterygium wilfordii Hook F in rats with acute
necrotizing pancreatitis. Chin J Gen Surg. 15:283–285. 2000.(In
Chinese).
|
|
4.
|
Brandt B, Siegel A, Waters G and Bloch MH:
Spectrophotometic assay for D-(-)-lactate in plasma. Anal Biochem.
102:39–46. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Schmidt J, Rattner DW, Lewandrowski K, et
al: A better model of acute pancreatitis for evaluating therapy.
Ann Surg. 215:44–56. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kazantsev GB, Hecht DW, Rao R, et al:
Plasmid labeling confirms bacterial translocation in pancreatitis.
Am J Surg. 167:201–206. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nettelbladt CG, Katouli M, Bark T,
Svenberg T, Möllby R and Ljungqvist O: Evidence of bacterial
translocation in fatal hemorrhagic pancreatitis. J Trauma.
48:314–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cicalese L, Sahai A, Sileri P, et al:
Acute pancreatitis and bacterial translocation. Dig Dis Sci.
46:1127–1132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lemaire LC, van Lanschot JJ, Stoutenbeek
CP, van Deventer SJ, Wells CL and Gouma DJ: Bacterial translocation
in multiple organ failure: cause or epiphenomenona still unproven.
Br J Surg. 84:1340–1350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ogawa M: Acute pancreatitis and cytokines:
‘second attack’ by septic complication leads to organ failure.
Pancreas. 16:312–315. 1998.
|
|
11.
|
Ding LA, Li JS, Li YS, Zhu NT, Liu FN and
Tan L: Intestinal barrier damage caused by trauma and
lipopolysaccharide. World J Gastroenterol. 10:2373–2378.
2004.PubMed/NCBI
|
|
12.
|
Murry J, Gonze D, Nowak R and Cobb CF:
Serum D-(-)-lactate levels as an aid to diagnosing acute intestinal
ischemia. Am J Surg. 167:575–578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sun XQ, Fu XB, Zhang R, et al: Plasma
D-lactate levels as a useful marker of increased intestinal
permeability after severe injuries. Zhongguo Wei Zhong Bing Ji Jiu
Yi Xue. 12:476–478. 2000.(In Chinese).
|
|
14.
|
Ammori BJ, Leeder PC, King RF, et al:
Early increase in intestinal permeability in patients with severe
acute pancreatitis: correlation with endotoxemia, organ failure,
and mortality. J Gastrointest Surg. 3:252–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wang XP, Wang BX, Wu JX, et al: Bacterial
translocation from gut in acute necrotizing pancreatitis:
beneficial effect of growth hormone. Chung Hua Hsiao Hua Tsa Chih.
20:171–174. 2000.(In Chinese).
|
|
16.
|
Cao M, Sun RQ, Wu DJ, et al: The research
progression of Chinese drug Tripterygium wilfordii hook.f.
Chinese Patent Medicine. 18:40–42. 1996.(In Chinese).
|
|
17.
|
Chang DM, Chang WY, Kuo SY and Chang ML:
The effects of traditional antirheumatic herbal medicines on immune
response cells. J Rheumatol. 24:436–441. 1997.PubMed/NCBI
|
|
18.
|
Zhao G, Vaszar LT, Qiu DV, Shi LF and Kao
PN: Anti-inflammatory effects of triptolide in human bronchial
epithelial cells. Am J Physiol Lung Cell Mol Physiol.
279:L958–L966. 2000.PubMed/NCBI
|
|
19.
|
Bao J and Dai SM: A Chinese herb
Tripterygium wilfordii Hook F in the treatment of rheumatoid
arthritis: mechanism, efficacy, and safety. Rheumatol Int.
31:1123–1129. 2011.
|
|
20.
|
Wang GM and Bao MS: The experiment
research of therapeutic alliance using Salvia miltiorrhiza
bunge and Tripterygium wilfordii hook f multiglycosides in
acute necrotizing pancreatitis. Journal of Shanxi Medical
University. 35:15–17. 2004.(In Chinese).
|