Introduction
Dental caries is a prevalent oral infectious
disease, which is associated with various pathogenic
microorganisms, including Streptococcus mutans and
Streptococcus sobrinus. S. mutans is considered a crucial
pathogen in the pathogenesis of dental caries (1). S. mutans produces
glucosyltransferases (Gtfs) and synthesizes glucans from sucrose.
Glucans are critical for bacterial accumulation on the tooth
surface and the formation of cariogenic biofilms (2). Furthermore, S. mutans survive
at low pH values and generate acids that result in the
demineralization of tooth enamel, thereby initiating dental caries
(3). Therefore, it has been
proposed that disruption of the ability of S. mutans to form
acids and glucans is an effective therapeutic approach for the
treatment of dental caries.
Eugenol (4-allyl-2-methoxyphenol) is an aromatic
molecule found in essential oils and various plants, including
cloves, bay leaves and cinnamon leaves. Numerous studies have
reported that eugenol possesses antibacterial, antiviral,
antioxidant, anti-inflammatory and analgesic effects (4–6).
Eugenol has been widely used in dentistry to treat toothache and
pulpitis (7). A previous study
indicated that eugenol may be an ideal natural agent for use in
oral care products. Eugenol inhibits the growth and insoluble and
soluble glucan synthesis of S. sobrinus (8). However, the therapeutic effect of
eugenol against dental caries has not been thoroughly evaluated.
Therefore, the aims of this study were: i) to examine the effects
of eugenol on the adherence, acid production and insoluble glucan
synthesis of S. mutans in vitro and ii) to determine the
inhibitory effects of eugenol on caries development in
vivo.
Materials and methods
Reagents
Eugenol and sodium fluoride were purchased from
Sigma (St. Louis, MO, USA). Hydroxyapatite beads were obtained from
Bio-Rad (Hercules, CA, USA). All other chemicals were of analytical
grade and commercially available.
Measurement of acid production by S.
mutans 25175
Acid production was performed as described
previously with slight modifications (9). Briefly, eugenol was added to 0.95 ml
phenol red broth containing 1% glucose. Then, the mixture was
inoculated with 0.05 ml S. mutans 25175 seed culture. After
incubation at 37°C for 24 h, the pH values of the cultures were
measured using a pH meter.
Measurement of water-insoluble glucan
synthesis by Gtfs
S. mutans 25175 was cultured at 37°C for 24 h
in tryptic soy broth. The culture supernatant was salted out with
solid ammonium sulfate to 70% saturation and then agitated at 4°C
for 1 h. After centrifugation at 13,500 × g for 20 min, the
precipitate was dialyzed against 10 mM potassium phosphate buffer
(pH 6.0). The crude Gtfs preparation was stored at −80°C until
further analysis.
Reaction mixtures consisting of 0.025 ml crude Gtfs
preparation and 0.175 ml eugenol (final concentration, 0, 4, 8 and
16 mg/ml) in 0.8 ml 0.0625 M potassium phosphate buffer containing
12.5 μg/l sucrose and 0.25 μg/l sodium azide were
incubated at 37°C for 18 h. The water-insoluble glucan was
sedimented and washed with distilled water and then ultrasonicated
for 6 sec. The absorbance was examined at 550 nm against a blank
control.
Bacterial adherence assay
The bacterial adherence assay was performed as
described previously (12).
Briefly, S. mutans 25175 was diluted in tryptic soy broth at
a density of 108 colony-forming units (CFU) per
milliliter. Human saliva was collected from an adult donor and
clarified by centrifugation. Hydroxyapatite beads were treated with
clarified human saliva and rotated at 7 × g for 1 h at room
temperature. The saliva-coated hydroxyapatite beads (S-HAs) were
washed three times with 0.01 M potassium phosphate buffer (pH 7.0).
Bacterial suspensions with or without various concentrations of
added eugenol were incubated with the S-HAs at 37°C for 90 min and
then the S-HAs were washed three times with potassium phosphate
buffer. The detached cells on S-HAs were dispersed, diluted and
spread on Mitis Salivarius agar plates containing bacitracin (3.2
mg/ml). The number of bacterial colonies was counted on each plate
after incubation at 37°C for 48 h and the CFU were then
calculated.
Animal experiments
The animal experiments were performed as described
previously (10,11). Briefly, specific pathogen-free male
Wistar rats (aged 19 days; Kunming Medical University, Kunming,
China) were infected daily for five consecutive days with a growing
culture of S. mutans 25175. Rats aged 25 days were randomly
divided into three groups (n=15) and their teeth were treated
topically using a camel hair brush twice daily for 5 weeks, as
follows: i) vehicle control [15% ethanol containing 1%
dimethylsulfoxide (DMSO)], ii) 16 mg/ml eugenol and iii) 250 ppm
fluoride. The rats were provided with cariogenic diet 2000 and 5%
sucrose water ad libitum. At the end of the 5-week
experimental period, the rats were deeply anesthetized and
sacrificed. The lower left jaw was collected aseptically, immerged
in 5.0 ml sterile saline solution and sonicated. The suspension was
plated on blood agar and on Mitis Salivarius agar plus streptomycin
to respectively estimate the number of total cultivable
microorganisms and S. mutans populations. The smooth-surface
and sulcal caries and their severities (Ds, dentin exposed; Dm, 3/4
of the dentin affected; Dx, whole dentin affected) were evaluated
by means of Larson’s modification of Keyes’s system (11,12).
The caries score was determined blindly with respect to the groups.
All procedures were performed in accordance with guidelines set for
the use of experimental animals by the Committee on Animal Care and
Use of Kunhua Hospital (Kunming, China).
Statistical analysis
All values are expressed as the mean ± standard
error of the mean (SEM). The data were analyzed by analysis of
variance (ANOVA) followed by Tukey-Kramer multiple comparisons test
using SPSS software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
In vitro effect of eugenol on the
cariogenic properties of S. mutans
In this study, we firstly investigated the
inhibitory effect of eugenol on acid production by S. mutans
25175. The cells were treated with various concentrations of
eugenol and the pH was then measured. As shown in Fig. 1, the acid production was
significantly suppressed by eugenol compared with the control
group.
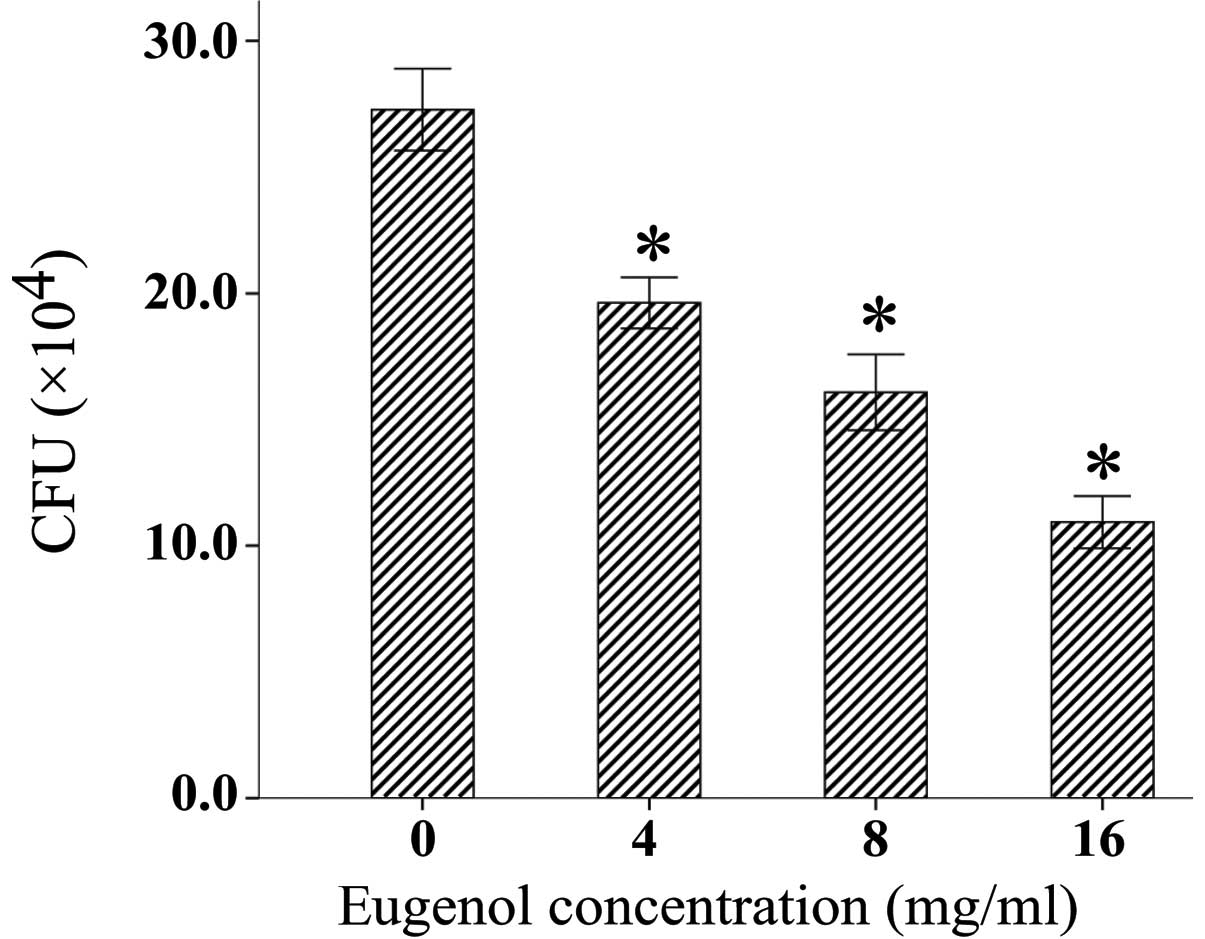
To assess the effects of eugenol on the adherence of
S. mutans 25175, a bacterial adherence assay was performed.
As shown in Fig. 2, eugenol
significantly inhibited the adherence of S. mutans to S-HAs
in a concentration-dependent manner.
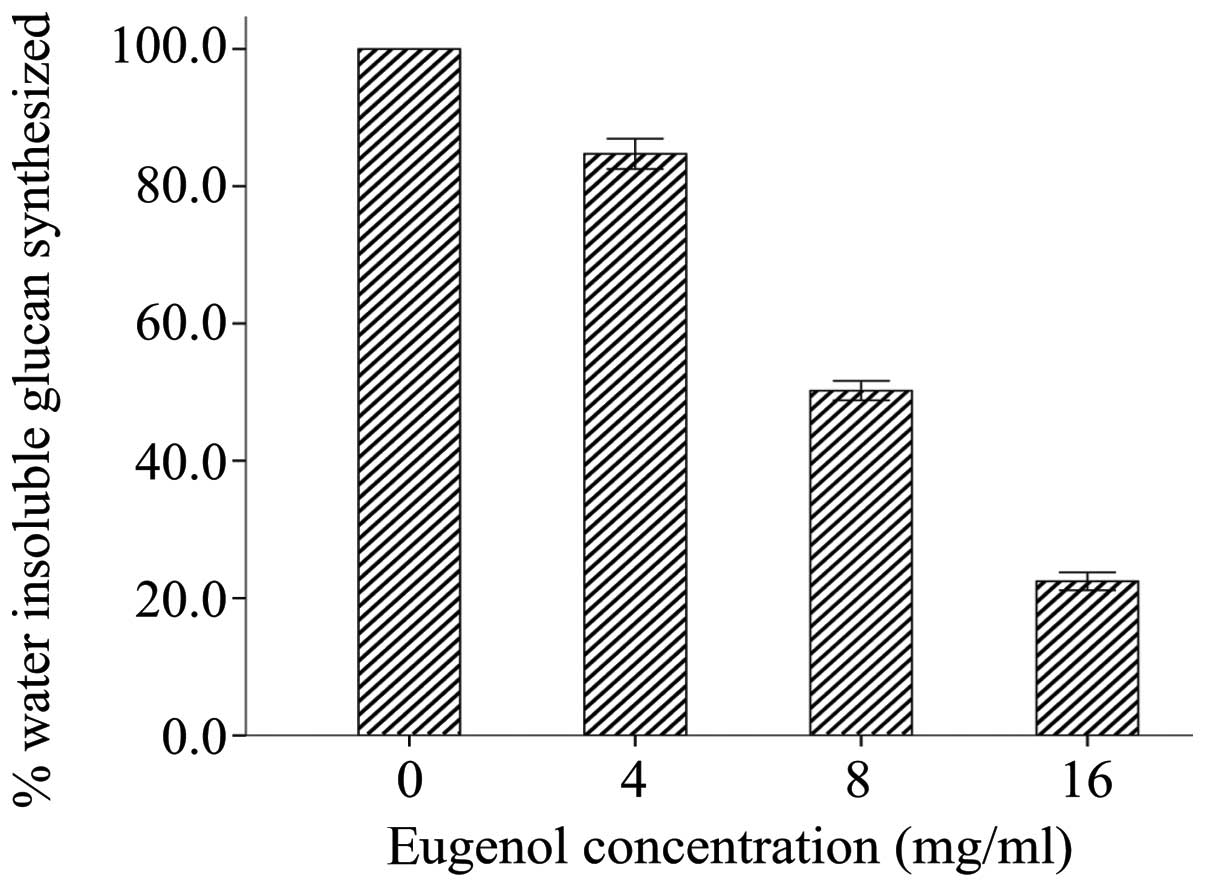
We also examined whether eugenol suppresses the
synthesis of water-insoluble glucans by crude Gtfs. As shown in
Fig. 3, a significant reduction in
water-insoluble glucan synthesis by crude Gtfs from S.
mutans was observed at concentrations of 4–16 mg/ml
eugenol.
Inhibitory effects of eugenol on dental
caries development in rats
In the animal experiment, the rats remained in good
health and gained weight during the 5 weeks of the study. No
significant differences in weight gain were identified among the
groups (P>0.05, data not shown).
The effects of eugenol on the total cultivable
flora, S. mutans viable populations and percentage of S.
mutans recovered from the rat jaws (as calculated from total
cultivable flora, S. mutans population) are shown in
Table I. The eugenol-treated group
exhibited significantly lower total flora counts compared with the
vehicle control. However, the number of CFUs and percentage of
S. mutans in the biofilms of the rats treated with eugenol
did not differ significantly from those of the vehicle control.
 | Table IEffects of eugenol on oral microbiota
in rats after a 5-week experiment. |
Table I
Effects of eugenol on oral microbiota
in rats after a 5-week experiment.
| Group | Total microorganisms
(×104 CFU/ml) | Streptococcus
Mutans 25175 (×104 CFU/ml) | Streptococcus
Mutans 25175 (%) |
|---|
| Vehicle | 4.3a
(1.6) | 3.0a
(1.8) | 69.9a
(20.1) |
| Eugenol (16
mg/ml) | 2.4b
(0.6) | 1.6a
(0.4) | 67.7a
(18.4) |
| 250 ppm fluoride | 2.2b
(0.5) | 1.7a
(0.3) | 77.3a
(16.2) |
Tables II and
III show the incidence and
severity of smooth-surface and sulcal caries. In the present study,
250 ppm fluoride was used as a positive control. The 250 ppm
fluoride treatment produced the lowest scores for incidence and
severity of smooth-surface and sulcal caries. Eugenol treatment
significantly reduced the incidence of smooth-surface and sulcal
caries compared with the vehicle control. Furthermore, the severity
scores of smooth-surface and sulcal caries were significantly lower
in the eugenol-treated group compared with those in the vehicle
control group.
 | Table IIEffects of treatments on dental caries
development (smooth surface and severity) in rats. |
Table II
Effects of treatments on dental caries
development (smooth surface and severity) in rats.
| Group | Smooth surface
total | Severity
|
|---|
| Ds | Dm | Dx |
|---|
| Vehicle | 63.1a
(5.7) | 41.6a
(6.7) | 18.7a
(4.3) | 7.4a
(5.1) |
| Eugenol (16
mg/ml) | 39.2b
(5.2) | 22.1b
(4.8) | 4.8b
(5.8) | 2.0b
(0.8) |
| 250 ppm fluoride | 23.2c
(3.1) | 17.7b
(5.7) | 0.7b
(0.5) | 0.1c
(0.4) |
 | Table IIIEffects of treatments on dental caries
development (sulcal surface and severity) in rats. |
Table III
Effects of treatments on dental caries
development (sulcal surface and severity) in rats.
| Group | Sulcal surface
total | Severity
|
|---|
| Ds | Dm | Dx |
|---|
| Vehicle | 37.8a
(4.8) | 26.3a
(4.5) | 20.3a
(3.7) | 13.4a
(2.2) |
| Eugenol (16
mg/ml) | 28.5b
(3.8) | 16.7b
(4.1) | 9.2b
(3.6) | 5.9b
(2.8) |
| 250 ppm fluoride | 18.9c
(3.1) | 9.5c
(4.1) | 2.9c
(1.4) | 0.3c
(0.2) |
Discussion
Despite advances in the development and improvement
of anti-caries chemotherapy, conventional therapeutic strategies
often fall short of the goal of controlling dental caries
progression. The use of natural products has been reported to be
one of the most successful strategies for the discovery of new
medicines (11,13). Previous studies have shown that
natural products are promising candidates for new anticariogenic
substances (9,13). The present study demonstrated that
eugenol, a naturally occurring agent, interferes with cariogenic
factors of S. mutans, including acid production, adherence
and water-insoluble glucan synthesis in vitro and inhibited
the development of dental caries in rats.
Acid production is an important dental
caries-related factor of S. mutans (14). In dental biofilms, S. mutans
metabolize sugars to produce organic acids, including lactic acid,
propionic acid and butyric acid, which may demineralize the tooth
surface and thereby induce dental caries (3). In the present study, eugenol
significantly inhibited the reduction of pH induced by S.
mutans. These results suggest that eugenol may reduce acid
production by S. mutans.
The adherence of S. mutans to the tooth
surface is one of the most important steps for dental plaque
formation (9,15). Disruption of the ability of S.
mutans to adhere to the surface of the tooth is considered an
important therapeutic approach for the prevention of plaque
formation. Therefore, we investigated the effect of eugenol on the
adhesion of S. mutans to S-HAs. At concentrations of 4–16
mg/ml, eugenol significantly reduces the adherence of S.
mutans to S-HAs. These data suggest that eugenol may be a novel
substance capable of modulating the activity of this important
dental caries-related factor.
The synthesis of extracellular polysaccharides,
including water-insoluble glucans, is one of the most important
virulent properties of S. mutans (16). Water-insoluble glucans promote the
adhesive interactions of bacteria with the tooth surface and
contribute to the formation of dental biofilms (17). Accordingly, we examined whether
eugenol inhibits the synthesis of water-insoluble glucans by crude
Gtfs. The results showed that the formation of water-insoluble
glucans by S. mutans was significantly suppressed in the
presence of eugenol. Our results correlate well with other reports
that eugenol suppresses the insoluble and soluble glucan synthesis
of S. sobrinus (8,18). S. mutans synthesizes glucans
from sucrose by the action of Gtfs. There are at least three types
of Gtfs in S. mutans: Gtf B, Gtf C and Gtf D. It has been
reported that Gtf B and C are important for the synthesis of
insoluble glucans by S. mutans (19,20).
Gtf B synthesizes primarily insoluble glucans, whereas Gtf C
synthesizes insoluble and soluble glucans. Although we identified
that eugenol inhibits water-insoluble glucan synthesis, it was not
confirmed whether eugenol inhibits Gtf B and/or Gtf C. This
requires further investigation.
The inhibitory effects of eugenol on the acid
production, adherence and water-insoluble glucan synthesis
activities of S. mutans may be beneficial for the prevention
of caries development in vivo. Therefore, we examined the
anti-caries activity of eugenol using a rat model of dental caries.
Topical application of eugenol reduces the incidence and severity
of carious lesions in rats without affecting the number and
percentage of S. mutans in the biofilms. In smooth-surface
caries, eugenol effectively reduced the number and severity of
caries, with results similar to those of 250 ppm fluoride (the
positive control). However, the inhibitory effects of eugenol in
sulcal caries were not as effective as those exhibited by 250 ppm
fluoride. These results suggest that eugenol is an effective agent
for the inhibition of dental caries development in vivo.
Natural products that inhibit cariogenic properties of S.
mutans may be used to control dental caries or even to enhance
the cariostatic effect of other recognized agents, including
fluoride. Therefore, further studies are required to investigate
the possible additive or synergistic anticariogenic effects of
eugenol and fluoride.
In conclusion, our data demonstrated that eugenol
significantly attenuates the acid production, adherence and
water-insoluble glucan synthesis activities of S. mutans and
suppresses dental caries development in rats. These results suggest
that eugenol is a promising naturally occurring agent for the
treatment of dental caries.
Acknowledgements
This study was supported by the
Science and Technology Joint Special Fund of Yunnan Province
(2011FB220).
References
|
1.
|
Loesche WJ: Role of Streptococcus
mutans in human dental decay. Microbiol Rev. 50:353–380.
1986.
|
|
2.
|
Hamada S and Slade HD: Biology,
immunology, and cariogenicity of Streptococcus mutans.
Microbiol Rev. 44:331–384. 1980.PubMed/NCBI
|
|
3.
|
Belli WA and Marquis RE: Adaptation of
Streptococcus mutans and Enterococcus hirae to acid
stress in continuous culture. Appl Environ Microbiol. 57:1134–1138.
1991.PubMed/NCBI
|
|
4.
|
Walsh SE, Maillard JY, Russell AD,
Catrenich CE, Charbonneau DL and Bartolo RG: Activity and
mechanisms of action of selected biocidal agents on Gram-positive
and -negative bacteria. J Appl Microbiol. 94:240–247. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chami N, Bennis S, Chami F, Aboussekhra A
and Remmal A: Study of anticandidal activity of carvacrol and
eugenol in vitro and in vivo. Oral Microbiol Immunol. 20:106–111.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yeon KY, Chung G, Kim YH, et al: Eugenol
reverses mechanical allodynia after peripheral nerve injury by
inhibiting hyperpolarization-activated cyclic nucleotide-gated
(HCN) channels. Pain. 152:2108–2116. 2011. View Article : Google Scholar
|
|
7.
|
Frisch J and Bhaskar SN: Tissue response
to eugenol-containing periodontal dressings. J Periodontol.
38:402–408. 1967.PubMed/NCBI
|
|
8.
|
Li MY, Lai GY, Wang J and Ye DX: The
inhibition of eugenol on glucan is essential for the biofilm
eradication effect on caries-related biofilm in an artificial mouth
model. Nat Prod Res. 26:1152–1155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Matsumoto M, Minami T, Sasaki H, Sobue S,
Hamada S and Ooshima T: Inhibitory effects of oolong tea extract on
caries-inducing properties of mutans streptococci. Caries Res.
33:441–445. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bowen WH, Madison KM and Pearson SK:
Influence of desalivation in rats on incidence of caries in intact
cagemates. J Dent Res. 67:1316–1318. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Koo H, Pearson SK, Scott-Anne K, et al:
Effects of apigenin and tt-farnesol on glucosyltransferase
activity, biofilm viability and caries development in rats. Oral
Microbiol Immunol. 17:337–343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Fukushima K, Motoda R and Ikeda T: Effects
of exogenous insoluble glucan primer on insoluble glucan synthesis
by Streptococcus mutans. J Dent Res. 60:1707–1712. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Harvey A: Strategies for discovering drugs
from previously unexplored natural products. Drug Discov Today.
5:294–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kuramitsu HK: Virulence factors of mutans
streptococci: role of molecular genetics. Crit Rev Oral Biol Med.
4:159–176. 1993.PubMed/NCBI
|
|
15.
|
Westergren G and Olsson J: Hydrophobicity
and adherence of oral streptococci after repeated subculture in
vitro. Infect Immun. 40:432–435. 1983.PubMed/NCBI
|
|
16.
|
Schilling KM and Bowen WH: Glucans
synthesized in situ in experimental salivary pellicle function as
specific binding sites for Streptococcus mutans. Infect
Immun. 60:284–295. 1992.PubMed/NCBI
|
|
17.
|
Madison KM, Bowen WH, Pearson SK and
Falany JL: Enhancing the virulence of Streptococcus sobrinus
in rats. J Dent Res. 70:38–43. 1991. View Article : Google Scholar
|
|
18.
|
Li M and Liu Z: In vitro effect of Chinese
herb extracts on caries-related bacteria and glucan. J Vet Dent.
25:236–239. 2008.PubMed/NCBI
|
|
19.
|
Yamashita Y, Bowen WH, Burne RA and
Kuramitsu HK: Role of the Streptococcus mutans gtf genes in
caries induction in the specific-pathogen-free rat model. Infect
Immun. 61:3811–3817. 1993.
|
|
20.
|
Koo H, Xiao J, Klein MI and Jeon JG:
Exopolysaccharides produced by Streptococcus mutans
glucosyltransferases modulate the establishment of microcolonies
within multispecies biofilms. J Bacteriol. 192:3024–3032. 2010.
|