Introduction
As one of the most frequently identified hearing
disorders, sensorineural hearing loss is mainly caused by the loss
of cochlear hair cells in the inner ear. In order to understand its
pathology and develop therapies for hearing restoration,
researchers have made great efforts to investigate different
methods for the regeneration of hair cells using appropriate animal
models, including the transdifferentiation of sustentacular cells
to hair cells or the conversion of multipotential stem cells to
hair cells (1,2). As the most frequently used and
extremely maturely applied gene in the study of this field, Atoh1
is a basic helix-loop-helix (bHLH) transcription factor required
for differentiation of hair cells in the inner ear. A previous
study demonstrated that ectopic expression of Atoh1 at various
time-points of tissue culture or in vivo culture of the
cochlear basilar membrane or vestibular sensory epithelium induces
additional regeneration of hair cells (3). Actin depolymerizing factor (ADF)
plays an important role in numerous cell treatment processes
requiring cytoskeletal rearrangement, including cell migration
(4,5). A number of studies have investigated
the function of ADF in morphogenetic events of organ polarity.
Research findings of Kuure et al demonstrated that knockout
of the ureteric bud (UB) epithelium cofilin1 gene (Cfl1) or
inactivating mutations of the destrin gene (Dstn) has no
effect on kidney morphogenesis; however, simultaneous deletion of
the two genes interrupts the morphogenesis of branching structures
in early development, and simultaneous deletion of UB epithelium
Cfl1 and Dstn (double knockout) leads to the
accumulation of filamentous actin, damage of the normal epithelial
structure and defects in cell migration (6). Twinstar (TSR) encodes
Drosophila cofilin/ADF. The retina of the Drosophila
TSR mutant is shorter compared with that of normal
Drosophila, which is due to a lack of the stretching process
required for retinal development. In the TSR mutant, the sensing
rod structure is not disordered; however, it is wider than the
normal structure. Adhesion connects photoreceptor cells with each
other; however, the structure remains wider than that in the normal
control group since the retinal stretching phenomenon is inhibited
(7,8). However, at present, no study has been
performed to investigate the expression of ADF/destrin in the
development of hair cells following ectopic regeneration induced by
the overexpression of Atoh1. Additionally it is also unknown
whether the ciliary structure of these ectopically regenerated hair
cells undergoes the planar cell polarity (PCP) process. The aim of
this study was to not only analyze the position changes of
ADF/destrin in hair cells following ectopic regeneration induced by
overexpression of Atoh1, but also to examine whether the kinetosome
position in these new hair cells [Myo7a(+)] following ectopic
regeneration has polar migration, similar to that of hair cells in
normal development. This should provide an important foundation for
future in-depth investigation of the polarized growth of ciliary
bundle structures and functional ion channels arising from ciliary
bundle development in hair cells following ectopic
regeneration.
Materials and methods
Sample collection, tissue culture and
viral transfection
Male and female healthy closed colony C57BL/6 mice
were provided by Shanghai SLAC Laboratory Animal Co., Ltd
(Shanghai, China). This study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee (IACUC) of Fudan University. The cochlear
basilar membrane, utricle and ampulla canalis semicircularis
were rapidly removed under the dissecting microscope. A sterilized
coverslip coated with 0.1% polylysine (Sigma, St. Louis, MO, USA)
was placed in a 35×10-mm culture dish (Falcon, Franklin Lakes, NJ,
USA), to which 1.0 ml serum culture fluid was added and the basilar
membrane was transferred in such a manner that it was adherent to
the wall; then it was incubated at 37°C overnight. The following
day, the serum culture fluid was replaced with serum-free medium
[Dulbecco’s modified Eagle medium/nutrient mixture F-12 (1:1),
supplemented with 20 ng/ml B27; Gibco, Carlsbad, CA, USA], to which
the Ad5-enhanced green fluorescent protein (EGFP)-Math1 or Ad5-EGFP
virus was transferred, so that the final concentration of virus
(SinoGene, Beijing, China) was 1.0×108 PFU. Then, the
culture fluid was replaced once every 1–2 days depending on the
growth of the tissue adherent cells, followed by incubation in a
quiet environment at 37°C and 95% humidity with 5% CO2.
On the sixth (DIV6) and twelth day (DIV12) after viral transfer,
the culture dish was removed for fixed observation. Myo7a(+) cells
(Atoh1-induced new ectopic hair cells) were obtained from the
cochlear basilar membrane. During culture of neonatal cochlear
basilar membrane in vitro, the basilar membrane culture was
transfected with (EGFP)-Math1 or Ad5-EGFP to induce new Myo7a(+)
cells in the culture in vitro.
Immunofluorescent staining of cells
The cells were stained as follows: i) The medium was
removed, the cells were washed once with phosphate-buffered saline
(PBS) and immobilized with 1 ml 2% paraformaldehyde for 15 min.
Then, the immobile liquid was removed, the cells were treated with
1 ml 0.2% Triton X-100 for 1 min, washed three times with PBS and
sealed with 1% bovine serum albumin (BSA) for 1 h. ii) The primary
antibody (monoclonal rabbit anti-ADF/destrin antibody; 1:200) was
formulated with 5% donkey serum [prepared with 0.1% TritonX-100 and
1X PBS (PBST)], added to the cells and then placed in a
refrigerator at 4°C overnight. iii) The cells were rinsed three
times with PBST solution at room temperature for 2 h each time. iv)
The secondary antibody (donkey anti-mouse Rho; 1:1,000) formulated
with PBST was added to cells and then placed in a refrigerator at
4°C overnight. v) The cells were rinsed three times with PBST
solution at room temperature for 2 h each time. vi) Fluorescein
isothiocyanate (FITC)-conjugated phalloidin (l:500) formulated with
PBST was added to cells which were then left at room temperature
for 30 min. vii) The cells were rinsed with PBST solution at room
temperature for 20 min; and viii) the tissue was transferred with
fluoromount-G slide mounting medium and a small amount of Dow
Corning high-vacuum grease was applied at the four corners. A
coverslip was placed onto the slide and nail polish was applied
around the edge.
Whole-mount preparation and
immunofluorescent staining of the tissue
The tissue was stained as follows: i) 10% donkey
serum was formulated with PBST, added to the tissue and left at
room temperature for 60 min. ii) The primary antibody (monoclonal
rabbit anti-ADF/destrin antibody; 1:200) was formulated with 5%
donkey serum (prepared with PBST), added to the tissue and then
placed in a refrigerator at 4°C overnight. iii) The tissue was
rinsed three times with PBST solution at room temperature for 2 h
each time. iv) The secondary antibody [donkey anti-mouse Cy5
(1:1,000) and donkey anti-mouse rhodamine (1:1,000)] was formulated
with PBST and added to the tissue which was then placed in a
refrigerator at 4°C overnight. v) The tissue was rinsed three times
with PBST solution at room temperature for 2 h each time. vi)
FITC-conjugated phalloidin (l:500) formulated with PBST was added
to tissue which was then left at room temperature for 30 min; vii)
the tissue was rinsed with PBST solution at room temperature for 20
min. viii) The tissue was transferred with fluoromount-G slide
mounting medium and a small amount of Dow Corning high-vacuum
grease was applied at the four corners. A coverslip was placed onto
the slide and nail polish was applied around the edges.
Laser scanning confocal microscopy
The cochlear basilar membrane was scanned layer by
layer from the uppermost layer down using a Zeiss LSM510 META laser
scanning confocal microscope (Carl Zeiss, Oberkochen, Germany),
with the laser at three wavelengths, specifically 488 nm (FITC),
543 nm (rhodamine) and 633 nm (Cy5), at a magnification of ×63,
with the selected layer thickness at 0.5 μm and an image
resolution of 2,048×2,048. Figs.
1–7 were processed using LSM
Image Browser (Carl Zeiss, Germany) and Adobe Photoshop 7.0.1 image
processing software (Adobe, San Jose, CA, USA).
Results
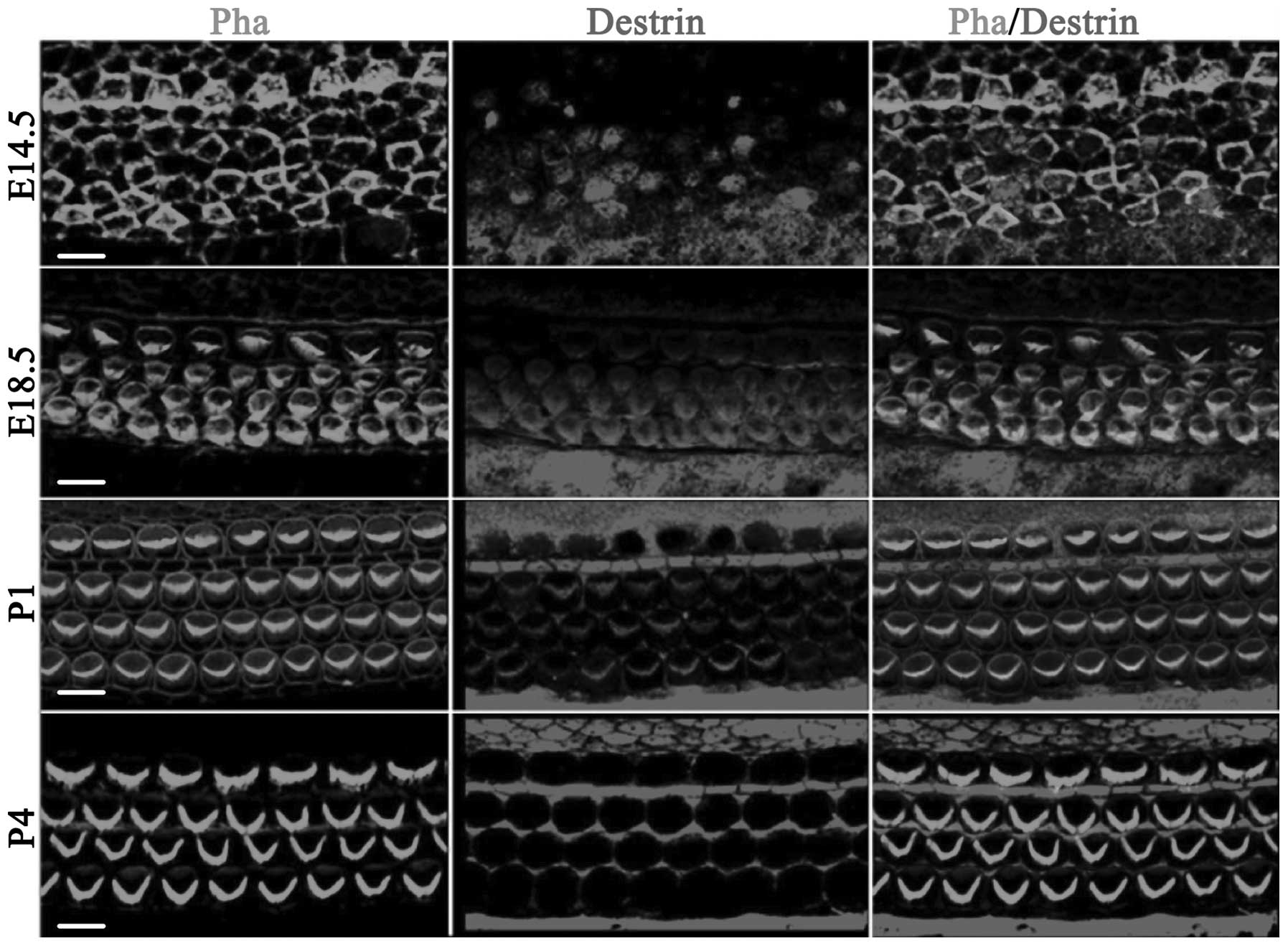
Analysis of the expression of ADF/destrin
in normal development of the cochlear basilar membrane in mice
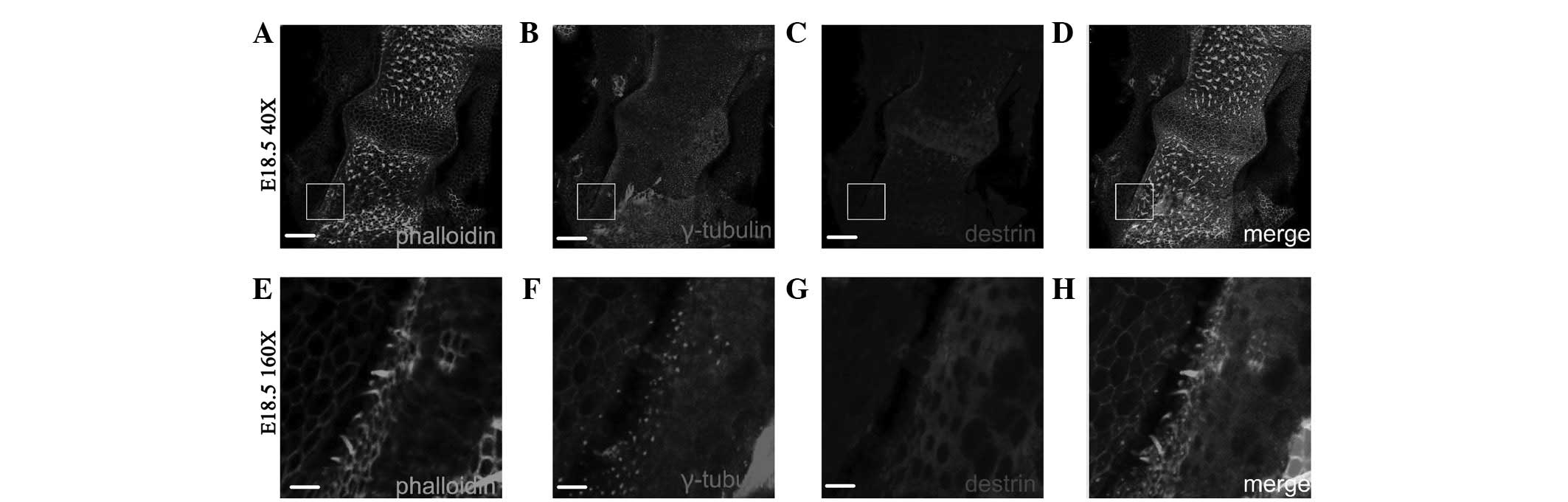
Fig. 1 shows the
distribution of ADF/destrin in the middle-bottom cochlea from day
14.5 of embryonic development (E14.5) to day 4 after birth (P4). On
E14.5, destrin was scattered and expressed in sustentacular cells
and hair cells, and on E18.5, destrin was expressed in cochlear
hair cells, mainly distributed in the cuticular plate of hair cells
and also expressed in a portion of the sustentacular cells. On P1,
destrin was mainly expressed in the cilia of hair cells and inner
phalangeal cells, and on P4, destrin was only expressed in the
cytoplasm of sustentacular cells and no longer expressed in hair
cells.
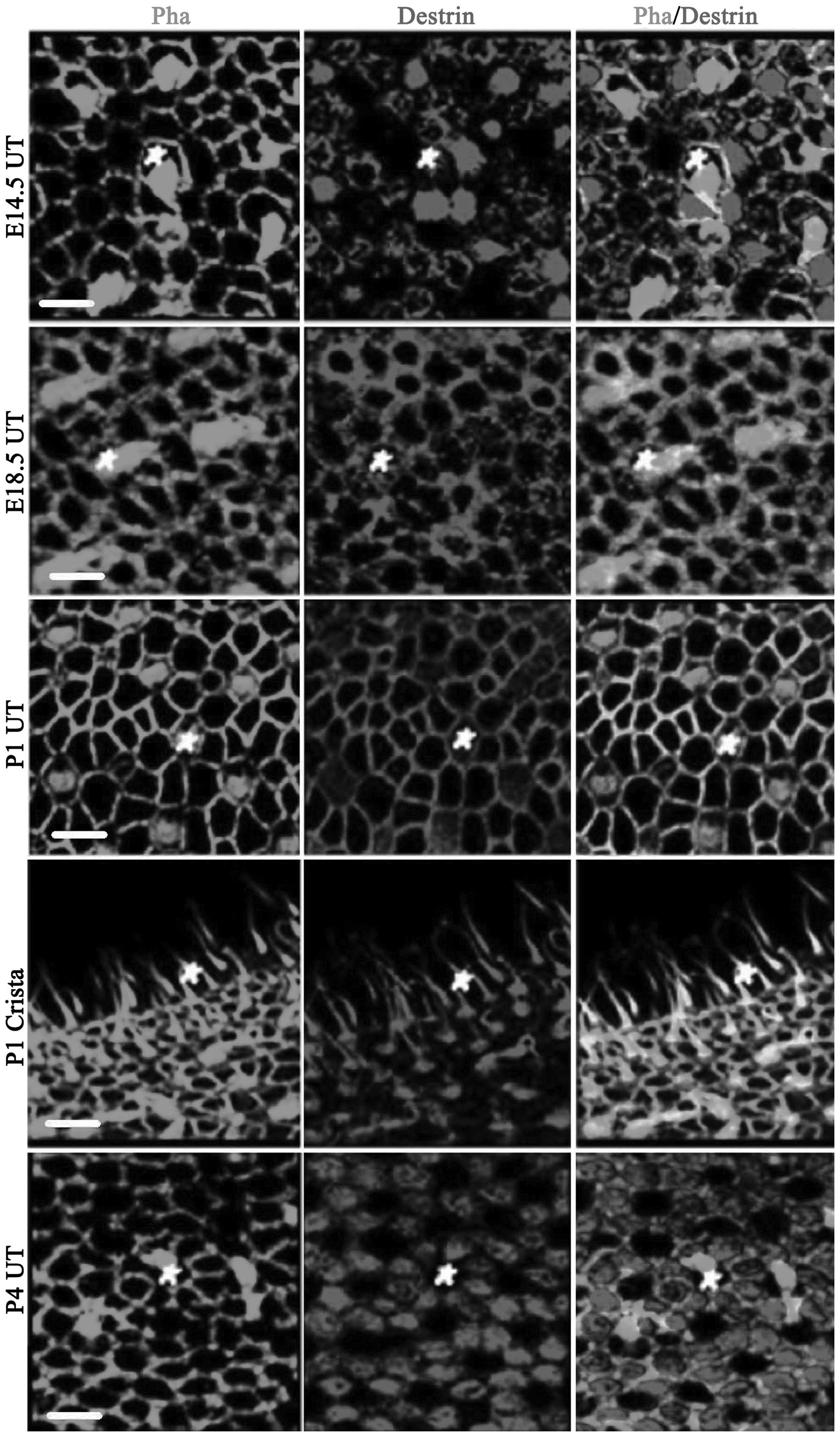
Analysis of ADF/destrin expression in the
normal development of vestibular utricles in mice
Fig. 2 shows the
expression of ADF/destrin in the vestibule mainly comprising
utricular macula epithelium, including crista ampullaris
staining at P1. On E14.5, destrin was expressed in utricular
epithelial hair cells and the surrounding sustentacular cells;
however, it was mainly expressed in sustentacular cells. On E18.5,
the cuticular plate of hair cells was stained and destrin was
expressed in hair cells, sustentacular cells and the junction
between sustentacular cells (co-localization of destrin and
phalloidin-labeled actin was observed and the color became yellow).
On P1, destrin was also significantly expressed in the cuticular
plate of hair cells and the junction between the cells on the
utricular macula. The kinocilium and cuticular plate were clearly
stained on the crista ampullaris. On P4, destrin was
significantly expressed in sustentacular cells (mainly in the
cytoplasm). In Fig. 2, the white
pentagons are hair cells.
Analysis of the expression of ADF/destrin
in normal development of the ampulla canalis semicircularis in
mice
Fig. 3 shows that
on E14.5, ADF/destrin was expressed in hair cells of the sensory
epithelium of the ampulla canalis semicircularis and
surrounding sustentacular cells. ADF/destrin was mainly located on
the edge of the cuticular plate and was mainly expressed in
sustentacular cells.
Fig. 4 shows that
on E18.5, ADF/destrin was mainly expressed in sustentacular cells
of the ampulla canalis semi-circularis. As shown in Fig. 4G–H, a cavernous fluorescent shadow
was observed where hair cells were located.
Changes of ADF/destrin expression in the
development of Myo7a(+) cells following ectopic regeneration
induced by overexpression of Atoh1 in the basilar membrane of
neonatal mice
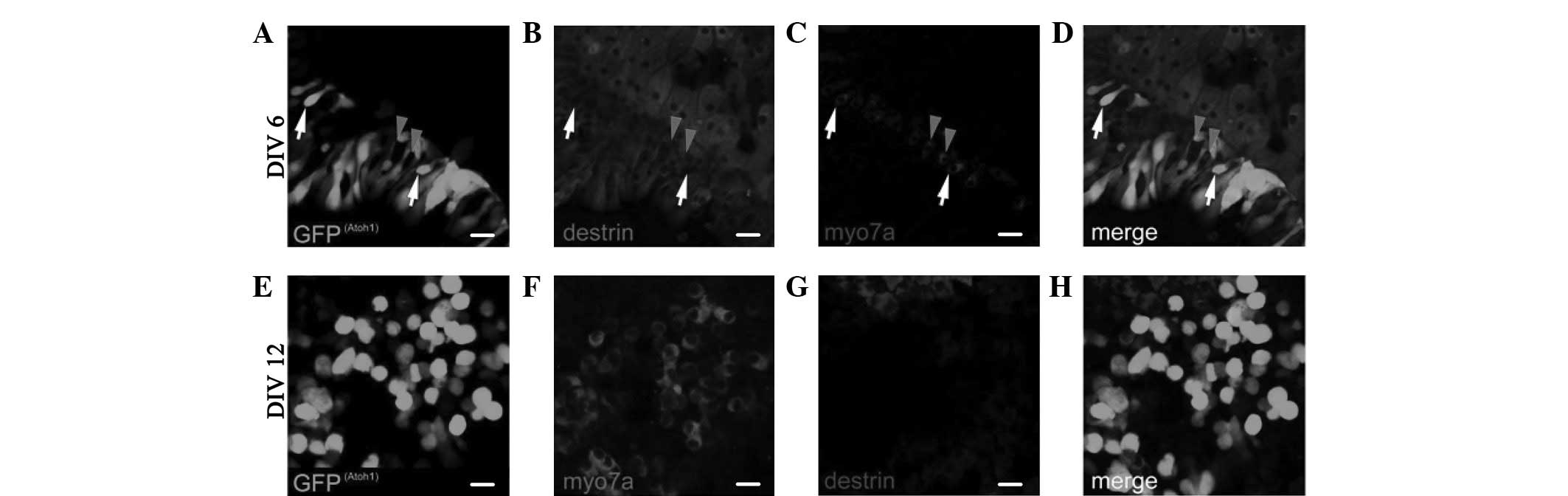
The expression of ADF/destrin in the greater
epithelial ridge (GER) cell area following retroviral
overexpression of Atoh1 and subsequent in vitro culture for
6 days (DIV6), as shown in Fig.
5A–D, indicates that destrin was also expressed in certain
areas of Myo7a(+) cells; however, it was not expressed in other
parts of Myo7a(+) cells. As shown in Fig. 5E–H of the expression of ADF/destrin
in the GER cell area following retroviral overexpression of Atoh1
and subsequent in vitro culture for 12 days (DIV12), destrin
was not expressed in any Myo7a(+) cells. No cell or area where
Myo7a (fluorescence) and destrin (fluorescence) coexisted was
observed (Fig. 5H).
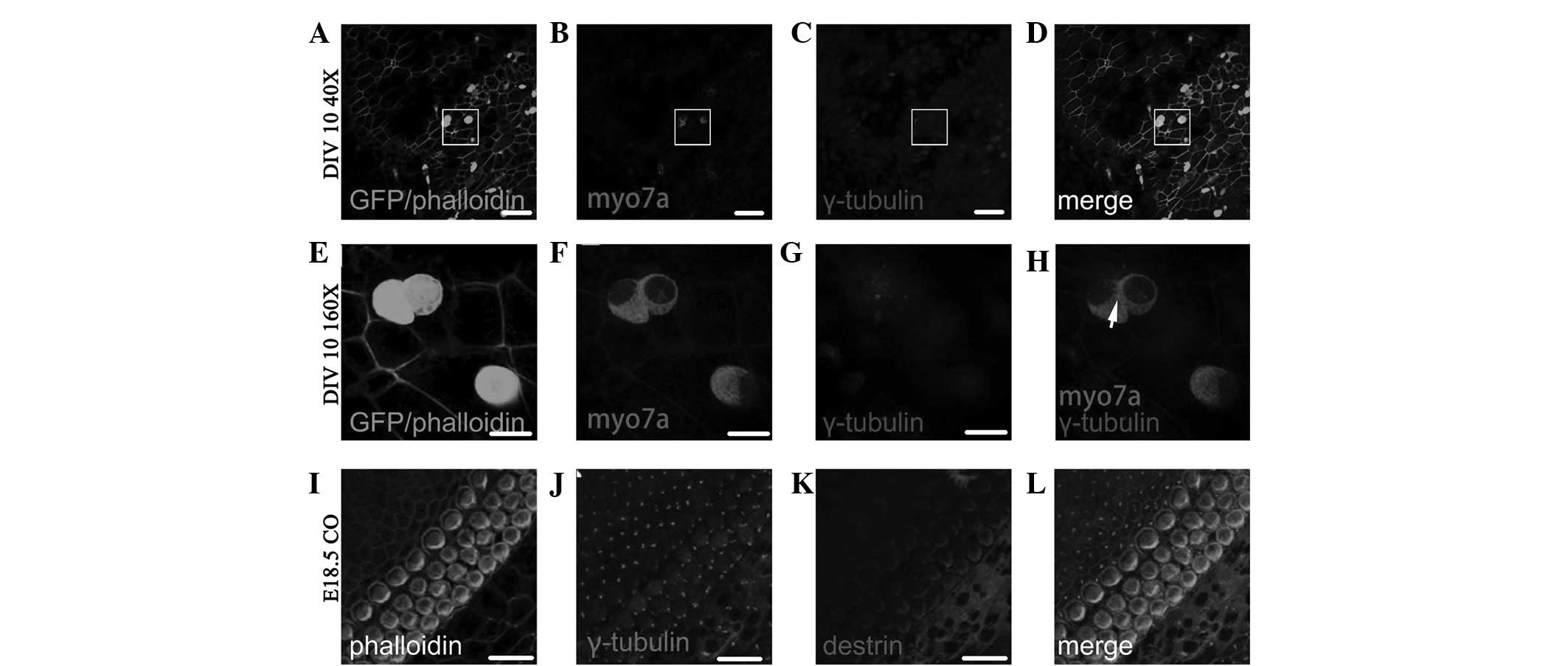
Polarity change of kinetosome position in
the development of Myo7a(+) cells following ectopic regeneration
induced by overexpression of Atoh1
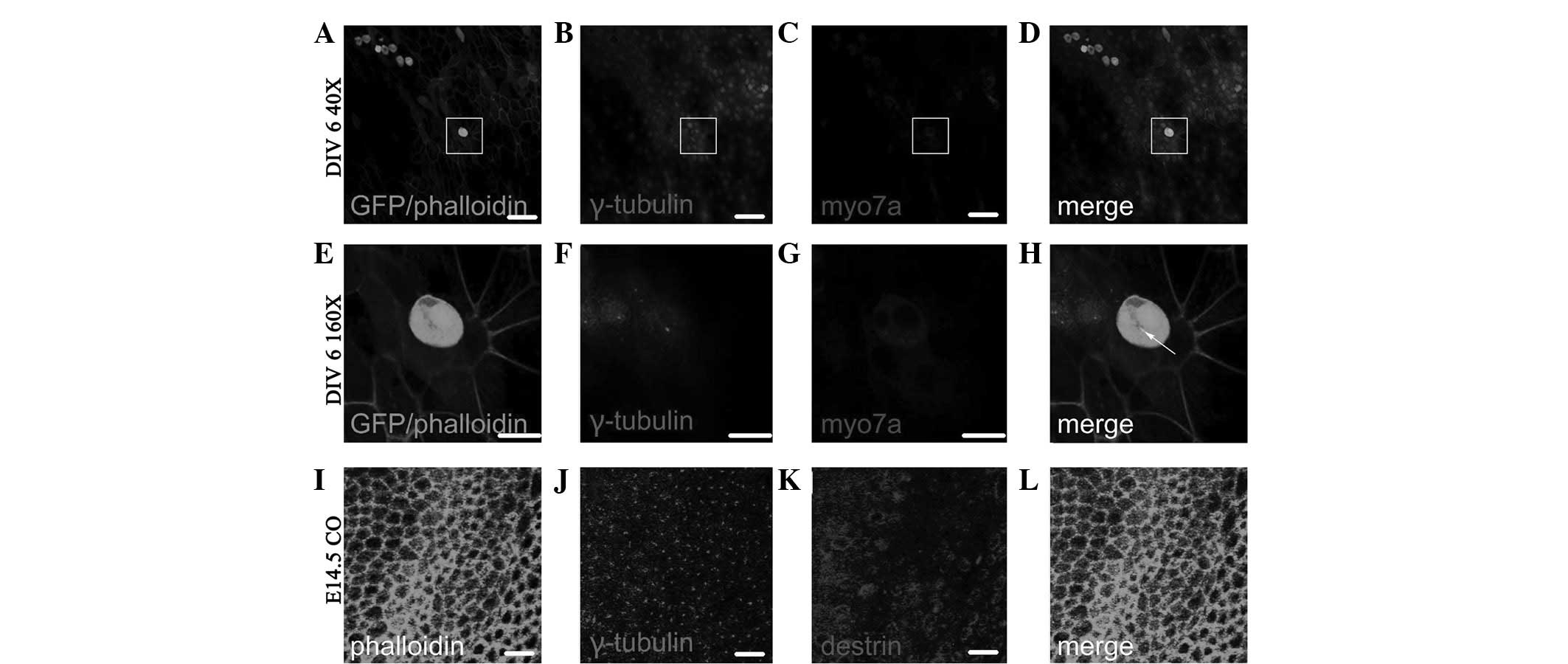
Fig. 6A–D shows the
Myo7a(+) cells following ectopic regeneration induced by retroviral
overexpression of Atoh1 and subsequent in vitro culture for
6 days (DIV 6; fluorescence colocalized area in Fig. 6D). As shown in the enlarged images
in Fig. 6E–H, which are four times
the area of the white squares in Fig.
6A–D, the γ-tubulin-labeled kinetosome is located in the middle
of individual Myo7a(+) cells (dot in Fig. 6H) and the Myo7a(+) cells therein
have two nuclei. Fig. 6I–L shows
the distribution of γ-tubulin and destrin of cochlear auditory
epithelium on E14.5 in normal development. As observed in the GER
side in the upper left side and lesser epithelial ridge (LER) side
in the lower right side of Fig.
6I–L, the majority of kinetosomes in hair cells and
sustentacular cells are located in the middle of the cuticular
plate, and the minority are located around the cuticular plate
(dots in Fig. 6J and L represent
the location of the kinetosome.
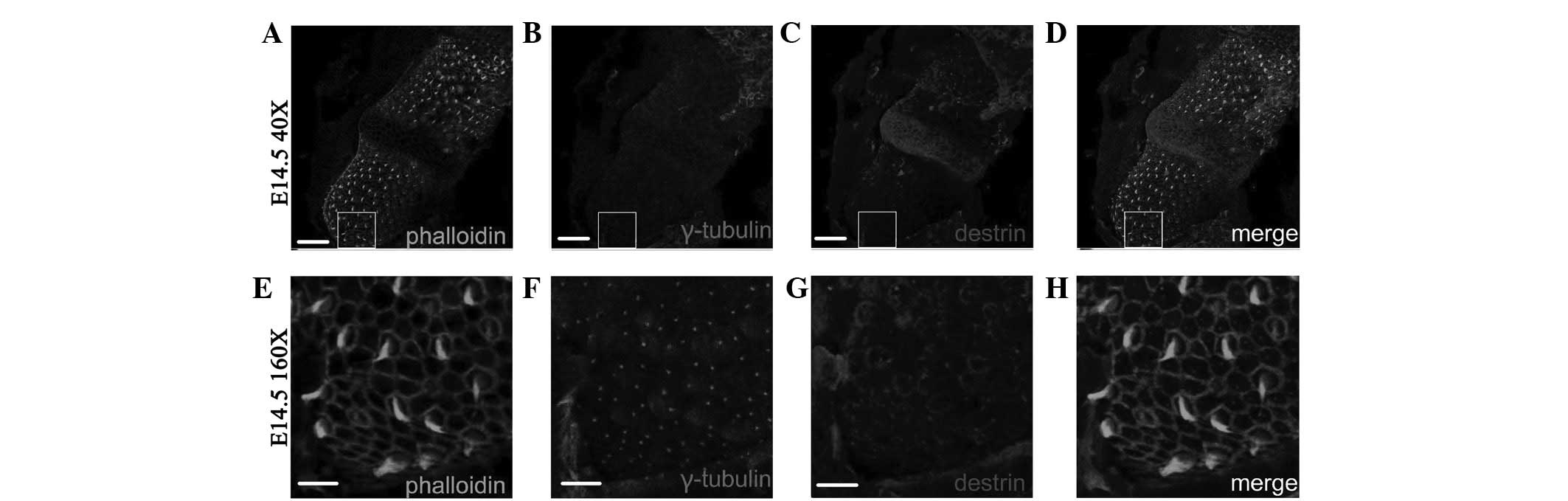
Fig. 7A–D shows the
Myo7a(+) cells following ectopic regeneration induced by retroviral
overexpression of Atoh1 and subsequent in vitro culture for
12 days (DIV 12; fluorescence colocalized area in Fig. 7D). As observed in the enlarged
images in Fig. 7E–H, which are
four times the area of the white squares in Fig. 7A–D, the γ-tubulin-labeled
kinetosome is located on the edge of individual Myo7a(+) cells (dot
in Fig. 7H). Fig. 7I–L shows the distribution of
γ-tubulin and destrin in the cochlear auditory epithelium on E18.5
in normal development. As observed in the GER side in the upper
left side and LER side in the lower right side of Fig. 7I–L, all kinetosomes in hair cells
were uniformly located on one side of the cuticular plate in order,
i.e., where the projection of the tallest stereocilia is located
(dots in Fig. 7J–L represent the
location of the kinetosome.
Discussion
This study aimed to investigate the effect of ADF
and position change of kinetosomes in the development of in
vitro cultured hair cells following ectopic regeneration. A
previous study observed that ADF/destrin expression has temporal
and spatial variation in the normal development of cochlear and
vestibular sensory epithelium in mice (9), suggesting that ADF/destrin is
involved in the development and maturation of hair cells in the
auditory and vestibular sensory epithelium, as well as the ciliary
bundle on the cuticular plate of sustentacular cells and hair cells
in mammalians. However, no studies have determined destrin
expression changes and position changes of kinetosomes in the
development of hair cells following ectopic regeneration in
vitro. By investigating the ectopic regeneration of hair cells
induced by overexpression of Atoh1 in the cochlear basilar membrane
of adenovirally transfected neonatal mice, we determined that
ADF/destrin is involved in the development of hair cells and the
ciliary bundle on their cuticular plate following ectopic
regeneration, as well as in the structural integration of
regenerated hair cells and surrounding sustentacular cells.
According to the experimental results, the transient
expression of ADF/destrin in cochlear hair cells in the embryonic
period and Myo7a(+) cells in early ectopic regeneration induced by
overexpression of Atoh1 suggests that ADF/destrin plays a role in
regulating the regeneration and circulation of cytoskeletal actin
in the early development of cochlear hair cells. Moreover, the
spatiotemporal distribution variation of ADF/destrin during in
vitro culture of Myo7a(+) cells following ectopic regeneration
is consistent with the phenomenon that destrin is expressed in hair
cells of the cochlear auditory epithelium in the embryonic period
(E14.5–E18.5) of normal development and not expressed after birth
(P4). ADF/destrin is only expressed in sustentacular cells on day 4
after birth or later in normal mice and destrin is not expressed in
Myo7a(+) cells following ectopic regeneration and subsequent in
vitro culture for 12 days. Destrin is only expressed in the
cells surrounding Myo7a(+) cells and ADF destrin expression tends
to be expressed first in hair cells and then in sustentacular cells
in the development of Myo7a(+) cells following Atoh1-induced
ectopic regeneration, suggesting that destrin is involved in the
structural development and structural integration of regenerated
hair cells and surrounding sustentacular cells. Additionally, we
identified that hair cells following ectopic regeneration induced
by overexpression of Atoh1, move their kinetosomes at different
culture times (specifically labelled by γ-tubulin) and
γ-tubulin-labelled kinetosomes appear in the middle of individual
Myo7a(+) cells following ectopic regeneration in early
overexpression of Atoh1 (cultured for <1 week after transfection
of the adenovirus). Furthermore, the kinetosome of ectopically
regenerated individual Myo7a(+) cells after being cultured for 1
week, moves to the cell edge, which may be the edge of the immature
cuticular plate (Figs. 6 and
7). This phenomenon is consistent
with the phenomenon that the kinetosome of normally developing hair
cells in the cochlear auditory epithelium moves to the cuticular
plate side in development.
A previous study identified that in dividing cells,
primary cilia may determine whether cells re-enter the cell cycle
or remain static (10). The
differential suggestion of cilia generation time may affect the
opportunities for the cells to respond to extracellular signals,
which affect the cell fate. Verdoni et al identified through
studies on Dstn mutant mice that a number of genes related
to the cell cycle are upregulated in this mutant, and mutant mice
mitotic period may be affected (11–13).
The results of the current study demonstrated that ADF is not
expressed in ectopically regenerated Myo7a(+) cells in later
transfection of Atoh1 (12 days after transfection); however, it is
expressed in the cell body closely adjacent to ectopically
regenerated Myo7a(+) cells. It is hypothesized that Myo7a(+) cells
and adjacent cells may have been in different differentiation
stages or have entered different cell cycles. In addition, previous
studies of our experimental group revealed that the polar core
protein Vangl2 and E-cadherin protein P120 in planar cells are
involved in convergent extension movements in the development of
auditory receptors and vestibular sensory epithelium, and the
deficiency of polar proteins in planar cells and cell adhesion
proteins seriously affect the establishment of a complete
cytoskeleton in the auditory epithelium and vestibular sensory
epithelium (14–16). Actin cytoskeleton remodeling plays
a direct and specific role in the cell location information
conversion process (17–21). The actin remodeling pathway is
involved in the process of decoding extracellular signal gradient
information to PCP (22–28). Blair et al emphasized the
genetic correlation between TSR (ADF/cofilin analogs) and the PCP
pathway, and inferred that actin remodeling is a key step in the
PCP generation mechanism. The required mechanism for their
redistribution remains unknown; however, actin remodeling is
involved in the redistribution of core PCP proteins (7,8).
There is no evidence to suggest that the kinetosome
of Myo7a(+) cells following ectopic regeneration in the late
culture of this experiment is located at the final projection of
the highest point of the cilia bundle; however, we determined that
as the incubation time progresses, the kinetosomes of individual
hair cells following ectopic regeneration move from the center of
the cell to the edge of the cell. This provides a foundation for
further in-depth investigation of the polarized development of
ciliary bundle structure and functional ion channels arising from
ciliary bundle development in hair cells following ectopic
regeneration, since the kinetosome position change is closely
related to the maturation of functional stereocilia in hair
cells.
The theoretical significance of the experimental
results includes: i) support of the conclusion that the
overexpression of Atoh1 may induce the ectopic regeneration of
immature hair cells with certain functions, and that regeneration
and circulation activities of actin in which ADF is involved exist
in the differentiation and maturation of these ectopically
regenerated hair cells; ii) actin regeneration activity has
spatiotemporal differences with the development of these
ectopically regenerated hair cells. Structural changes of the
cytoskeleton caused by the spatiotemporal differences in the
regeneration activities of this actin are likely to be involved in
polar migration of kinetosomes or the ciliary bundle on the
cuticular plate of regenerated hair cells; and iii) contribution to
the study of development and maturation of differentiated ciliary
bundles in hair cells, polarity development of ciliary bundle in
ectopically regenerated hair cells and its potential mechanism.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (No.
81028003/H1305, 81271084/H1304, 81000413/H1305), the Key Basic
Research Project of Shanghai Committee of Science and Technology
(No. 10JC1402500), Shanghai Rising-Star Program (A type)
11QA1401100, the Major State Basic Research Development Program of
China (973 Program; No. 2011CB504500 and 2011CB504506).
References
|
1.
|
Sinkkonen ST, Chai R, Jan TA, et al:
Intrinsic regenerative potential of murine cochlear supporting
cells. Sci Rep. 1:262011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pan N, Jahan I, Kersigo J, Duncan JS,
Kopecky B and Fritzsch B: A novel Atoh1 ‘self-terminating’ mouse
model reveals the necessity of proper Atoh1 level and duration for
hair cell differentiation and viability. PLoS One.
7:e303582012.
|
|
3.
|
Han Z, Yang JM, Chi FL, Cong N, Huang YB,
Cao Z and Li W: Survival and fate of transplanted embryonic neural
stem cells by Atoh1 gene transfer in guinea pigs cochlea.
Neuroreport. 21:490–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bernstein BW and Bamburg JR: ADF/cofilin:
a functional node in cell biology. Trends Cell Biol. 20:187–195.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Herde MK, Friauf E and Rust MB:
Developmental expression of the actin depolymerizing factor ADF in
the mouse inner ear and spiral ganglia. J Comp Neurol.
518:1724–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kuure S, Cebrian C, Machingo Q, et al:
Actin depolymerizing factors cofilin1 and destrin are required for
ureteric bud branching morphogenesis. PloS Genet. 6:e10011762010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Blair A, Tomlinson A, Pham H, Gunsalus KC,
Goldberg ML and Laski FA: Twinstar, the Drosophila homolog of
cofilin/ADF, is required for planar cell polarity patterning.
Development. 133:1789–1797. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pham H, Yu H and Laski FA: Cofilin/ADF is
required for retinal elongation and morphogenesis of the Drosophila
rhabdomere. Dev Biol. 318:82–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Görlich A, Wolf M, Zimmermann AM, et al:
N-cofilin can compensate for the loss of ADF in excitatory
synapses. PLoS One. 6:e267892011.PubMed/NCBI
|
|
10.
|
Han YG and Alvarez-Buylla A: Role of
primary cilia in brain development and cancer. Curr Opin Neurobiol.
20:58–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Verdoni AM, Aoyama N, Ikeda A and Ikeda S:
The effect of destrin mutations on the gene expression profile in
vivo. Physiol Genomics. 34:9–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kawakami-Schulz SV, Verdoni AM, Sattler
SG, Ikeda A and Ikeda S: Differences in corneal phenotypes between
destrin mutants are due to allelic difference and modified by
genetic background. Mol Vis. 18:606–616. 2012.
|
|
13.
|
Zhang W, Zhao J, Chen L, Urbanowicz MM and
Nagasaki T: Abnormal epithelial homeostasis in the cornea of mice
with a destrin deletion. Mol Vis. 14:1929–1939. 2008.PubMed/NCBI
|
|
14.
|
Rida PCG and Chen P: Line up and listen:
Planar cell polarity regulation in the mammalian inner ear. Semin
Cell Dev Biol. 20:978–985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chacon-Heszele MF, Ren D, Reynold AB, Chi
F and Chen P: Regulation of cochlear convergent extension by the
vertebrate planar cell polarity pathway is dependent on
p120-catenin. Development. 139:968–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jones C, Roper VC, Foucher I, et al:
Ciliary proteins link basal body polarization to planar cell
polarity regulation. Nat Genet. 40:69–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Manor U and Kachar B: Dynamic length
regulation of sensory stereocilia. Semin Cell Dev Biol. 19:502–510.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matsumoto N, Kitani R, Maricle A, Mueller
M and Kalinec F: Pivotal role of actin depolymerization in the
regulation of cochlear outer hair cell motility. Biophys J.
99:2067–2076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tanimoto M, Ota Y, Inoue M and Oda Y:
Origin of inner ear hair cells: morphological and functional
differentiation from ciliary cells into hair cells in zebrafish
inner ear. J Neurosci. 31:3784–3794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Miyoshi J and Takai Y: Structural and
functional associations of apical junctions with cytoskeleton.
Biochim Biophys Acta. 1778:670–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Schwander M, Kachar B and Müller U: The
cell biology of hearing. J Cell Biol. 190:9–20. 2010. View Article : Google Scholar
|
|
22.
|
Manor U, Disanza A, Grati M, et al:
Regulation of stereocilia length by myosin XVa and whirlin depends
on the actin-regulatory protein Eps8. Curr Biol. 21:167–172. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wansleeben C and Meijlink F: The planar
cell polarity pathway in vertebrate development. Dev Dyn.
240:616–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Van Troys M, Huyck L, Leyman S, Dhaese S,
Vandekerkhove J and Arnpe C: Ins and outs of ADF/cofilin activity
and regulation. Eur J Cell Biol. 87:649–667. 2008.PubMed/NCBI
|
|
25.
|
Wallingford JB and Mitchell B: Strange as
it may seem: the many links between Wnt signaling, planar cell
polarity, and cilia. Genes Dev. 25:201–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Oteiza P, Köppen M, Krieg M, et al: Planar
cell polarity signalling regulates cell adhesion properties in
progenitors of the zebrafish laterality organ. Development.
137:3459–3468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Corbit KC, Shyer AE, Dowdle WE, et al:
Kif3a constrains beta-catenin-dependent Wnt signalling through dual
ciliary and non-ciliary mechanisms. Nat Cell Biol. 10:70–76. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
He X: Cilia put a brake on Wnt signalling.
Nat Cell Biol. 10:11–13. 2008. View Article : Google Scholar : PubMed/NCBI
|