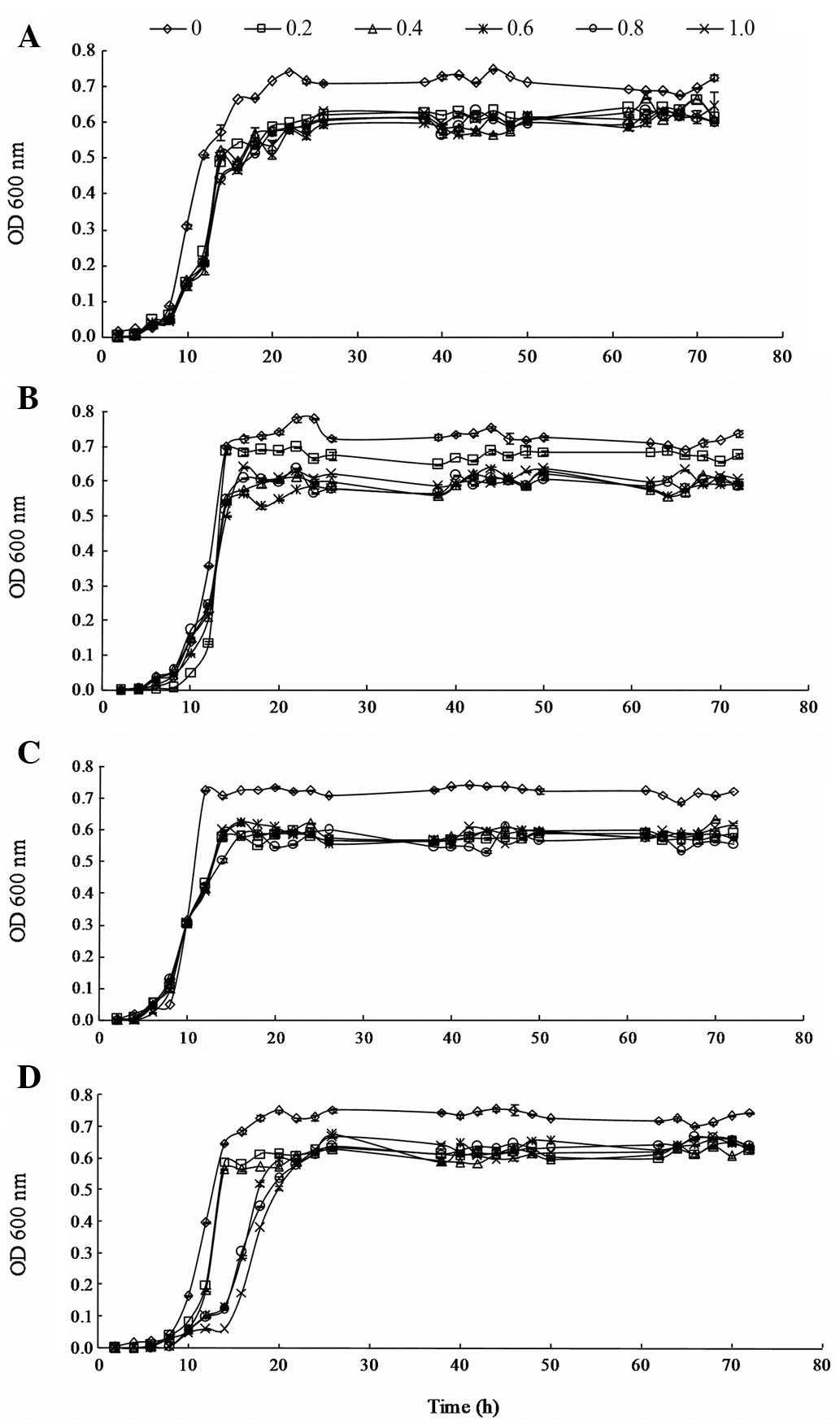
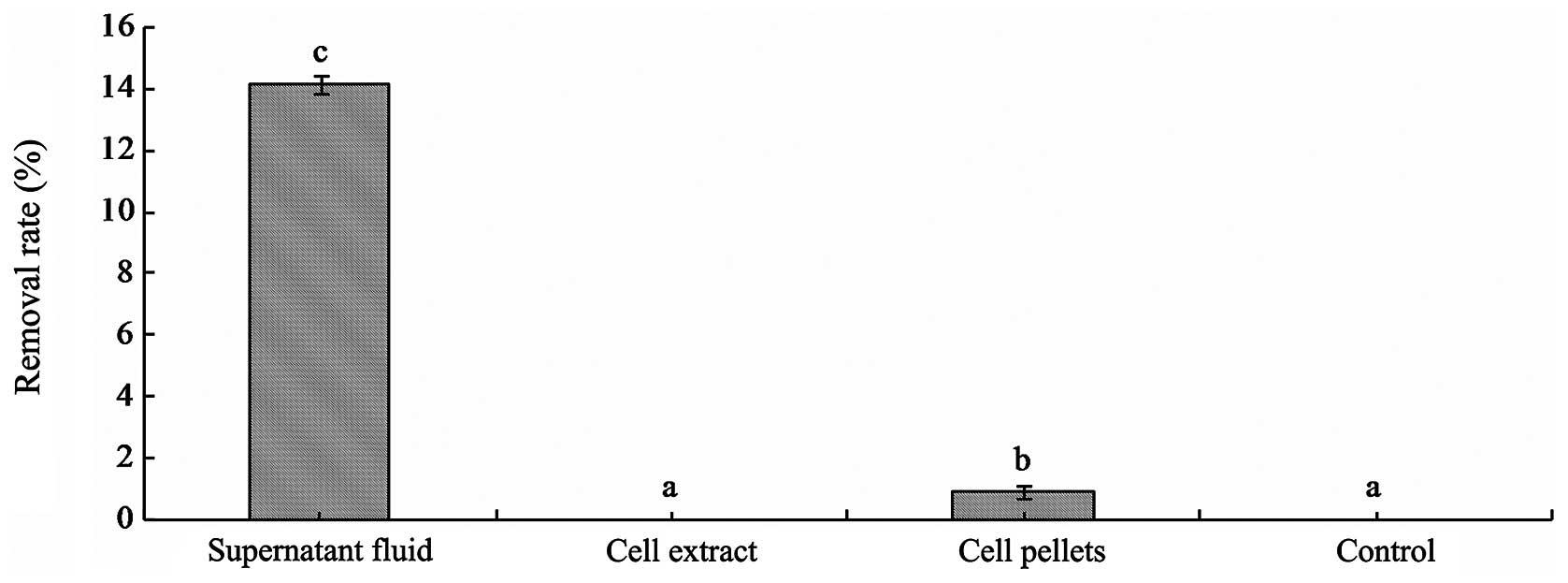
|
1.
|
Barton Gade PA: Meat and fat quality in
boars, castrates and gilts. Livest Prod Sci. 16:187–196. 1987.
|
|
2.
|
Bonneau M, Dufour R, Chouvet C, Roulet C,
Meadus W and Squires EJ: The effects of immunization against
luteinizing hormone-releasing hormone on performance, sexual
development, and levels of boar taint-related compounds in intact
male pigs. J Anim Sci. 72:14–20. 1994.
|
|
3.
|
Babol J, Squires EJ and Lundström K:
Relationship between metabolism of androstenone and skatole in
intact male pigs. J Anim Sci. 77:84–92. 1999.PubMed/NCBI
|
|
4.
|
Babol J, Squires EJ and Lundström K:
Hepatic metabolism of skatole in pigs by cytochrome P4502E1. J Anim
Sci. 76:822–828. 1998.PubMed/NCBI
|
|
5.
|
Jensen MT, Cox RP and Jensen BB:
3-Methylindole (skatole) and indole production by mixed populations
of pig fecal bacteria. Appl Environ Microbiol. 61:3180–3184.
1995.PubMed/NCBI
|
|
6.
|
Yokoyama MT and Carlson JR: Microbial
metabolites of tryptophan in the intestinal tract with special
reference to skatole. Am J Clin Nutr. 32:173–178. 1979.PubMed/NCBI
|
|
7.
|
Lunde K, Skuterud E, Hersleth M and
Egelandsdal B: Norwegian consumers’ acceptability of boar tainted
meat with different levels of androstenone or skatole as related to
their androstenone sensitivity. Meat Sci. 86:706–711. 2010.
|
|
8.
|
Chen G, Zamaratskaia G, Andersson HK and
Lundström K: Effects of raw potato starch and live weight on fat
and plasma skatole, indole and androstenone levels measured by
different methods in entire male pigs. Food Chem. 101:439–448.
2007. View Article : Google Scholar
|
|
9.
|
Yuan YJ, Lu ZX, Huang LJ, Li Y, Lu FX, Bie
XM, et al: Biodegradation of nicotine from tobacco waste extract by
Ochrobactrum intermedium DN2. J Ind Microbiol Biotechnol.
34:567–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bai YH, Sun Q, Zhao C, Wen D and Tang X:
Aerobic degradation of pyridine by a new bacterial strain,
Shinella zoogloeoides BC026. J Ind Microbiol Biotechnol.
36:1391–1400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Patil PS, Shedbalkar UU, Kalyani DC and
Jadhav JP: Biodegradation of Reactive Blue 59 by isolated bacterial
consortium PMB11. J Ind Microbiol Biotechnol. 35:1181–1190. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kohda C, Ando T and Nakai Y: Isolation and
characterization of anaerobic indole- and skatole-degrading
bacteria from composting animal wastes. J Gen Appl Microbiol.
43:249–255. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yin B, Huang L and Gu JD: Biodegradation
of 1-methylindole and 3-methylindole by mangrove sediment
enrichment cultures and a pure culture of an isolated
Pseudomonas aeruginosa Gs. Water Air Soil Poll. 176:185–199.
2006. View Article : Google Scholar
|
|
14.
|
Gu JD and Berry DF: Metabolism of
3-methylindole by a methanogenic consortium. Appl Environ
Microbiol. 58:2667–2669. 1992.PubMed/NCBI
|
|
15.
|
Gu JD, Fan Y and Shi H: Relationship
between structures of substituted indolic compounds and their
removal by marine anaerobic microorganisms. Mar Pollut Bull.
45:379–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
El-Nezami HS, Polychronaki N, Salminen S
and Mykkänen H: Binding rather than metabolism may explain the
interaction of two food-grade Lactobacillus strains with
zearalenone and its derivative ά-zearalenol. Appl Environ
Microbiol. 68:3545–3549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Niderkorn V, Boudra H and Morgavi DP:
Binding of Fusarium mycotoxins by fermentative bacteria
in vitro. J Appl Microbiol. 101:849–856. 2006.
|
|
18.
|
Cheng B, Wan C, Yang S, Xu H, Wei H, Liu
J, et al: Detoxification of deoxynivalenol by Bacillus
strains. J Food Safety. 30:599–614. 2010.
|
|
19.
|
Lu Q, Liang X and Chen F: Detoxification
of zearalenone by viable and inactivated cells of Planococcus
sp. Food Control. 22:191–195. 2011. View Article : Google Scholar
|
|
20.
|
El-Nezami HS, Kankaanpaa PE, Salminen S
and Ahokas J: Ability of dairy strains of lactic acid bacteria to
bind a common food carcinogen, afatoxin B1. Food Chem Toxicol.
36:321–326. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Topcu A, Bulat T, Wishah R and Boyaci IH:
Detoxification of aflatoxin B1 and patulin by Enterococcus
faecium strains. Int J Food Microbiol. 139:202–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tuomola M, Vahva M and Kallio H:
High-performance liquid chromatography determination of skatole and
indole levels in pig serum, subcutaneous fat, and submaxillary
salivary glands. J Agr Food Chem. 44:1265–1270. 1996. View Article : Google Scholar
|
|
23.
|
Dreizen S and Spies TD: Further studies on
the association between the products of protein putrefaction and
dental caries activity. J Dent Res. 27:305–315. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tittsler RP, Sandholzer LA and Callahan
ET: The bacteriostatic action of skatole on Gram-negative enteric
bacilli. J Infect Dis. 57:57–60. 1935. View Article : Google Scholar
|
|
25.
|
Li P, Tong L, Liu K and Wang YX:
Biodegradation of 3-methylindole by Pseudomonas putida LPC24
under oxygen limited conditions. Fresenius Environ Bull.
19:238–242. 2010.
|
|
26.
|
Squires EJ and Lundström K: Relationship
between cytochrome P4502E1 in liver and levels of skatole and its
metabolites in intact male pigs. J Anim Sci. 75:2506–2511.
1997.PubMed/NCBI
|
|
27.
|
Matal J, Matuskova Z, Tunkova A,
Anzenbacherova E and Anzenbacher P: Porcine CYP2A19, CYP2E1 and
CYP1A2 forms are responsible for skatole biotransformation in the
reconstituted system. Neuro Endocrinol Lett. 30:36–40.
2009.PubMed/NCBI
|
|
28.
|
Diaz GJ and Squires EJ: Metabolism of
3-methylindole by porcine liver microsomes: responsible cytochrome
P450 enzymes. Toxicol Sci. 55:284–292. 2000. View Article : Google Scholar : PubMed/NCBI
|