Introduction
Periodontal disease is one of the most common
infectious diseases and the primary cause of tooth loss. Currently,
14,000,000 individuals live on plateaus, where morbidity due to
periodontal disease increases with altitude (1). A survey conducted by the Health
Organization revealed that the morbidity of periodontal disease is
50% on the plains, whereas it increases to 70.4% on plateaus
(2). One of the most important
causes of the high morbidity is the low oxygen conditions on
plateaus (3), as the air is thin
and therefore oxygen content is low. Thus, the blood becomes dense,
sticky and cohesive, leading to various degrees of hypoxia in body
tissues (4). Changes in oxygen
concentrations, an important physiological and pathological
regulator, may affect the entire life span, from embryogenesis and
development to the maintenance of normal function, dysfunction,
disease and aging. Physiological oxygen tension (pO2) in
normal tissues ranges from 24 to 66 mmHg (3–9% O2)
(5) on plateaus, particularly at a
higher altitude and ambient atmosphere. The oxygen that is utilized
by metazoan gradually decreases, resulting in pathological hypoxia
(pO2<3%). The periodontium is particularly sensitive
to hypoxia, and a series of pathological and physiological
reactions to hypoxia lead to the reduction of tissue defenses
(6). Furthermore, hypoxia results
in the decrease of redox potential, which leads to the gathering of
anaerobes (7). The interaction of
these factors accelerates the initiation and development of
periodontal disease. Studies regarding plateau periodontal disease
have mostly been epidemiological surveys of small sectors and
samples (8,9). Few studies regarding the initiation,
development and outcomes of the disease have been conducted, and
even fewer studies have been conducted on the biological behaviors
of periodontium cells under plateau circumstances, such as hypoxia
or ultraviolet rays.
Of all the cells in the periodontium, human
periodontal ligament fibroblasts (HPLFs) are the most numerous and
have the most important role (10). They constantly produce new
principal fibers and dental cement and reconstruct alveolar bones.
HPLFs also synthesize the extracellular matrix, which not only
holds the cells but also has special biological functions such as
participating in cell conglutination, transportation and
mineralization (10,11). If the number of HPLFs is reduced or
the structure is broken, the periodontal support tissue may be
damaged. This damage further induces or aggravates periodontal
disease (12). Therefore, studies
on the proliferation and mineralization activities of HPLFs are
important in improving our understanding of the etiology and
treatment of periodontal disease.
For the reasons stated previously, HPLFs were
selected as the subject of this study. The effects of hypoxia on
the proliferation, mineralization and ultrastructure of HPLFs at
various time points were investigated by imitating different
hypoxic conditions. To a certain extent, the results reveal the
biological changes of HPLFs under hypoxic conditions. Therefore,
this study provides an experimental basis for additional study on
plateau-hypoxia-induced periodontal disease.
Materials and methods
Culture and identification of HPLFs
Sourcing and culturing of HPLFs
In this study, periodontal ligament tissues were
isolated from the premolar teeth extracted from 6 donors (mean age:
13 years and 3 months) for orthodontic treatment. Informed consent
was obtained from all of the patients prior to the beginning of
experiments. Permission for a series of experiments was granted by
the Ethics Committee of The Third Military Medical University
(Chongqing, China). The patients were asked to gargle with
chlorhexidine prior to the extraction of the tooth. Subsequently,
the tooth was disinfected with 1% iodine and 72% alcohol. Following
extraction, the tooth was repeatedly washed with sterile PBS and
placed into a DMEM culture solution (Gibco, Carlsbad, CA, USA)
containing 5% FBS. The tooth was subsequently sent to the super
clean bench.
The tooth was placed in a sterile culture dish, and
a small quantity of DMEM containing antibiotics (100 U/ml
penicillin, 100 μg/ml streptomycin and 5 μg/ml amphotericin B;
North China Pharmaceutical, Shijiazhuang, China) was added in order
to keep the root face moist. The central third of the periodontal
tissue on the root was scraped and cut into small sections (~1
mm3 each). The sections were spread evenly at the bottom
of a 25 mm2 sterile culture bottle with DMEM
infiltration. The bottom of the bottle was turned upward.
Subsequently, 4 ml DMEM containing 5% FBS and antibiotics (Gibco)
was carefully added to the bottle, which was subsequently placed
into a 37°C CO2 incubator for 2–4 h. The bottom of the
bottle was turned downward after the tissue sections had adhered in
order to allow the culture solution to slowly cover the tissue
sections. The solution was cultured again in the incubator and
changed every four days. The growth condition of the cells was
observed each day under an inverted microscope (Olympus, Tokyo,
Japan). The cells were passaged at a ratio of 1:2 when they
detached from the tissue sections and covered 80–90% of the bottom
of the bottle.
Growth curve and doubling time of
HPLFs
The HPLFs (2.0×104/ml, fourth passage)
were inoculated into a 24-well culture plate (Shanghai Jiang Lai
Biotechnology Co., Ltd., Shanghai, China) at 1 ml for each well.
Three wells were selected for cell counting every day. The growth
curve was created following eight days of continuous
observation.
The doubling time was TD = t × log2 /
(logNt-logN0), where t is the culture time,
N0 is the number of cells at the beginning of the
culture period, and Nt is the number of cells at the end
of the culture period.
Identification of HPLFs using
avidin-biotin complex (ABC) immunohistochemistry (IHC)
The HPLFs (1×l05/ml, fourth passage) were
inoculated into a culture dish with a glass slide in order to
create a cell climbing slide. Paraformaldehyde was used for
fixation and exclusive serum (bovine serum albumin 1 g,
phosphate-buffered saline 100 ml, sodium azide 0.08 g) for closure.
The first, second and third antibodies were added in sequence. DAB
was primarily used in the color reaction, which was terminated by
PBS. Subsequently, haematoxylin was used for the counterstaining
process, and neutral balsam was used in the sealing process. Images
were captured under a microscope (Olympus).
HPLF groups according to various
hypoxic conditions
HPLFs were assigned into four groups, as follows:
slight hypoxia group, 5% O2 content; middle hypoxia
group, 2% O2 content; severe hypoxia group, 1%
O2 content; and the control group, 21% O2
content. HPLFs in the three hypoxia groups were cultivated in a
three-gas (CO2/O2/N2) incubator
(NuAire Inc., Plymouth, MN, USA), and the HPLFs in the control
group were cultivated in a common CO2 incubator (NuAire,
Inc., Plymouth, MN, USA).
Effects of different hypoxic
conditions on the proliferation of HPLFs at various time
points
HPLFs (1.7×104/ml, fifth passage) were
inoculated in a 96-well culture plate at 200 μl/well. Each group
contained four duplicate holes. The HPLFs in the different groups
were cultivated according to their corresponding circumstances.
Firstly, 20 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (5 g/l) was added to each tested well 12, 24, 48 and 72 h
after cultivation. Subsequently, cultivation was continued for a
further 4 h at 37°C. The culture solution was discarded, and 20 μl
DMSO was added to each tested well. The optical density (OD) of
each tested well was measured using an ELISA plate reader
(Precision Microplate Reader, Molecular Devices, Bio-Rad Inc.,
Hercules, CA, USA) at 490 nm following agitation for 10 min.
Effects of different hypoxic
conditions on the ALP activity of HPLFs at various time points
HPLFs (3.7×104/ml, fifth passage) were
inoculated into a 96-well culture plate at 200 μl/well. Each group
contained four duplicate holes. The HPLFs in the different groups
were cultivated according to their corresponding circumstances. The
culture solution was discarded 12, 24, 48 and 72 h after
cultivation and the cells were washed with PBS.
Triton X-100 (Henan Sino-American Biotechnology Co.,
Ltd., Henan, China) was used to dissolve the cells. The lysates
were maintained at 4°C overnight. The lysates from each well were
moved to an Eppendorf tube (Eppendorf, Hamburg, Germany) for
centrifugation at 997 × g for 5 min. Subsequently, 30 μl of
supernatant fluid was extracted for the ALP activity detection
using the ALP kit (R&D Systems, Minneapolis, MN, USA),
according to the manufacturer’s instructions in the. An ultraviolet
spectrophotometer (Unico, Franksville, WI, USA) was used to measure
the OD at 520 nm.
Effects of severe hypoxia on the
morphology of HPLFs at various time points
HPLFs (fifth passage) were assigned to the severe
hypoxia and control groups, and the growth conditions were observed
using an inverted microscope 12, 24, 48 and 72 h post-cultivation.
Following trypsinization and centrifugation, the HPLFs were fixed
using glutaraldehyde, dehydrated with acetone, and embedded using
epoxy resin-618 (Chenguang Research Institute, Sichuan, China). The
HPLFs were subsequently solidified in an oven (60°C) and sliced
into 1 μm samples using an ultrasonic wave slicer. Subsequently,
ultrathin 50–70 nm slices were created. HPLF ultrastructures were
observed using a transmission electron microscope (TEM) (Olympus)
after being coloured, washed and dried.
Statistical analysis
Data were expressed as the means ± SD. Mean values
were compared by single factor analysis of variance (ANOVA) and a
paired t-test using SPSS 13.0 statistical software (SPPS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cultivation and identification of
HPLFs
HPLFs began to migrate from the edge of the tissues
following 48 h of cultivation. The coronal outgrowth appeared and
extended after seven days, and the tissues disintegrated after two
weeks. The HPLFs were fusiform in shape and in a good condition
after being passaged. The cells were arranged in a sarciniform or
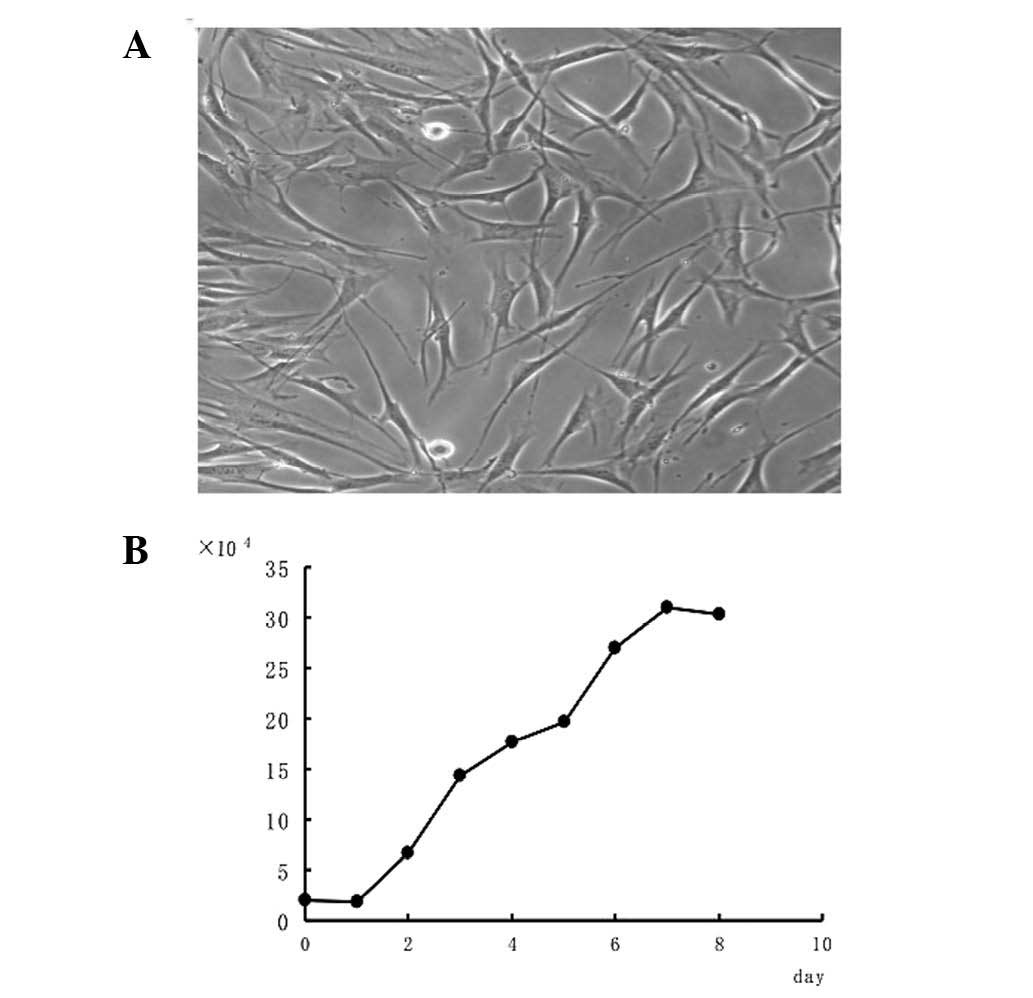
swirl pattern (Fig. 1A). The HPLF
growth curve was similar to an ‘S’, with arrest, logarithmic growth
and plateau phases (Fig. 1B). HPLF
multiplication was completed within 35.6 h.
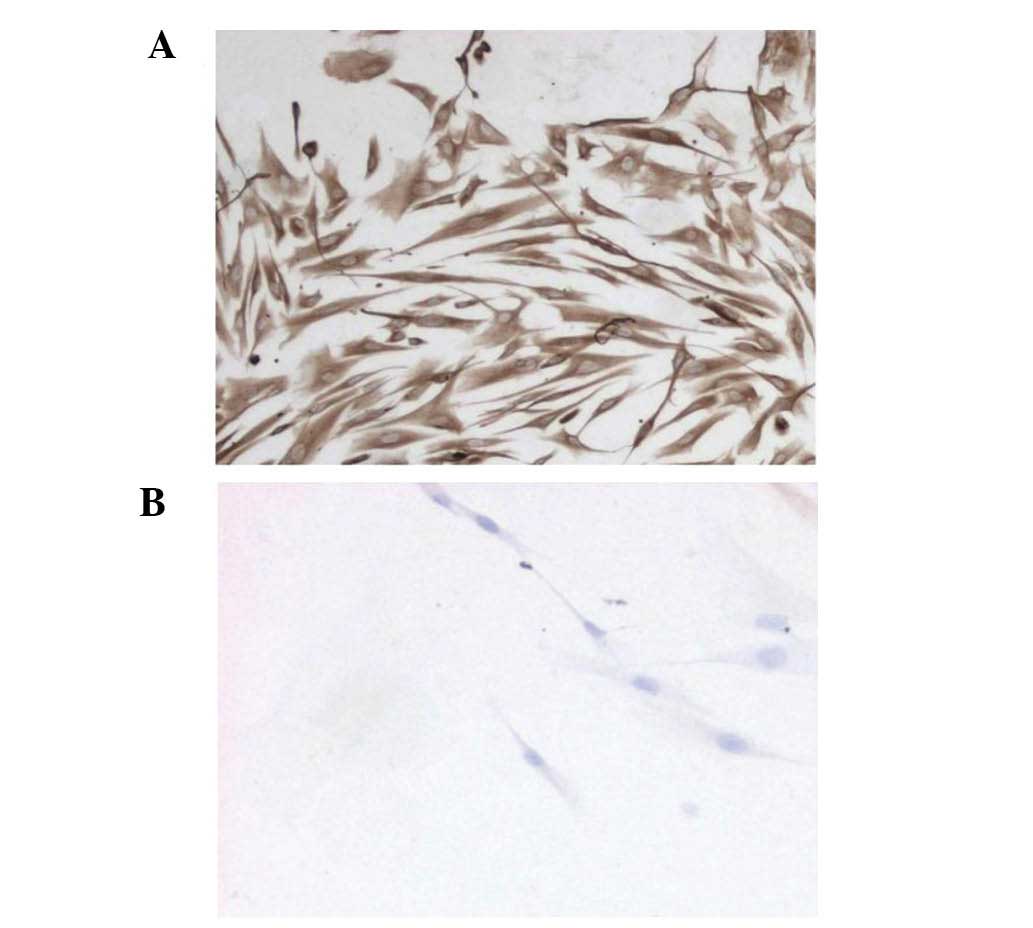
IHC testing of the HPLFs (fourth passage) revealed
that the cytoplasm was positive for vimentin, indicated with a
yellow-brown color (Fig. 2A).
However, keratin was not found in the cytoplasm (Fig. 2B). The results demonstrated that
HPLFs are mesenchymal cells derived from the embryonic
mesoderm.
Effects of different hypoxic conditions
on the proliferation of HPLFs at various time points
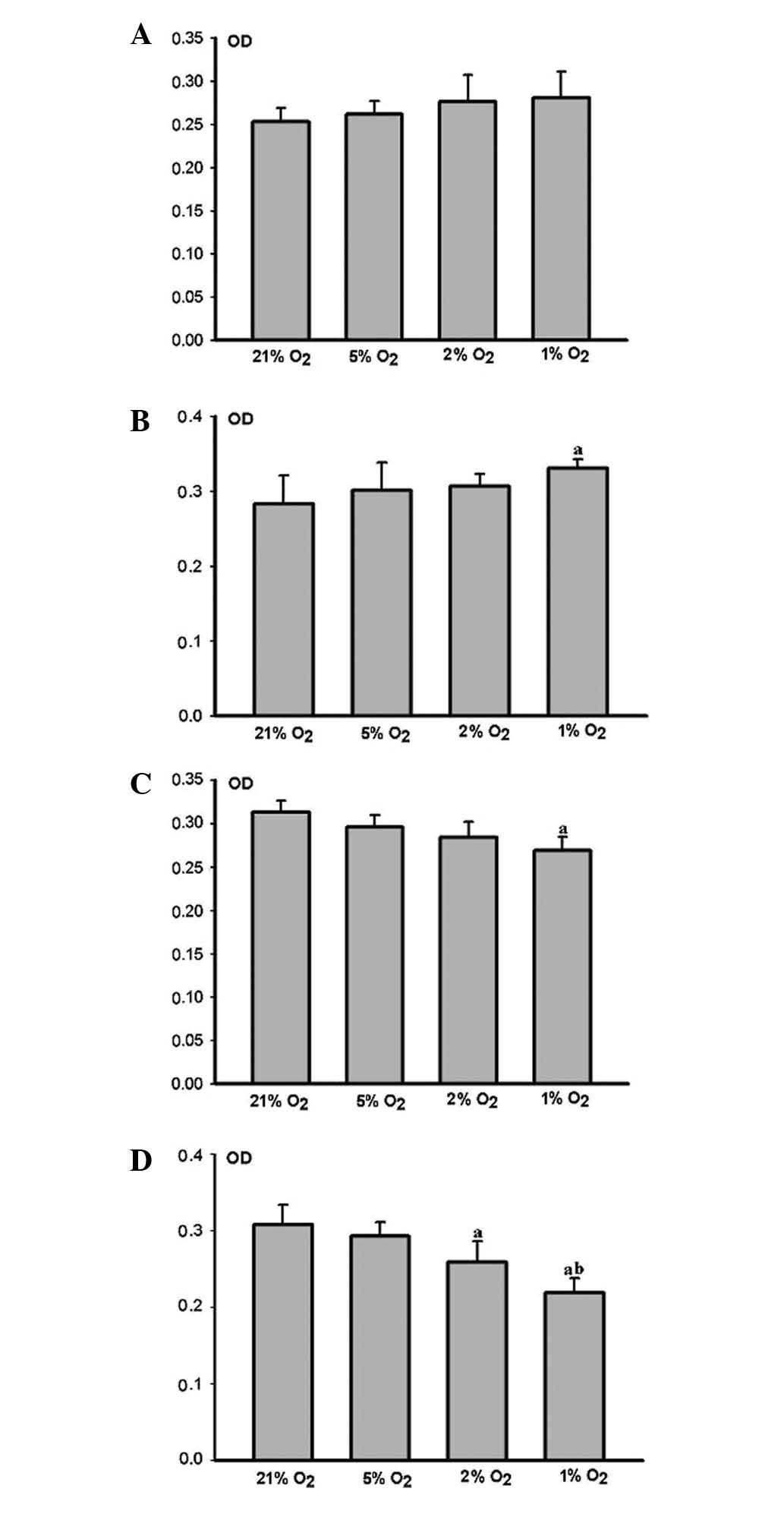
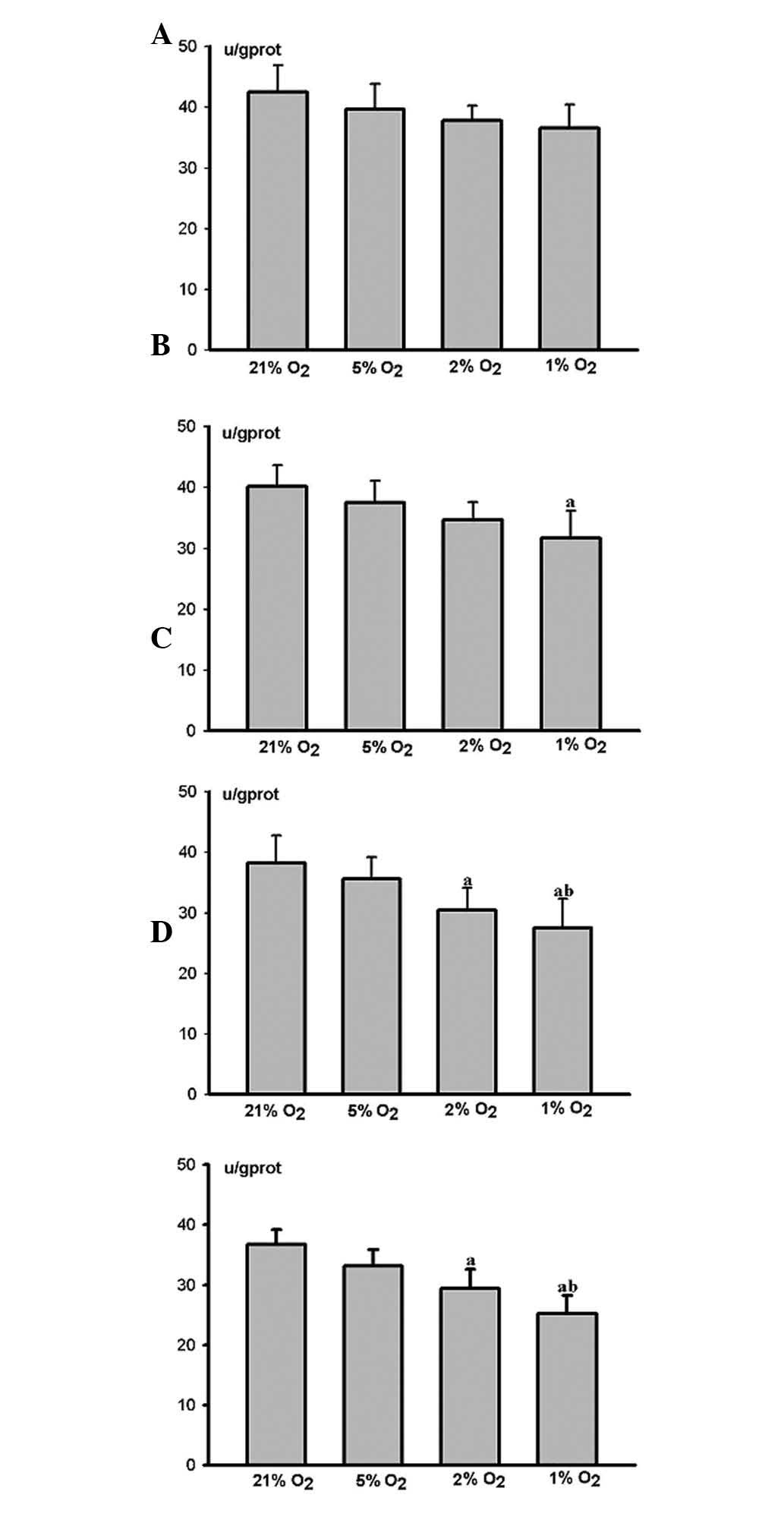
The HPLFs grew more rapidly as the degree of hypoxia
increased, when compared with the matched control group 12 h
(Fig. 3A) and 24 h (Fig. 3B) post cultivation. Cell
proliferation in the severe hypoxia group 24 h post-cultivation was
considered to be significant (P<0.05) (Fig. 3B).
HPLF growth was restrained as the degree of hypoxia
increased, when compared with the matched control group 48 h
(Fig. 3C) and 72 h (Fig. 3D) post-cultivation. Cell
proliferation in the middle and severe hypoxia groups 72 h
post-cultivation was markedly restrained (P<0.05) (Fig. 3D). However, the restraint was more
marked in the severe hypoxia group (P<0.05) (Fig. 3D).
Effects of different hypoxic conditions
on the ALP activity of HPLFs at various time points
ALP activity decreased at each time point as the
degree of hypoxia increased. No marked difference was observed
between the hypoxic and control groups after 12 h (Fig. 4A). The ALP activity of the HPLFs in
the severe hypoxia group was markedly restrained (P<0.05) after
24 h (Fig. 4B), whereas that of
the HPLFs in the middle and severe hypoxia groups was restrained
(P<0.05) after 48 h (Fig. 4C)
and 72 h (Fig. 4D). However, the
restraint was more marked in the severe hypoxia group (P<0.05)
(Fig. 4C and D).
Effects of severe hypoxia on the
morphology of HPLFs at various time points
Inverted microscopy at 12 h post-cultivation
(Fig. 5A) revealed that the HPLFs
adhered completely and were either fusiform or dendroid in shape
and assembled as a monolayer. Following 24 h (Fig. 5B), the HPLFs grew vigorously, had
full cell bodies, clear nuclei, and two or three fine cytoplasmic
processes. Following 48 h (Fig.
5C) and 72 h (Fig. 5D), the
HPLFs became contracted and sparse, and their cytoplasmic processes
were reduced. Their cytoplasms were vesiculated, and a section of
the HPLFs dismantled and disappeared.
 | Figure 5Effects of severe hypoxia on the
morphology of human periodontal ligament fibroblasts (HPLFs). (A–D)
Inverted microscopy was performed at various time points. (A) At 12
h post-cultivation the cells were in good condition, flat fusiform
or dendroid in shape and were arranged in a monolayer with normal
cell spacing; original magnification, ×100. (B) At 24 h
post-cultivation, the cells grew vigorously with full cell bodies,
clear nuclei and two or three fine cytoplasmic processes; original
magnification, ×100. (C) At 48 h post-cultivation, the cells became
contracted and sparse, their cytoplasmic processes were reduced and
their cytoplasms became vesiculated; original magnification, ×100.
(D) At 72 h post-cultivation, the cells were in a worse condition
and sections disassembled and disappeared; original magnification,
×100. (E–J) Transmission electron microscope (TEM) was also
performed at various time points. At 12 h post-cultivation revealed
that the organelles remained normal in cells, and the cytoplasm of
the HPLFs contained numerous mitochondria and rough endoplasmic
reticula (RER); original magnification, ×6200. (F) At 24 h
post-cultivation showed a marked increase in the number of
mitochondria and RER; original magnification, ×6200. (G) At 48 h
post-cultivation, the number of mitochondria and RER decreased. The
mitochondria increased in size, the cristae appeared to be vague
and RER structural disorder was observed; original magnification,
×6200. (H) At 72 h post-cultivation, degeneration occurred, and the
number of mitochondria and RER further decreased with the broken
membrane structure. Mitochondrial cristae were disassembled,
vacuolar degeneration occurred and particles of the RER were
reduced with increasing number of lysosomes; original
magnification, ×6200. (I) At 24 h post-cultivation, the cells had
numerous cytoplasmic processes; original magnification, ×5800. (J)
At 72 h post-cultivation, the cell had few cytoplasmic processes;
original magnification, ×6200. |
TEM demonstrated at 12 h post-cultivation (Fig. 5E) that the HPLF cell organelles
remained normal with clear nuclei and karyotheca. The cytoplasms of
the HPLFs contained numerous rough endoplasmic reticula (RER) and
mitochondria. Following 24 h (Fig.
5F), the number of mitochondria and RER significantly
increased, and the mitochondria and RER exhibited mild expansions
with complete membrane structures. The cell with a large or double
nuclei exhibited more cytoplasmic processes (Fig. 5I). After 48 h (Fig. 5G), the number of mitochondria and
RER decreased. The mitochondria increased in size, the cristae
appeared vague, and the RER were structurally disordered. The
number of cytoplasmic processes also decreased. Following 72 h
(Fig. 5H), the HPLFs degenerated,
and the number of mitochondria and RER decreased further with
broken membrane structures. The mitochondrial cristae were broken,
vacuolar degeneration occurred, RER particles reduced as the number
of lysosomes increased and the number of cytoplasmic processes
decreased further (Fig. 5J).
Discussion
HPLFs were isolated and cultured according to the
tissue culture method. IHC test results revealed that the cells
were derived from the embryonic mesoderm. The HPLFs grew vigorously
with full cell bodies and clear nuclei prior to the 10th passage.
The HPLFs were fusiform in shape and in a good condition. The
fourth to seventh passages were selected for this study as the
cells in this period proliferated vigorously and had the best
activity.
Cell proliferation is one of the most important
factors in maintaining the balance of cell numbers and maintaining
normal organism functions. We investigated the cell proliferation
statuses under various hypoxic conditions at 12, 24, 48 and 72 h,
respectively. In this study, O2 conditions ≤5% were
termed hypoxic and 21% O2 conditions were termed control
conditions. Cell viability was assessed following HPLF exposure to
hypoxic conditions for various periods of time. Our results
demonstrated that HPLF growth accelerated with an increase in the
degree of hypoxia during acute hypoxia. However, growth was
restrained as the degree of hypoxia increased over time. These
findings are in accordance with several other studies (13–15).
Harada et al(16) found
that short-term hypoxia promoted the proliferation of fibroblasts
in the heart. Lennon et al(17) found that the number of osteoblasts
increased under short-term hypoxia. Ren et al(18) concluded that the number of bone
marrow stromal cells markedly increased compared with the control
group under short-term hypoxic conditions (8% oxygen content).
However, the overall number of cells decreased over time. Piret
et al(19) investigated
whether hypoxia creates protective or destructive effects. The
effects are directly related to the duration and degree of hypoxia.
The effects of hypoxia on cell proliferation are determined using
hypoxia inducible factor-1 (HIF-1) (20). HIF-1 is mostly composed of
oxygen-sensitive (HIF-1α) and oxygen-insensitive subunits (HIF-1β)
(20). HIF-1α may be degraded
rapidly by hydroxyprolinase under normal conditions. However,
hydroxylation is halted under hypoxic conditions (21). An excessive quantity of HIF-1α is
capable of both promoting (short-term hypoxia) and restraining
(extension of time) cell proliferation (21).
ALP is important for differentiating osteoblast-like
cells, whose degree of activity reflects the mineralization ability
of tissues and cells and the parameters for the formation of
osteogenic property (22). This
study revealed that ALP activity was decreased at each time point
as the degree of hypoxia increased. The restraint was observed in
the middle and severe hypoxia groups over time. These findings are
consistent with those of earlier studies. Ren et al(18) stated that hypoxia was capable of
restraining the mineralization ability of bone marrow stromal
cells. Utting et al(23)
demonstrated that the biological activities of osteoblasts in
vitro were entirely oxygen dependent. Furthermore, hypoxia may
markedly reduce the ALP activity of osteoblasts and the mRNA
expressions of ALP and osteocalcin over time. The formation rate of
bone-mineralized nodules was significantly reduced as the degree of
hypoxia increased (24,25).
To demonstrate the effects of hypoxia on HPLFs, we
used an inverted microscope and TEM to observe structural changes
in HPLFs under severe hypoxic conditions. Furthermore, the effects
of hypoxia on HPLFs were explored by observing the cell
morphologies. It was observed under an inverted microscope that
over time the HPLFs became smaller under severe hypoxic conditions.
The growth and metabolism of HPLFs were suppressed, and the
structures were broken (even necrotic). TEM revealed that the
mitochondrial structures and RER of the HPLFs were broken as the
time period under which the cells were exposed to severe hypoxic
conditions was prolonged. Additionally, the number of cytoplasmic
processes decreased while the number of lysosomes increased. The
broken structure of the mitochondria directly affects the energy
metabolism and protein synthesis (26). The changes in the RER revealed the
slow rate of cell proliferation and division. The changes also
revealed a dysfunction in protein synthesis (27). The reduced number of cytoplasmic
processes demonstrated that the number of substances secreted and
synthesized by cells were reduced. The increased number of
lysosomes was a sign of cytotoxity. It revealed that large
quantities of aging organoid and external harmful substances had
gathered inside the cells (28).
Therefore, cell proliferation and protein synthesis were restrained
and an increased cytotoxicity occurred as the period of hypoxia was
prolonged.
In conclusion, short-term and slight hypoxic
conditions had relatively small effects on HPLFs, whereas long-term
and middle or severe hypoxic conditions had negative effects on the
proliferation and mineralization of HPLFs. Furthermore, the
mitochondria and RER of HPLFs were broken under long-term severe
hypoxic conditions. Therefore, middle or severe hypoxia in the long
term is capable of affecting the reconstruction and recovery of
periodontal tissues and may further initiate or aggravate
periodontal disease.
Acknowledgements
This study was supported by grants from the
Scientific and Technological Projects of PLA, China (project no.
2006MB252), National Natural Science Foundation of China (project
no. 31070863). Dr Yu-qi Gao of the Department of High-Altitude
Medicine, The Third Military Medical University provided help with
the experimental design. Dr Wen-qi Huang provided technical
assistance with TEM. ‘Enpapers’ provided editorial assistance with
this manuscript.
Abbreviations:
|
ALP
|
proliferation and alkaline
phosphatase
|
|
HPLFs
|
human periodontal ligament
fibroblasts
|
|
RER
|
rough endoplasmic reticulum
|
References
|
1
|
Xiao X, Li Y, Zhang G, Gao Y, Kong Y, Liu
M and Tan Y: Detection of bacterial diversity in rat’s periodontal
disease model under imitational altitude hypoxia environment. Arch
Oral Biol. 57:23–29. 2012.
|
|
2
|
Yong Liu, Qin-Tao WANG, Gang Li, et al:
Epidemiological survey on periodontal healthy status of residents
in Highland. Endodontic Journal of Periodontology. 17:282–285.
2007.
|
|
3
|
Pichon A, Zhenzhong B, Favret F, et al:
Long-term ventilatory adaptation and ventilatory response to
hypoxia in plateau pika (Ochotona curzoniae): role of nNOS
and dopamine. Am J Physiol Regul Integr Comp Physiol.
297:R978–R987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou ZN, Zhuang JG, Wu XF, Zhang Y and
Cherdrungsi P: Tibetans retained innate ability resistance to acute
hypoxia after long period of residing at sea level. J Physiol Sci.
58:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewis JS, Lee JA, Underwood JC, Harris AL
and Lewis CE: Macrophage responses to hypoxia: relevance to disease
mechanisms. J Leukoc Biol. 66:889–900. 1999.PubMed/NCBI
|
|
6
|
Park HJ, Baek KH, Lee HL, et al: Hypoxia
inducible factor-1α directly induces the expression of receptor
activator of nuclear factor-κB ligand in periodontal ligament
fibroblasts. Mol Cells. 31:573–578. 2011.
|
|
7
|
Amemiya H, Matsuzaka K, Kokubu E, Ohta S
and Inoue T: Cellular responses of rat periodontal ligament cells
under hypoxia and re-oxygenation conditions in vitro. J
Periodontal Res. 43:322–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adegbembo AO, Adeyinka A, Danfillo IS, et
al: National pathfinder survey of periodontal status and treatment
needs in The Gambia. SADJ. 55:151–157. 2000.PubMed/NCBI
|
|
9
|
Desvarieux M, Demmer RT, Rundek T, et al:
Relationship between periodontal disease, tooth loss, and carotid
artery plaque: the Oral Infections and Vascular Disease
Epidemiology Study (INVEST). Stroke. 34:2120–2125. 2003. View Article : Google Scholar
|
|
10
|
Choe Y, Yu JY, Son YO, et al: Continuously
generated H2O2 stimulates the proliferation
and steoblastic differentiation of human periodontal ligament
fibroblasts. J Cell Biochem. 113:1426–1436. 2012.
|
|
11
|
Yu Y, Mu J, Fan Z, et al: Insulin-like
growth factor 1 enhances the proliferation and osteogenic
mineralization of human periodontal ligament stem cells via ERK and
JNK MAPK pathways. Histochem Cell Biol. 137:513–525. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scheres N, Laine ML, Sipos PM, et al:
Periodontal ligament and gingival fibroblasts from periodontal
disease patients are more active in interaction with
Porphyromonas gingivalis. J Periodontal Res. 46:407–416.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chakravarthy MV, Spangenburg EE and Booth
FW: Culture in low levels of oxygen enhances in vitro proliferation
potential of satellite cells from old skeletal muscles. Cell Mol
Life Sc. 58:1150–1158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao T, Zhu LL, Zhao HQ, Li HS and Fan M:
Effects of hypoxia on the proliferation of rat myoblast in vitro.
In: Proceedings of 5th congress of Chinese society for
neuroscience; pp. 2902003
|
|
15
|
Yun Z, Lin Q and Giaccia AJ: Adaptive
myogenesis under hypoxia. Mol Cell Biol. 25:3040–3055. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harada M, Itoh H, Nakagawa O, et al:
Significance of ventricular myocytes and nonmyocytes interaction
during cardiocyte hypertrophy: evidence for endothelin-1 as a
paracrine hypertrophic factor from cardiac nonmyocytes.
Circulation. 96:3737–3744. 1997. View Article : Google Scholar
|
|
17
|
Lennon DP, Edmison JM and Caplan AI:
Cultivation of rat marrow-derived mesenchymal stem cells in reduced
oxygen tension: effects on in vitro and in vivo
osteochondrogenesis. J Cell Physiol. 187:345–355. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren H, Cao Y, Zhao Q, et al: Proliferation
and mineralization of bone marrow stromal cells under hypoxic
conditions. Biochem Biophys Res Commun. 347:12–21. 2006. View Article : Google Scholar
|
|
19
|
Piret JP, Mottet D, Raes M and Michiels C:
Is HIF-1α a pro- or an anti-apoptotic protein? Biochem Pharmacol.
64:889–892. 2002.
|
|
20
|
Mylonis I, Sembongi H, Befani C, Liakos P,
Siniossoglou S and Simos G: Hypoxia causes triglyceride
accumulation via HIF-1-mediated stimulation of lipin 1 expression.
J Cell Sci. 125:3485–3493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hisada T, Ayaori M, Ohrui N, et al: Statin
inhibits hypoxia-induced endothelin-1 via accelerated degradation
of HIF-1α in vascular smooth muscle cells. Cardiovasc Res.
95:251–259. 2012.PubMed/NCBI
|
|
22
|
Pae A, Kim SS, Kim HS and Woo YH:
Osteoblast-like cell attachment and proliferation on turned,
blasted, and anodized titanium surfaces. Int J Oral Maxillofac
Implants. 26:475–481. 2011.PubMed/NCBI
|
|
23
|
Utting JC, Robins SP, Brandao-Burch A,
Orriss IR, Behar J and Arnett TR: Hypoxia inhibits the growth,
differentiation and bone-forming capacity of rat osteoblasts. Exp
Cell Res. 312:1693–1702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawato Y, Hirao M and Ebina K:
Nkx3.2-induced suppression of Runx2 is a crucial mediator of
hypoxia-dependent maintenance of chondrocyte phenotypes. Biochem
Biophys Res Commun. 416:205–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ontiveros C, Irwin R, Wiseman RW and
McCabe LR: Hypoxia suppresses runx2 independent of modeled
microgravity. J Cell Physiol. 200:169–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zenebe WJ, Nazarewicz RR, Parihar MS and
Ghafourifar P: Hypoxia/reoxygenation of isolated rat heart
mitochondria causes cytochrome c release and oxidative stress;
evidence for involvement of mitochondrial nitric oxide synthase. J
Mol Cell Cardiol. 43:411–419. 2007. View Article : Google Scholar
|
|
27
|
Chen X, Sans MD, Strahler JR, et al:
Quantitative organellar proteomics analysis of rough endoplasmic
reticulum from normal and acute pancreatitis rat pancreas. J
Proteome Res. 9:885–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walls KC, Ghosh AP, Franklin AV, et al:
Lysosome dysfunction triggers Atg7-dependent neural apoptosis. J
Biol Chem. 285:10497–10507. 2010. View Article : Google Scholar : PubMed/NCBI
|