Introduction
Rheumatoid arthritis (RA) is a chronic disabling
autoimmune disease that causes chronic, progressive inflammatory
joint synovial damage, which largely encroaches upon the synovium
of the joint. The pathological changes include synovial cell
hyperplasia, expansion, congestion, hypertrophy of vessel walls,
inflammatory cell infiltration, fibrous tissue hyperplasia,
transparency and degeneration (1).
The primary goal of RA therapy is to control inflammation and joint
erosion. The pathogenesis of RA has not been fully elucidated, but
may be associated with the immune system and inflammatory reaction.
Traditional medicine may slow the development of RA to a certain
extent; however, numerous side-effects are often observed.
Therefore, the focus of RA research is the identification of a safe
and effective medicine.
Bizhongxiao decoction (BZXD) is a traditional
Chinese medicine (TCM), which was formulated by the Department of
Traditional Chinese Medicine (Changsha, China). The medicine has
been used clinically to treat RA for a number of years and has
demonstrated good clinical efficacy (2). The reported actions of BZXD include
the regulation of the immune system, the inhibition of inflammatory
cytokines, synovial angiogenesis and bone destruction, and the
modulation of the abnormal expression of genes and proteins
(3–8). However, since BZXD is a constituent
of numerous TCMs and has a complex chemical composition, the
comprehensive study of its mechanism of action in the treatment of
RA is limited. Radix Paeoniae Alba is an important component of
BZXD. It is obtained from a type of peony and is slightly cold,
bitter and sour, with efficacy in calming the liver, relieving
pain, nourishing menstruation, astringing Yin and hidroschesis
(9). Medicinal Radix Paeoniae Alba
is the dried root of the Ranunculaceae plant, Paeonia
lactiflora Pall, from which the skin has been removed (10). Previous pharmacological studies of
Radix Paeoniae Alba have shown that it has anti-inflammatory,
analgesic, antispasmodic, liver protection and immune regulatory
functions (11). The effective
components of Radix Paeoniae Alba are mainly composed of a series
of aminoglycoside substances, including paeoniflorin,
hydroxy-paeoniflorin, peony glucoside, albiflorin and
benzoylpaeoniflorin, which are collectively referred to as the
total glucosides of peony (TGP). Paeoniflorin accounts for >90%
of the total glucosides in Radix Paeoniae Alba and is the main
effective component. Paeoniflorin has been found to mediate a wide
range of pharmacological effects, including hypoglycemic,
antitumor, immunomodulatory, anti-inflammatory and neuronal
protection actions (12). One
study demonstrated the ability of paeoniflorin to inhibit the
generation of interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α)
and PGE2 in peritoneal macrophages in rats with adjuvant arthritis
(AA) (13). In addition, orally
administered paeoniflorin has been shown to significantly reduce
paw edema in rats with collagen-induced arthritis (CIA), thereby
improving the inflammation of multiple joints (14).
At present, the use of the ultra performance liquid
chromatography and photo diode array (UPLC-PDA) method to determine
the paeoniflorin composition in Radix Paeoniae Alba decoction, and
in plasma following the intragastric administration of Radix
Paeoniae Alba decoction to rats, is rarely reported in the
literature. However, the present study used the UPLC-PDA method for
this purpose and also explored the therapeutic effect of
paeoniflorin when administered to rats with CIA. The aim of the
study was to lay the foundations for further studies of the
mechanism of paeoniflorin and the TCM, BZXD, in the treatment of
RA.
Materials and methods
UPLC-PDA analysis of paeoniflorin in
Radix Paeoniae Alba decoction and in rat plasma following the oral
administration of Radix Paeoniae Alba decoction
Preparation of drugs and
standards
Radix Paeoniae Alba was purchased from the Xiangya
Hospital of Central South University (Changsha, China). It passed
identification by the Research Institute for Pharmacology of
Traditional Chinese Medicine of Xiangya Hospital, Central South
University. Radix Paeoniae Alba was crushed into powder and then
pure water was added in the ratio of 1:8 of powder to water. The
aqueous composition was boiled for 30 min, filtered to obtain the
liquid and then rotary evaporated at 60°C and low pressure to
provide a concentrated aqueous solution containing only one
traditional Chinese medicine. A freeze dryer was used to transform
the concentrate into a freeze-dried powder, with a yield of 18.5%.
The powder was sealed and stored at 4°C. A reference substance of
paeoniflorin was purchased from The National Institute For The
Control of Pharmaceutical and Biological Products (Beijing, China)
and the mass fraction was >98%.
Chromatographic conditions
UPLC was performed using an Acquity UPLC system
(Waters Corporation, Milford, MA, USA), which included a binary
pump processor, sample processor, column oven, PDA detector and
Empower chromatography workstation. The mobile phase consisted of
acetonitrile and 1% acetic acid in the ratio 22:78 under the
following conditions: Detection wavelength, 190–480 nm; flow rate,
0.25 ml/min; column temperature, 40°C; and injection volume, 5 μl.
The analysis time was 4 min. The number of theoretical plates was
calculated using the paeoniflorin peak and was not <5,000.
Acetic acid, acetonitrile and methanol were AR grade and
self-prepared triple-distilled water was used.
Preparation of the reference substance
solution
Paeoniflorin was weighed to 0.41 mg accurately, put
into a 10-ml brown volumetric flask and methanol was added for
ultrasonic dissolution. The solution was diluted to scale and
shaken. A paeoniflorin reference stock solution was obtained with a
concentration of 0.041 mg/ml. The reference solution was sealed and
stored at 4°C for later use.
Preparation of the test solution
Radix Paeoniae Alba freeze-dried powder was weighed
accurately to 5 g with a 1% electronic balance (equivalent to 27.03
g crude drug). The powder was ultrasonically dissolved in 200 ml
water for 10 min and Radix Paeoniae Alba decoction, with a
concentration of 0.135 g crude drug/ml, was obtained. Next, 2 ml
decoction was measured accurately, placed into a 10-ml volumetric
flask and 7 ml methanol was added followed by 30 min ultrasonic
oscillation. After maintaining at room temperature for 30 min, 7 ml
methanol was added with shaking and the resultant mixture was
filtered. The filtrate was filtered using a 0.45-μm membrane and
the test solution comprising Radix Paeoniae alba (0.027 g crude
drug/ml) was obtained.
Preparation of the plasma sample
Experiments were performed using male Sprague Dawley
(SD) rats (weight, 200–220 g) provided by the Animal Experimental
Center of the Hunan People’s Hospital (Changsha, China). All
experiments conformed to the Regulations for the Administration of
Affairs Concerning Experimental Animals (1988) and were approved by
the Animal Experimental Center for Central South University. Normal
SD rats were divided into a Radix Paeoniae Alba gastric perfusion
group and a blank control group, and were fasted for 12 h. An oral
decoction of Radix Paeoniae Alba was administered to the Radix
Paeoniae Alba group at a dose of 1.35 g/kg crude drug/body weight
(converted according to the surface area of a 70 kg human)
(15). To the blank control group
was administered a dose of double-distilled water by gavage. After
30 min, the rats were sacrificed and blood samples were collected
in anticoagulant tubes. The samples were allowed to stand at room
temperature for 2 h, prior to centrifugation at 1,000 × g for 15
min, in order to obtain the rat plasma. Following this, 2 ml ethyl
acetate, 4 ml acetonitrile and 1.2 ml acetone was added to 2 ml rat
plasma and the resulting mixture was irradiated with ultrasound for
20 min. Next, the solutions were centrifuged at 3,000 rpm for 20
min, the supernatant was obtained and placed in a water bath at
room temperature. The supernatant was dried with nitrogen,
redissolved in 50 μl acetic acid solution (20%) and 50 μl methanol,
then centrifuged at 15,000 × g for 20 min. The resulting
supernatant was the required plasma sample.
Screening of the gavage dose of
paeoniflorin
Animals
The Animal Experimental Center of the Hunan People’s
Hospital provided 25 male and female healthy SD rats (clean grade;
age, 45–50 days; weight, 150–180 g). Rats had access to food ad
libitum and were maintained in a 12/12 h light/dark cycle
(light time, 6:00–18:00). Background noise was maintained at 40±10
db and the temperature was 20±3°C. The rats were acclimatized to
these conditions for 1 week.
Replication of the CIA rat model
Following the instructions provided with bovine
II-type collagen (BIIC; immunization grade; lot, 120197; Chondrex,
Inc., Redmond, WA, USA), replicated CIA models were constructed for
20 rats randomly selected from the 25 SD rats. Firstly, 10 mg BIIC
was completely mixed with 5 ml acetic acid (0.05 M) to form a 2
mg/ml BIIC solution. Next, 5 ml of this solution was mixed with 5
ml complete Freund’s adjuvant (070M8704; Sigma-Aldrich, St. Louis,
MO, USA), to produce a 1 mg/ml BIIC solution. This BIIC solution
(0.2 ml) was then subcutaneously injected into the tail root of
each rat for immunization. After 7 days, 5 ml BIIC and 5 ml
incomplete Freund’s adjuvant (101M8711; Sigma-Aldrich) were mixed
using the same method to prepare 1 mg/ml BIIC solution and a
subcutaneous injection of the BIIC solution (0.1 ml) was
administered at the tail root of each rat for reimmunization.
Animal grouping
Two weeks following the initial injection for
immunization, the animal models were randomly divided into low,
middle and high dose groups for paeoniflorin treatment. The model
control group (model group) and the normal control group (normal
group) were also established.
Delivery methods
Following immunization (14 days), the treatment
groups were treated with the paeoniflorin standards. The
administration dosage for the rats was converted according to the
surface area of a 70 kg human body. The concentrations of the
paeoniflorin standards for the low, middle and high dose groups
were 0.5, 1 and 2 mg/kg/day, respectively. The model and normal
group rats were able to drink water freely.
Detection of serum inflammatory
cytokines in the rats
On day 42 following immunization, the rats were
fasted for 12 h and then sacrificed. Blood samples were collected
and left at room temperature for 2 h. Centrifugation at 111 × g for
15 min was used to obtain the required serum. An ELISA kit (lot,
G12030317; Wuhan Huamei Biotech Co., Ltd, Wuhan, China) was used to
test IL-1β and TNF-α levels.
Effect of paeoniflorin on CIA model
rats
Animals and replication of the CIA rat
model
The animal experimental center at the Hunan People’s
Hospital provided 60 male and female healthy SD rats (age, 45–50
days; weight, 150–180 g). Rats were maintained as described
previously. A total of 40 SD rats were selected for replication of
the model, using the modeling methods described earlier.
Animal grouping and delivery
method
Two weeks following immunization, the animal models
were randomly divided into the paeoniflorin treatment (PF group),
model and normal groups. According to the screened gavage dose, the
concentration of the paeoniflorin standard was 1 mg/kg/day. The
model and normal groups were able to drink water freely.
Weight and growth of the rats
Body weight was measured on days 7, 14, 21, 28, 35
and 42 following immunization in each group. The increase in body
weight was calculated by subtracting the previous weight for every
week. General observations of the rats were also recorded,
including the mental state, hair quality, diet and activity of the
rats.
Joint symptom score
A joint symptom score was calculated using the
arthritis index (AI) integral method (16). The AI is an objective index,
reflecting the occurrence and development of arthritis. Joint
redness, the extent and degree of swelling and the joint
deformation of rats with arthritic disease were analyzed to
determine a grade of between 0 and 4 (0, no arthritis; 1, mild
swelling of joints following redness; 2, moderate swelling; 3,
severe joint swelling; and 4, severe joint swelling and the
inability to be loaded). Higher arthritis exponential integrals
represent joint symptoms of greater severity.
Measurement of the degree of paw
swelling
Every week following immunization, the thickness of
the right rear foot of each rat was measured in a fixed position
using a fine angle compass and millimeter ruler.
Joint synovial histopathology
[hematoxylin and eosin (H&E) staining]
On days 14, 28 and 42 following immunization, the
rats were sacrificed by cervical dislocation (using anesthesia 10%
chloral hydrate) and blood samples were collected. The bilateral
knee joint and the whole rear paw, including the ankle joint, were
removed. The fur and muscle fiber were removed and the paw was
fixed in 10% neutral formalin for 24 h. This was followed by
decalcification in 14% EDTA decalcifying fluid for 5 days and
neutralization in 5% sodium thiosulfate for 3 h. The samples were
washed for 12 h, embedded with dehydrate paraffin and cut into
5–6-μm sections (longitudinal). Sections were placed in a 60°C oven
for 30 min, then soaked with xylene twice for 20 min. Next,
sections were soaked with 95% ethanol for 3 min and then 80%
ethanol for 1 min, washed with distilled water for 1 min, stained
with hematoxylin for 15 min and washed. Acid alcohol was used for
differentiation for 3 sec, followed by washing with tap water for
10 min. Eosin solution was then used to stain the sections for 3
min, followed by washing. Next, 80% ethanol, 95% ethanol and
ethanol were used successively for gradient dehydration. The
samples were mounted with a neutral gum and the pathological
changes were observed using a light microscope (CX21; Olympus,
Tokyo, Japan).
Joint X-ray imaging
The presence of joint destruction was investigated
in the rat joints after modeling. An intraperitoneal injection of
10% chloral hydrate (3.5 ml/kg) was administered to the rats for
anesthesia. The joints of the rat limbs were stretched as far as
possible and placed in an X-ray machine for image data
collection.
Detection of serum inflammatory
cytokines
On days 28 and 42, the levels of serum inflammatory
cytokines were measured as described earlier.
Statistical Analysis
SPSS 19.0 software was used for statistical analysis
(SPSS, Inc., Chicago, IL, USA). All data are expressed as the mean
± SD. Group comparison adopts analysis of variance. Prior to
analysis of variance, the data had already passed the variance
homogeneity and normality tests. Each time point index difference
in the group was analyzed by the analysis of variance of repeated
measurement design. P<0.05 was considered to indicate a
statistically significant result.
Results
Paeoniflorin composition
UPLC-PDA was performed to detect the presence of
paeoniflorin in the Radix Paeoniae Alba decoction and in the plasma
of rats following Radix Paeoniae Alba gavage. Following a number of
preliminary experiments, the optimum chromatographic conditions for
the separation of paeoniflorin were selected. Using the UPLC-PDA
method, under the selected chromatographic conditions and according
to the retention time and characteristic UV spectrum of the
corresponding ingredients of the standard and Radix Paeoniae Alba
Decoction, the paeoniflorin ingredient was separated well in <4
min. The paeoniflorin component was detected in the plasma of
healthy SD rats, 30 min following Radix Paeoniae Alba gavage.
Fig. 1 shows typical chromatograms
of the paeoniflorin standard, Radix Paeoniae Alba decoction and a
rat plasma sample following the oral administration of Radix
Paeoniae Alba, as well as a blank plasma sample.
Determination of a suitable gavage dose
of paeoniflorin for absorption
The serum IL-1β and TNF-α levels in the model and PF
groups were greatly increased compared with those of the normal
group. Among the low, medium and high dose paeoniflorin groups, the
IL-1β and TNF-α serum levels of the medium dose group were the
lowest. Table I shows the mean
IL-1β and TNF-α serum levels in each group.
 | Table ISerum levels of TNF-α and IL-1β in
rats (pg/ml, mean ± SD). |
Table I
Serum levels of TNF-α and IL-1β in
rats (pg/ml, mean ± SD).
| Groups | IL-1β | TNF-α |
|---|
| Normal | 19.51±1.09 | 21.92±1.66 |
| Model | 60.41±2.56a | 75.21±1.86a |
| Low dose PF | 34.77±1.72a–c | 39.44±2.12a–c |
| Middle dose PF | 24.72±1.98a,b | 31.38±2.40a,b |
| High dose PF | 31.77±1.97a–c | 36.76±1.92a–c |
Effect of paeoniflorin on rats with
CIA
Certain rats had joint swelling and ecchymosis 5–7
days following the initial immunization. Following reimmunization
on day 7, the joint disease worsened. On day 14, toe joint swelling
also worsened and the surface skin of specific joints was shiny and
hyperemia was present. On day 42, certain rats had difficulty in
weight bearing and movement. During the disease process, rats
presented low spirits, somnolence, slow actions, reduced dietary
intake and dry hair in the later stages. By evaluating the model
rats 2 weeks following immunization, the successful replication
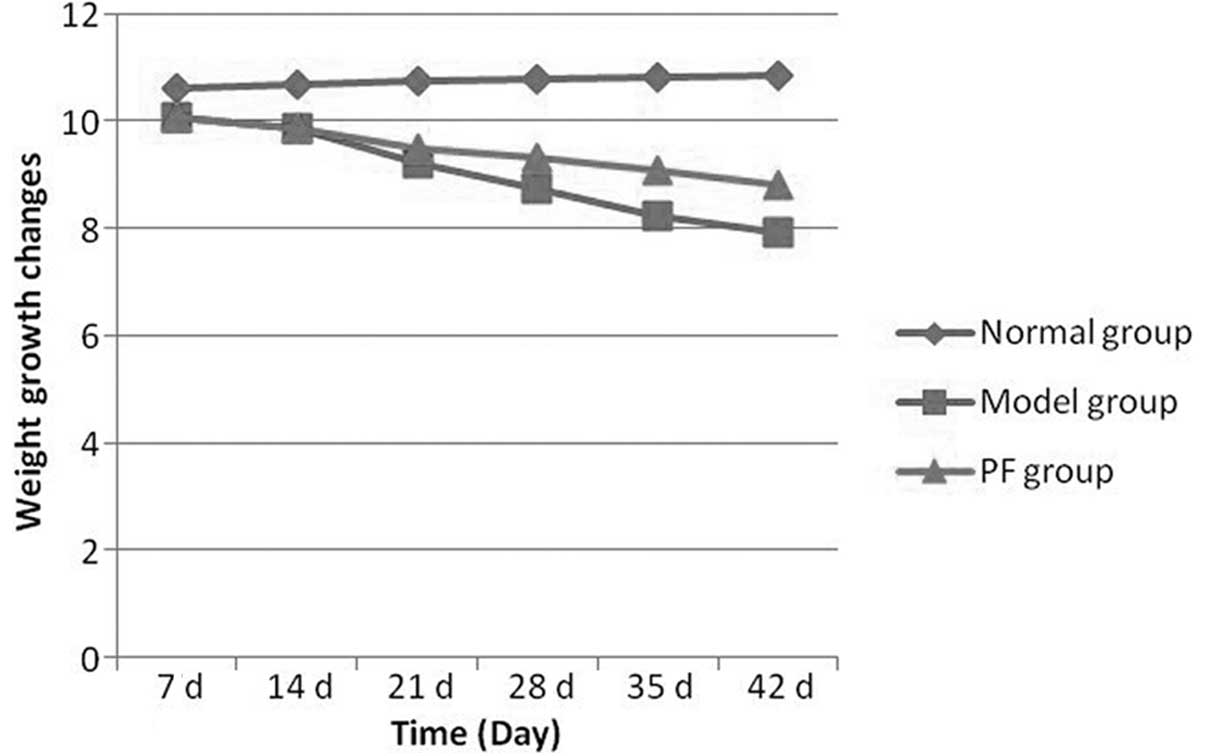
rate of the CIA model reached 90%. The rate of weight increase of
the normal group did not differ from that prior to modeling.
However, 7 days following immunization, the rate of weight increase
of the model group rats began to reduce compared with that of the
normal group. Following 2 weeks of paeoniflorin treatment, the
joint swelling and ecchymosis in the rats with CIA was eased
compared with that of the model group. In addition, after 4 weeks,
the joint swelling gradually eased, the shiny and hyperemic skin
reduced in area, the food intake and activity improved and the hair
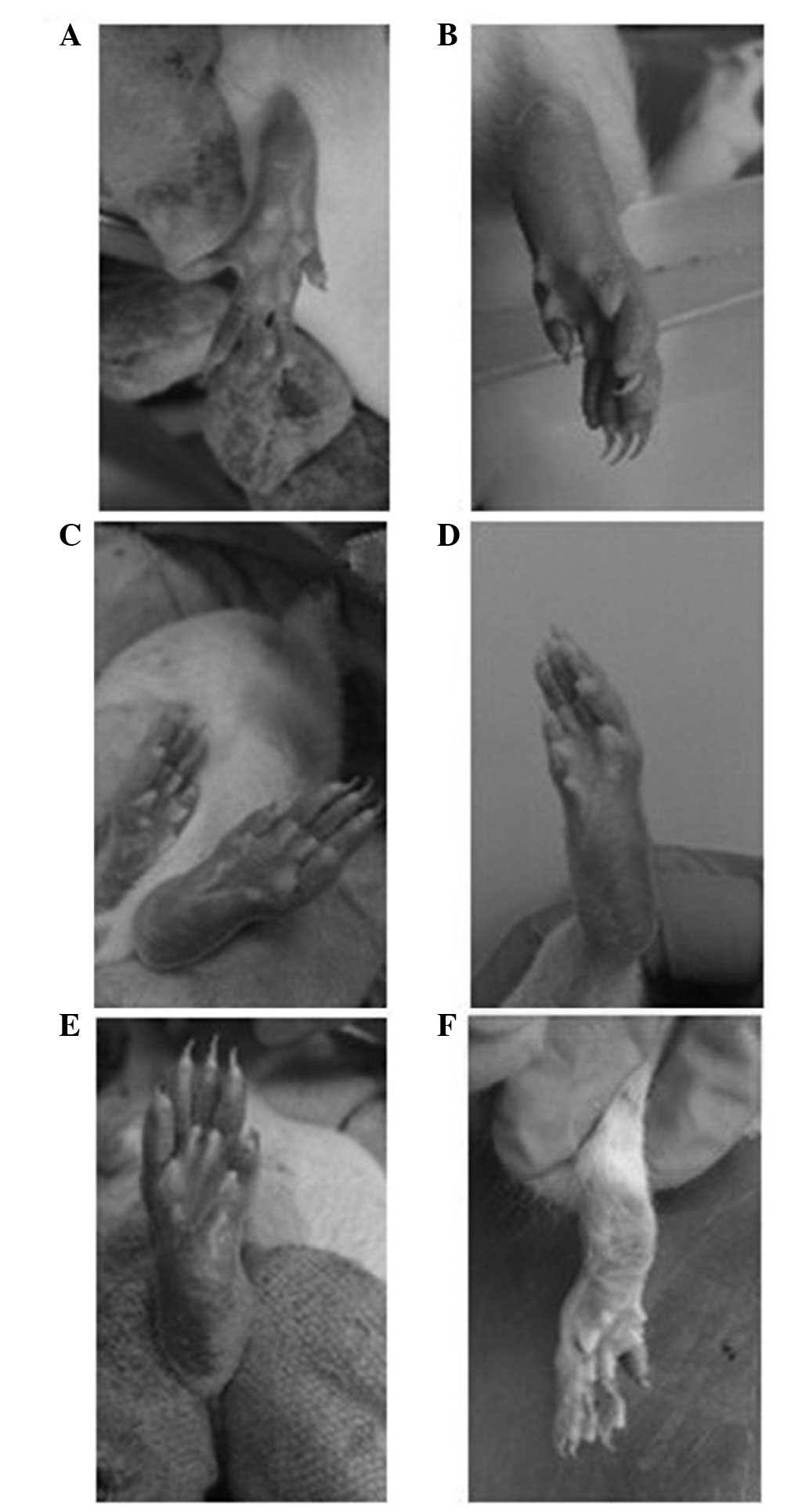
became shiny (Fig. 2). On days 35
and 42, the weight growth rate of the PF group was more marked than
that of the model group. Fig. 3
shows the weight growth changes of the rats in each group.
Comparison of AI integrals
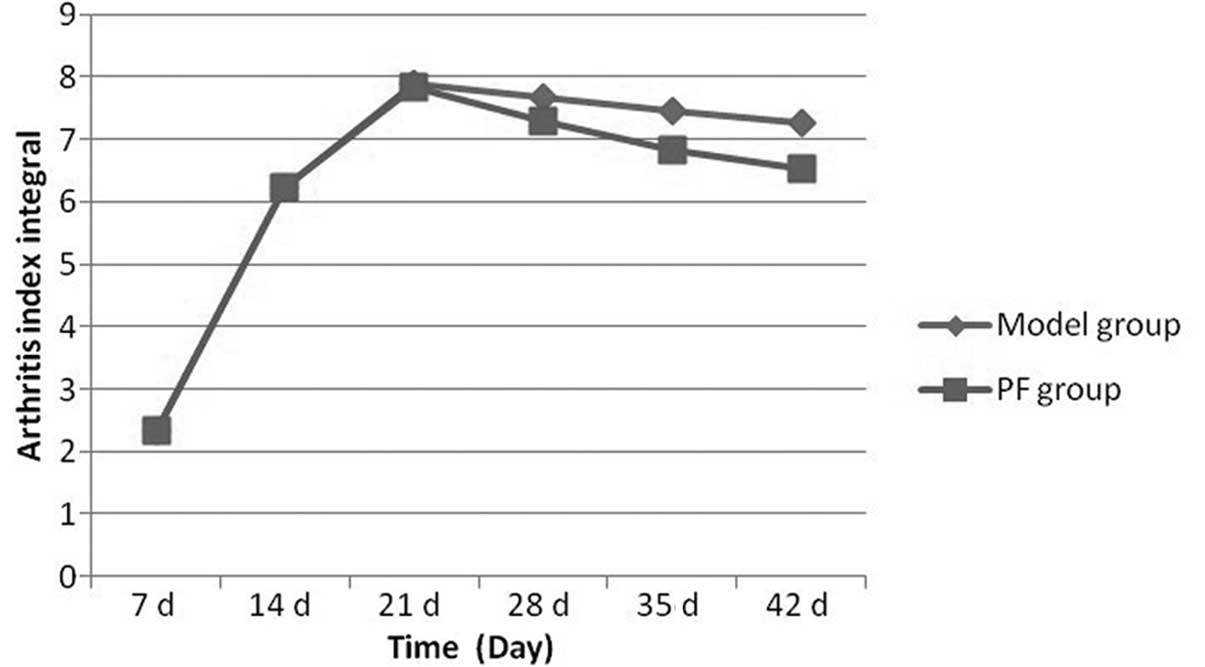
Signs of arthritis in the rats occurred between 5–7
days after the initial immunization. The AI integral of the model
group increased compared with that of the normal group. With the
extension in immunization time, the AI integral of the model and PF
groups greatly increased and peaked at day 21. However, the AI
integral of the PF group was lower than that of the model group. On
day 28, the AI integral of the model and PF groups began to
decrease and the reduction was more marked in the PF group than in
the model group. On day 35 the difference between the model and PF
groups was significant. Fig. 4
shows the AI integrals at different time points.
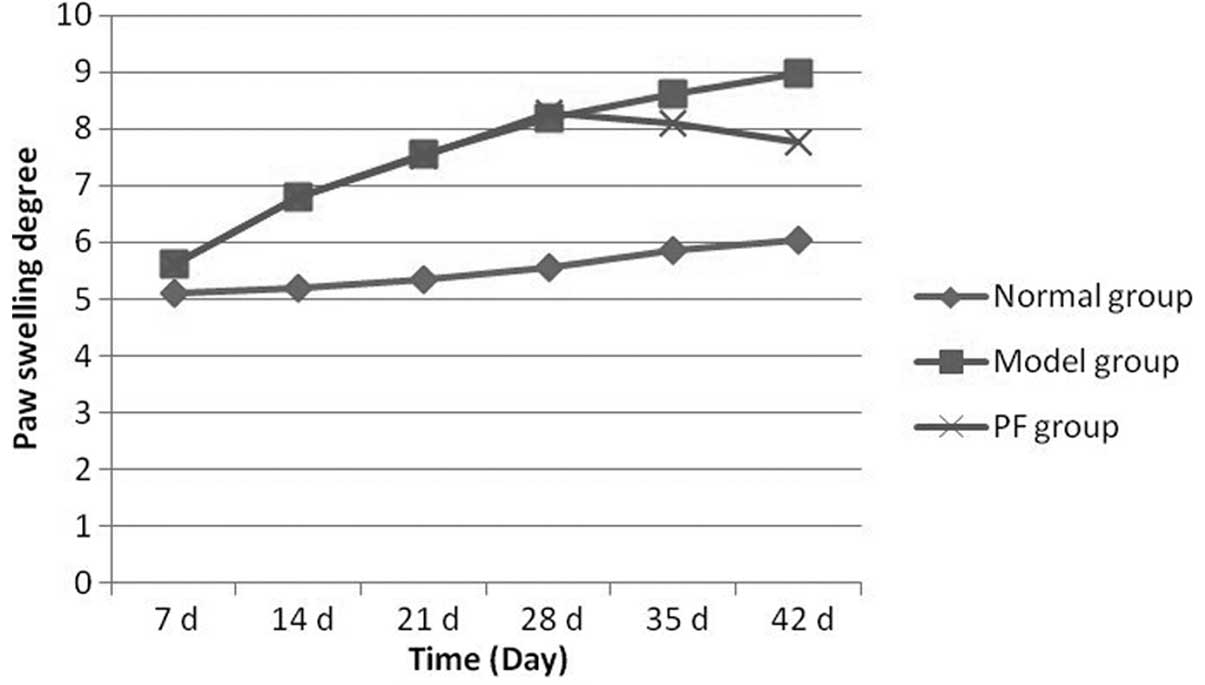
Measurement of paw swelling
In the first week of immunization, the foot swelling
in the rats was significant. The swelling in the model group was
significantly different compared with that in the PF group. With
the extension in immunization time, foot swelling in the PF group
eased compared with that in the model group. In week 3 of treatment
(week 5 of immunization) the difference between the PF and model
groups was significant. Fig. 5
shows paw swelling in the rats of each group.
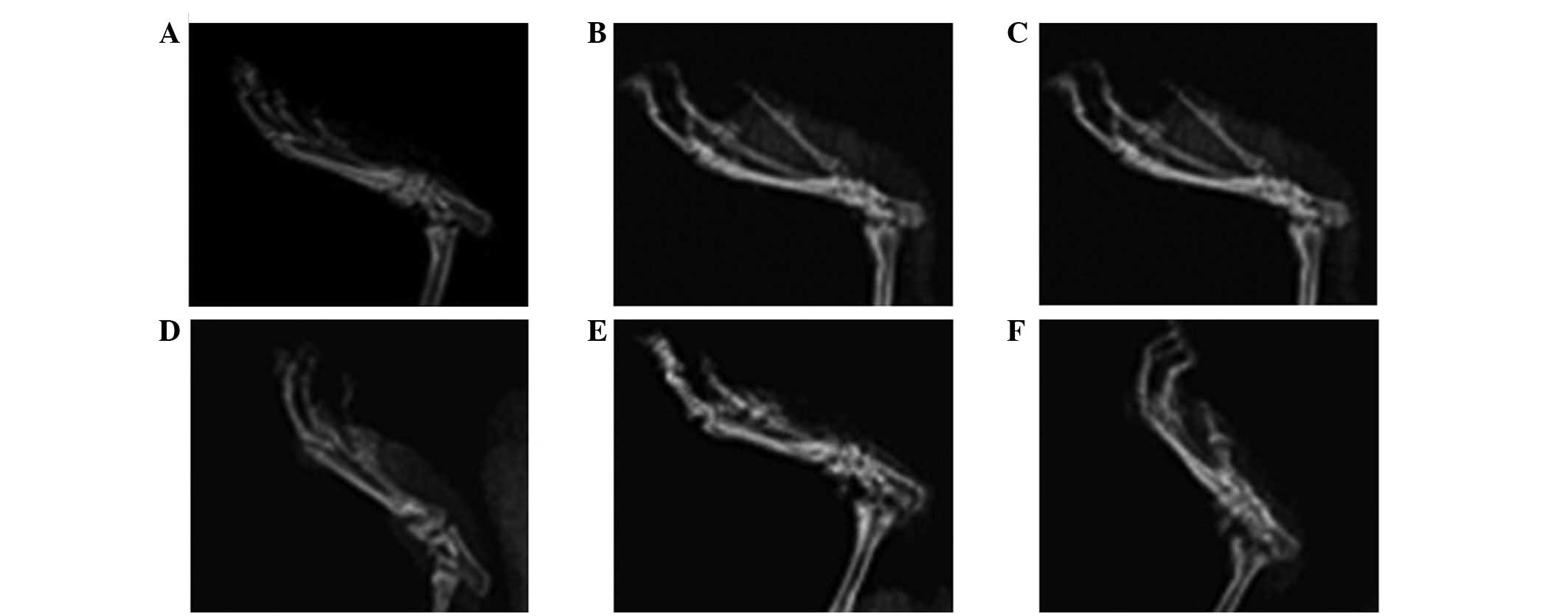
Analysis of joint X-rays
Normal rats had no swelling of the ankle soft
tissue, no bone destruction, an intact structure and clear foot
joint space. The X-rays at 2 weeks following immunization (14±2
days) show that the model group rats had tissue swelling around the
ankle joint. With the extension of immunization time, the foot
joint space was obscured, with narrowing and fusion. In the model
group, the first bone change occurred in week 4 (26±4 days)
following initial immunization. On day 42, certain areas of joint
soft tissue remained moderately swollen and bone destruction was
evident. The tissue swelling around the joints in rats with
arthritis in the PF group was reduced compared with that in the
model group, and the degree of bone destruction was greatly
reduced. Fig. 6 shows X-ray images
of the joints of rats from each group.
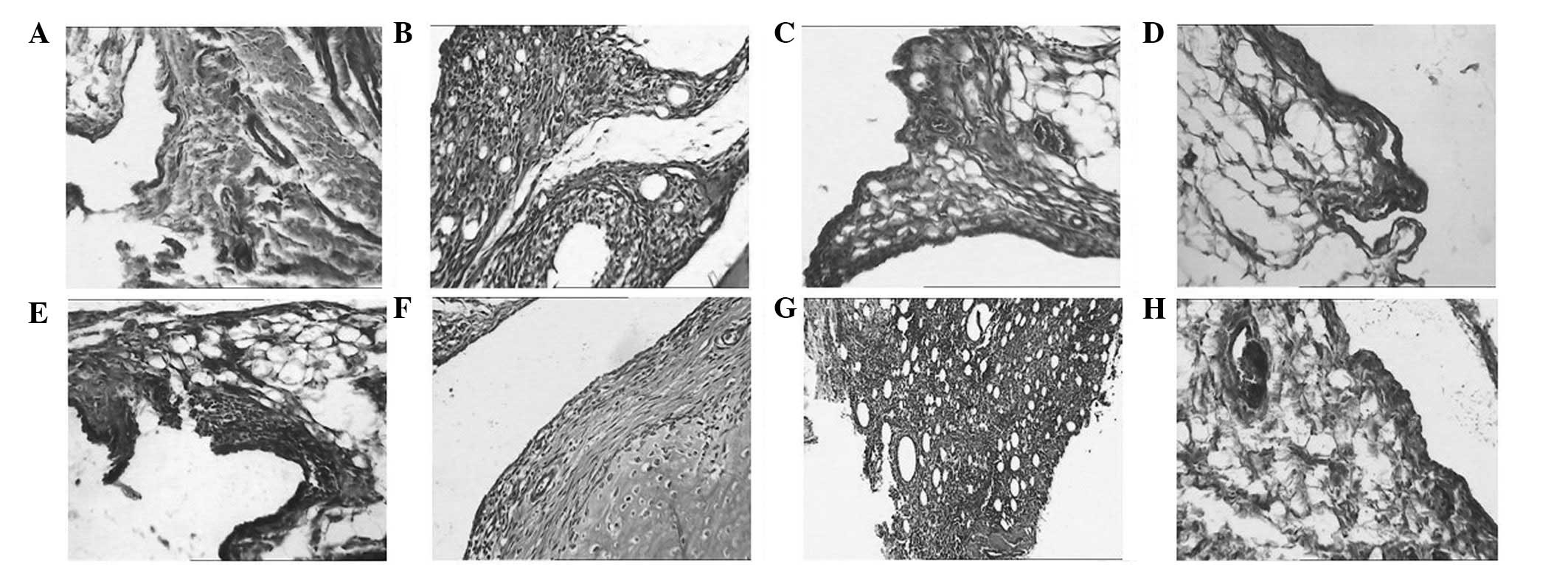
Histopathological observations
The synovial tissue of normal rats had no
significant abnormalities 14 days following the initial
immunization, whereas that of the model rats exhibited inflammatory
cell infiltration. With the extension of immunization time, large
amounts of pannus were generated on day 28 and vascular
proliferation and synovial thickening occurred at day 42. The
deterioration of arthritis caused an increase in the number of new
vessels, vascular dilatation and congestion with large quantities
of infiltrating lymphocytes. With regard to the PF group, on day
28, a small number of new vessels and inflammatory cell
infiltration in the synovial tissue were observed and the synovium
had thickened a little. With the extension of the treatment time,
on day 42 the number of new vessels was reduced and the
inflammation had eased. Fig. 7
shows H&E staining of the synovial tissue in rats from each
group.
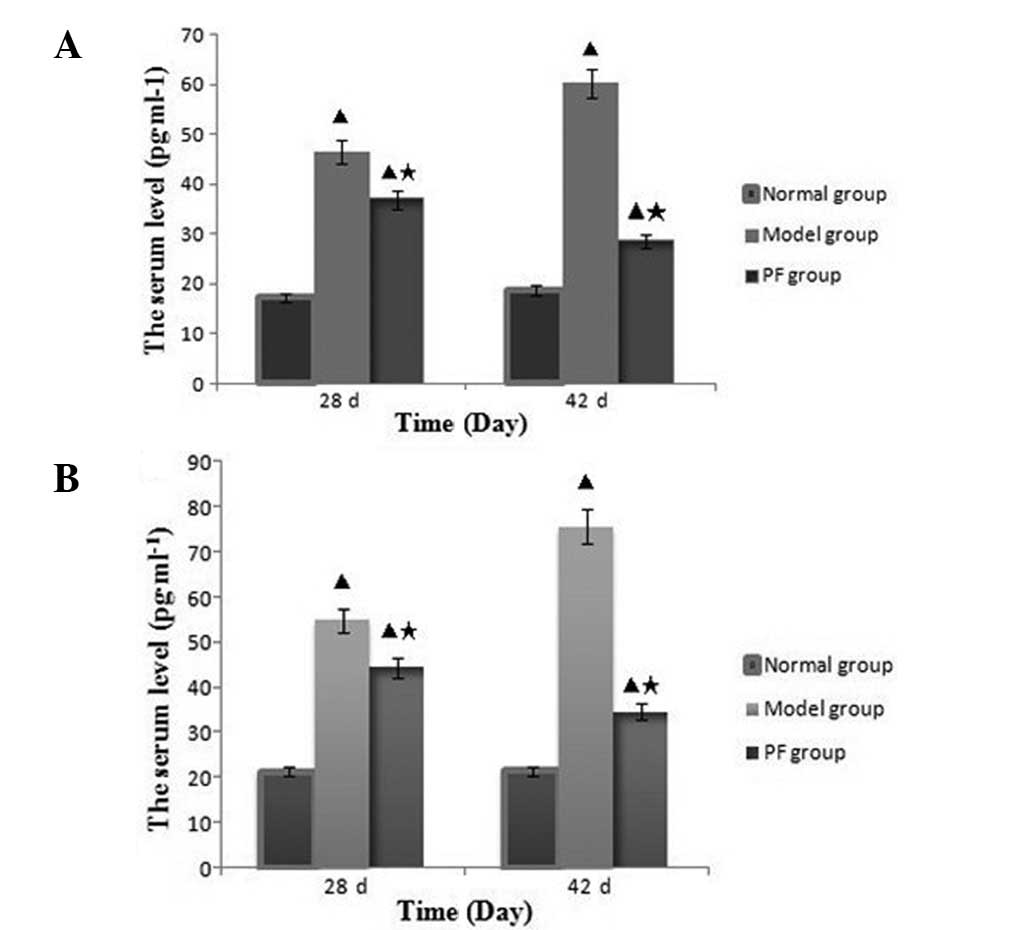
Levels of inflammatory cytokines in rat
serum
On day 28 following immunization (14 days following
treatment), the IL-1β and TNF-α serum levels of the model and PF
groups were markedly increased compared with those of the normal
group. However, at this time, the serum IL-1β and TNF-α levels in
the PF group were significantly decreased compared with those in
the model group (P<0.01). On day 42, the serum IL-1β and TNF-α
levels in the model group had increased further, while those of the
PF group had decreased; the differences in the IL-1β and TNF-α
levels between the groups of rats were significant at 28 and 42
days (P<0.01). Fig. 8 shows the
serum IL-1β and TNF-α levels in the normal, model and PF
groups.
Discussion
The development of modern analytical instruments and
technology indicates broad prospects for the determination of the
compositions of TCMs. In 2004, Waters Corporation introduced the
world’s first UPLC instrument. This revolutionary technology
eliminated the limitations of traditional chromatographic analysis
systems, solving the problem of pressure proofing the whole system.
In addition, UPLC has a high column efficiency, which increases the
degree of separation, speed of analysis and detection sensitivity
to unprecedented degrees (17,18).
UPLC not only improves the quality of analysis results, it also
reduces the running time and the quantity of solvent ≥10-fold,
thereby saving considerable time, money, energy and space, and
improving the efficiency overall (19,20).
As a result, UPLC plays a vital role in the pharmaceutical and
biomedical fields (21,22). The combined UPLC-PDA detector is a
convenient and widely used technology utilized for the routine
detection of a variety of components of plant-based drugs. UPLC is
now considered to be one of the most promising analysis techniques
in the chromatographic field. For the separation and determination
of the effective components of TCMs, UPLC has certain advantages
compared with conventional HPLC, including high speed, high
sensitivity, high resolution, time saving and reduced consumption
(23). In the present study, the
detection of paeoniflorin in a Radix Paeoniae Alba decoction by a
UPLC-PDA method was completed in <4 min. This separation time is
lower than the previously reported time of ~4 min (24). The experiments in which Radix
Paeoniae Alba decoction was administered to rats indicate that
paeoniflorin is an active ingredient of the decoction that is
absorbed by rat plasma. To the best of our knowledge, this has not
been reported previously.
RA in TCM belongs to the Chinese medicine
‘arthralgia category’. TCM theory considers RA to be an asthenic
disease, including deficiency and excess mixing as the main
manifestations (25). TCM has a
long history and rich resources in the treatment of RA. In previous
years, a number of studies have shown that specific TCM components
are useful in the treatment of RA (26). These components have been reported
to downregulate cytokine expression, alleviate inflammation in RA,
inhibit fibroblast-like synoviocytes (FLS) proliferation and
promote apoptosis, thereby reducing synovial hyperplasia,
inhibiting human chondrocyte and cartilage degradation and
preventing joint bone destruction; multiple targets and pathways in
RA are affected (26). The
analysis of the effects of TCM components also provides a
theoretical basis for studies of the mechanisms of Chinese herbal
compounds, which contain these effective components.
BZXD is a TCM that was formulated by our department.
It has been used clinically for a number of years to treat RA and
has good clinical efficacy (2).
Previous studies have shown that BZXD is involved in the regulation
of the immune system, the inhibition of inflammatory cytokines,
synovial angiogenesis and bone destruction, and the adjustment of
the abnormal expression of genes and proteins (3–8). The
chemical constituents of TCMs are complex and the majority are not
absorbed by the body following oral administration. The functioning
component is the material that is absorbed into the blood, i.e.,
the transitional ingredients in the serum. A previous study on the
serum pharmacochemistry of TCM considered that only the ingredients
that are absorbed into the blood are the effective components
(27). The current study analyzed
rat plasma following the oral administration of Radix Paeoniae Alba
decoction and demonstrated that paeoniflorin is an active
ingredient that is absorbed by rat plasma. These observations
indicate that paeoniflorin is likely to be one of the active
substances of BZXD and may play a vital role in the treatment of
RA. At present, studies concerning the measurement of the
paeoniflorin content of plasma are limited.
Radix Paeoniae Alba is an important component of
BZXD. It has a number of functions, including nourishing the blood
and liver, reducing pain and astringing Yin sweat (9). The effective components of Radix
Paeoniae Alba mainly include a series of aminoglycoside substances,
including paeoniflorin, hydroxy-paeoniflorin, peony glucoside,
albiflorin and benzoylpaeoniflorin, which are collectively referred
to as TGP. In Radix Paeoniae Alba, paeoniflorin accounts for
>90% of the total glucosides and is the main effective
component. Numerous studies on the pharmacological effect of
paeoniflorin have been performed, which have revealed that
paeoniflorin functions as an anti-free radical agent, exhibiting
antineurotoxic properties and inhibiting intracellular calcium
overload (28). In vivo
studies have shown that paeoniflorin has numerous biological
effects, including blood viscosity reduction, the inhibition of
platelet aggregation, the dilation of blood vessels and improvement
of the microcirculation and functions as an antioxidant and
anticonvulsant with limited side-effects (29,30,31).
Studies concerning the pharmacological effects of paeoniflorin have
mainly focused on the effects it has on the nervous system
(32,33). With regard to the role of
paeoniflorin in RA, studies have shown that paeoniflorin has
significant inhibitory effects in rats with AA and rats with CIA
and secondary paw swelling, markedly improves the pain reaction
associated with polyarthritis and the paw of secondary arthritis,
and exhibits a regulatory effect on imbalanced inflammatory
cytokine secretion (34–36). However, there are also a small
number of studies indicating that paeoniflorin inhibits the bone
erosion associated with the pathological process of RA. In the
present study, rats with CIA were treated with paeoniflorin, which
led to significant reductions in the swelling, joint AI and the
levels of inflammatory cytokines, IL-1β and TNF-α. In addition, the
inflammatory reaction in rats with CIA was inhibited. IL-1β and
TNF-α play key roles in the synovitis and cartilage and bone
destruction of RA (37,38). IL-1β promotes osteoblast expression
and osteoclast absorption (4).
IL-1β is a major cytokine that amplifies the inflammatory response
of RA and transforms it into a damaging reaction. TNF-α is a
proinflammatory cytokine that is important for the pathogenesis of
RA; it is involved in the pathogenic mechanism of RA, including
endothelial cell activation, cytokine induction, white blood cell
aggregation, activation of osteoclasts and the destruction of
cartilage and bone cells (39) and
leads to a continued inflammatory reaction and the progressive
destruction of cartilage and bone.
The results of the present study show that
paeoniflorin reduced the levels of the inflammatory cytokine IL-1β
and TNF-α, and reduced the soft tissue swelling of the joints of
the arthritic rats. The degree of joint bone destruction decreased
significantly compared with that in the model group, indicating
that paeoniflorin may inhibit bone erosion in RA. These
observations provide a scientific basis for the use of paeoniflorin
in the treatment of RA, laying the foundations for future studies
into the mechanism of action of Radix Paeoniae Alba and providing a
new method for the further study of the biologically active
ingredients of the efficacy of BZXD. This method, which combines
the analysis of the effective components of a TCM with UPLC-PDA and
with intervention in animal models, is likely to provide a new
approach for the study of effective TCM components in the future.
In addition, it may aid the identification of new drugs for the
treatment of RA.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (no. 81102564), Ph.D. Programs
Foundation of Ministry of Education of China (no. 20110162120004),
Central South University Plans to Boost the Freedom to Explore
Young Teachers Project (no. 2011QNZT154) and Key-Discipline
Construct Programs of Hunan Province and SATCM.
References
|
1
|
Li RZ: The observation of pathological
manifestations (50 cases) and immunohistochemical (5 cases) of
rheumatoid arthritis. Zhonghua Gu Ke Za Zhi. 1:35–36. 1989.(In
Chinese).
|
|
2
|
Liang Q, Tang T and Zhang H: Clinical
investigation of effects of bizhongxiao decoction (BZX) on
rheumatoid arthritis on active phase. Hunan Yi Ke Da Xue Xue Bao.
25:449–452. 2000.(In Chinese).
|
|
3
|
Liang QH, Zhang HX and Tang T: The effects
of bizhongxiao decoction (BZX) on T-lymphocyte subsets in the
peripheral blood of patients with rheumatoid arthritis. Hunan Yi Ke
Da Xue Xue Bao. 26:534–536. 2001.(In Chinese).
|
|
4
|
Liang QH, Luo X, Chen CH, Wang AY, He JH,
Tan Y and Bao TC: A clinical observation on the effects of
Bizhongxiao Decoction on the interleulkin-1β level in the serum of
patients with rheumatoid arthritis. Hunan Zhong Yi Xue Yuan Xue
Bao. 6:43–45. 2003.(In Chinese).
|
|
5
|
Tang TF, Liang QH, Luo X, Chen J and Li
XL: Effects of bizhongxiao decoction on serum matrix
metalloproteinase 3 and its tissue inhibitors of metalloproteinase
1 in patients with rheumatoid arthritis at active phase. Zhongguo
Lin Chuang Kang. 9:114–116. 2005.(In Chinese).
|
|
6
|
Liang QH, Chen J, He JH, Li XL, Zhang HX,
Wang AY and Tan Y: Correlation between plasma contents of tumor
necrosis factor-α and expressions of vascular endothelium growth
factor in collagen II-induced arthritis rats. Zhonghua Feng Shi
Bing Xue Za Zhi. 7:655–658. 2003.(In Chinese).
|
|
7
|
Wang AY, Liang QH, Tang FQ, Li CY, Chen J,
Bao TC, Luo X and Yang B: Effect of bizhongxiao decoction on the
synovitis gene expression profiles of rats with collagen induced
arthritis: a study on the cDNA microarrays. Zhongguo Lin Chuang
Kang Fu. 9(11): 42–43. 2005.(In Chinese).
|
|
8
|
Yang B, Liang QH, Cai Y, Xie W, He JH and
Liu XC: Effect of bizhongxiao decoction on protein in synovitis of
rats with collagen-induced arthritis. Zhongguo Lin Chuang Kang Fu.
10(15): 74–78. 2006.(In Chinese).
|
|
9
|
Li WY, Huang SJ and Wang R: Advances in
research on pharmacological actions and quality control of Paeoniae
Radix Alba. Pharm Care Res. 12:118–122. 2012.(In Chinese).
|
|
10
|
State Pharmacopoeia Committee of the PRC.
Pharmacopoeia of the People’s Republic of China 2010. 1. China
Medical Science and Technology Press; Beijing: pp. 96–97. 2010, (In
Chinese).
|
|
11
|
Gao XR and Tian GY: Active principles of
Paeonia lactiflora Pall. Chinese Journal of New Drugs.
6:416–418. 2006.(In Chinese).
|
|
12
|
Hu N, Xu HY, Chen ZW and Xing GH: The
pharmacology research progress of Paeoniflorin. Qiqihar Yi Xue Yuan
Yuan Bao. 28:1093–1095. 2007.(In Chinese).
|
|
13
|
Wang XY, Wei W, Tang LQ, Wu Hong, Yang YQ
and Chen Y: Effect of paeoniflorin on peritoneal macrophage from
rats of adjuvant-induced arthritis. Anhui Yi Ke Da Xue Xue Bao.
42:189–192. 2007.(In Chinese).
|
|
14
|
Zhang LL, Wei W, Wang NP, Wang QT, Chen
JY, Chen Y, Wu H and Hu XY: Paeoniflorin suppresses inflammatory
mediator production and regulates G protein-coupled signaling in
fibroblast-like synoviocytes of collagen induced arthritic rats.
Inflamm Res. 57:388–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei W, Wu XM and Li YJ: Experimental
Methodology of Pharmacology. People’s Medical Publishing House;
Beijing: pp. 1698–1699. 2010, (In Chinese).
|
|
16
|
Hang XF, Ma BL, Zhang JY, Bai J, Wang L
and Hao AM: Establishment of autoimmune experimental animal model
for rheumatoid arthritis. Shanghai Mian Yi Xue Za Zhi. 21:330–333.
2001.(In Chinese).
|
|
17
|
Nguyen DT, Guillarme D, Heinisch S,
Barrioulet MP, Rocca JL, Rudaz S and Veuthey JL: High throughput
liquid chromatography with sub-2 microm particles at high pressure
and high temperature. J Chromatogr A. 1167:76–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nováková L, Matysová L and Solich P:
Advantages of application of UPLC in pharmaceutical analysis.
Talanta. 68:908–918. 2006.PubMed/NCBI
|
|
19
|
Wren SA and Tchelitcheff P: Use of
ultra-performance liquid chromatography in pharmaceutical
development. J Chromatogr A. 1119:140–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guillarme D, Nguyen DT, Rudaz S and
Veuthey JL: Method transfer for fast liquid chromatography in
pharmaceutical analysis: application to short columns packed with
small particle. Part II: gradient experiments. Eur J Pharm
Biopharm. 68:430–440. 2008. View Article : Google Scholar
|
|
21
|
Guan J, Lai CM and Li SP: A rapid method
for the simultaneous determination of 11 saponins in Panax
notoginseng using ultra performance liquid chromatography. J
Pharm Biomed Anal. 44:996–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han LF, Wu B, Pan GX, Wang YF, Song XB and
Gao XM: UPLC-PDA analysis for simultaneous quantification of four
active compounds in crude and processed rhizome of Polygonum
multiflorum Thunb. Chromatographia. 70:657–659. 2009.
View Article : Google Scholar
|
|
23
|
Wu T, Wang C, Wang X, Xiao HQ, Ma Q and
Zhang Q: Comparison of UPLC and HPLC for analysis of 12 phthalates.
Chromatographia. 68:803–806. 2008. View Article : Google Scholar
|
|
24
|
Lu XY, Chu C and Yan JZ: Simultaneous
determination of paeoniflorin and albiflorin in Radix Paeoniae Alba
by UPLC. Zhong Nan Yao Xue. 10:98–100. 2012.(In Chinese).
|
|
25
|
Zhang C and Yang ZS: TCM syndrome
differentiation and experience in rheumatoid arthritis. Zhongguo
Lin Chuang Yi Sheng Za Zhi. 35:66–67. 2007.(In Chinese).
|
|
26
|
Li JL, Sun L, Xi ZX, Li X and Sun LN:
Research development of traditional Chinese medicine monomer
composition on the treatment of rheumatoid arthritis. Zhong Yao
Cai. 35:1355–1360. 2012.(In Chinese).
|
|
27
|
Huang Y: Advances in methods of quality
control in Chinese materia medica formula. Yao Xue Shi Jian Za Zhi.
26:11–13. 2008.(In Chinese).
|
|
28
|
Sun R, Lv LL, Guo SD and Liu GQ: Study on
effects of paeoniflorin on blood brain barrier and pathological
changes during reperfusion of CMAO model in rats. Journal of Harbin
University of Commerce (Natural Sciences Edition). 4:405–410.
2005.(In Chinese).
|
|
29
|
Zheng SC, Li XY, Ou YB and Sun R: Research
development on pharmacological of paeoniflorin. Zhongguo Yao Wu
Jing Jie. 2:100–103. 2012.(In Chinese).
|
|
30
|
Ma W, Ma WD, Miao ZH and Tian JY:
Protective effect of paeoniflorin on the Aβ1–40-induced
neurotoxicity in cultured PC12 cells. Journal of Ningxia Medical
University. 2:132–135. 2011.(In Chinese).
|
|
31
|
Guo P, Wang JF and Wang SQ: Effects of
paeoniflorin on Epo and G-CSF gene expression in bone marrow of
irradiated blood deficiency mice. Journal of Shandong University of
TCM. 3:236–239. 2005.(In Chinese).
|
|
32
|
Sun R, Zuo YF, Li XY and Ou YB: Progress
of paeoniflorin against neuronal injury pharmacological research.
Shangdong Zhong Yi Yao Da Xue Xue Bao. 36:454–456. 2012.(In
Chinese).
|
|
33
|
Hu ZY, Xu L, Yan R, Huang Y, Liu G, Zhou
WX and Zhang YX: Advance in studies on effect of paeoniflorin on
nervous system. Zhongguo Zhong Yao Za Zhi. 38:297–301. 2013.(In
Chinese).
|
|
34
|
Liu ZQ, Jiang ZH, Chan K, Zhou H, Wong YF,
Bian ZX, Xu HX and Liu L: Pharmacokinetic interaction of
paeoniflorin and sinomenine: pharmacokinetic parameters and tissue
distribution characteristics in rats and protein binding ability in
vitro. J Pharmacol Sci. 99:381–391. 2005. View Article : Google Scholar
|
|
35
|
Chen LC, Chou MH, Lin MF and Yang LL:
Pharmacokinetics of paeoniflorin after oral administration of
Shao-yao Gan-chao Tang in mice. Jpn J Pharmacol. 88:250–255. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Gu Q, Qiu F and Zhong D: Rapid
determination of metformin in human plasma by liquid
chromatography-tandem mass spectrometry method. J Chromatogr B
Analyt Technol Biomed Life Sci. 802:377–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strand V and Kavanaugh AF: The role of
interleukin-1 in bone resorption in rheumatoid arthritis.
Rheumatology (Oxford). 43(Suppl 3): iii10–iii16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zwerina J, Hayer S, Tohidast-Akrad M,
Bergmeister H, Redlich K, Feige U, Dunstan C, Kollias G, Steiner G,
Smolen J and Schett G: Single and combined inhibition of tumor
necrosis factor, interleukin-1, and RANKL pathways in tumor
necrosis factor-induced arthritis: effects on synovial
inflammation, bone erosion, and cartilage destruction. Arthritis
Rheum. 50:277–290. 2004. View Article : Google Scholar
|
|
39
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|