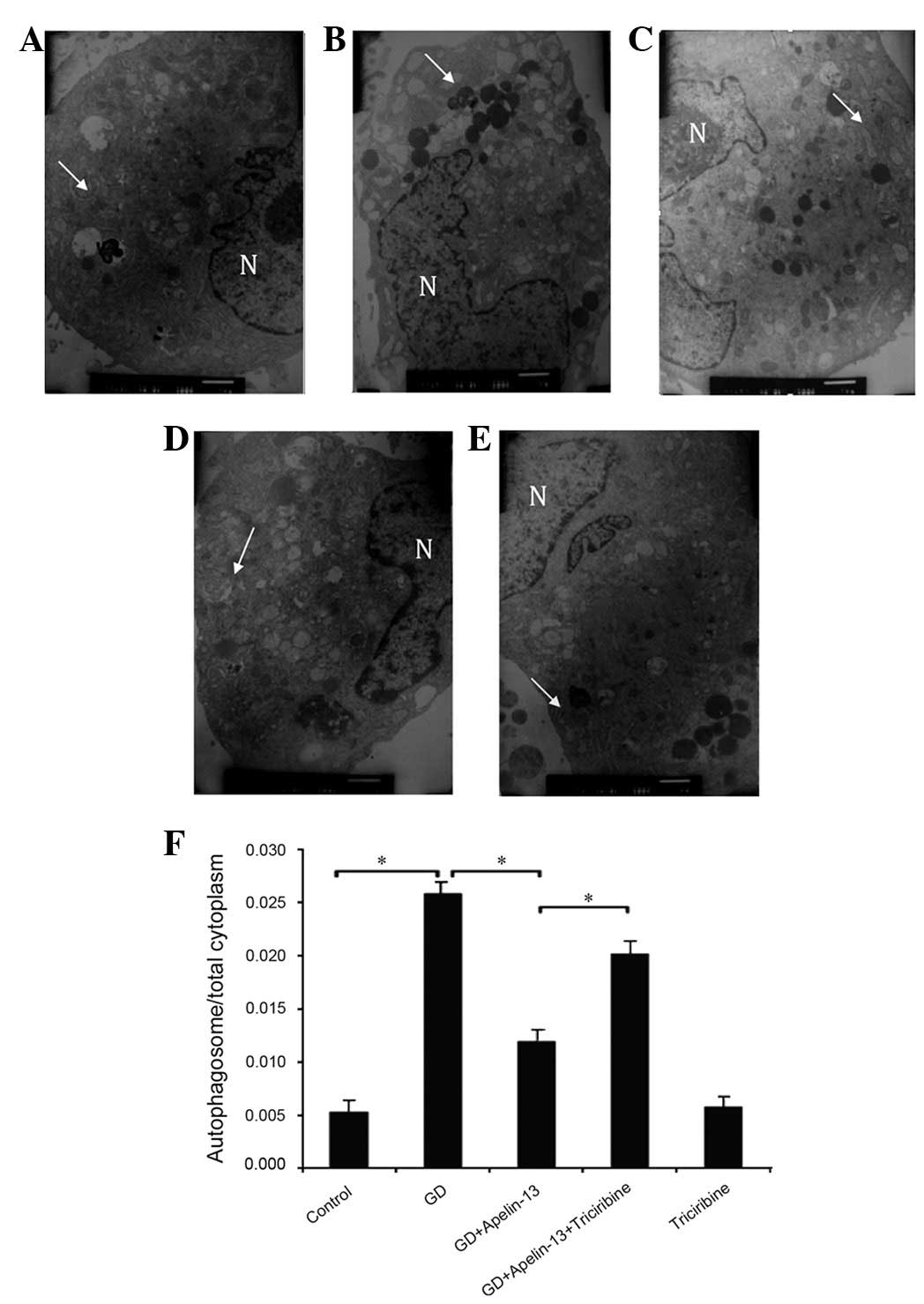
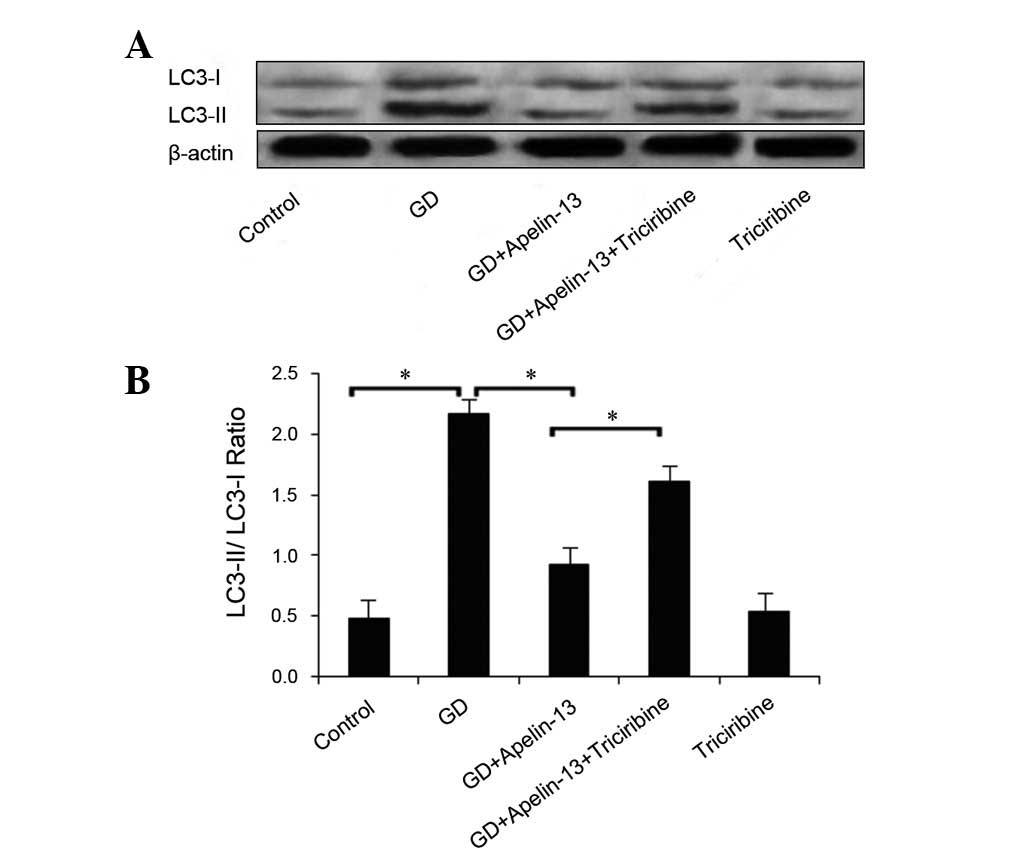
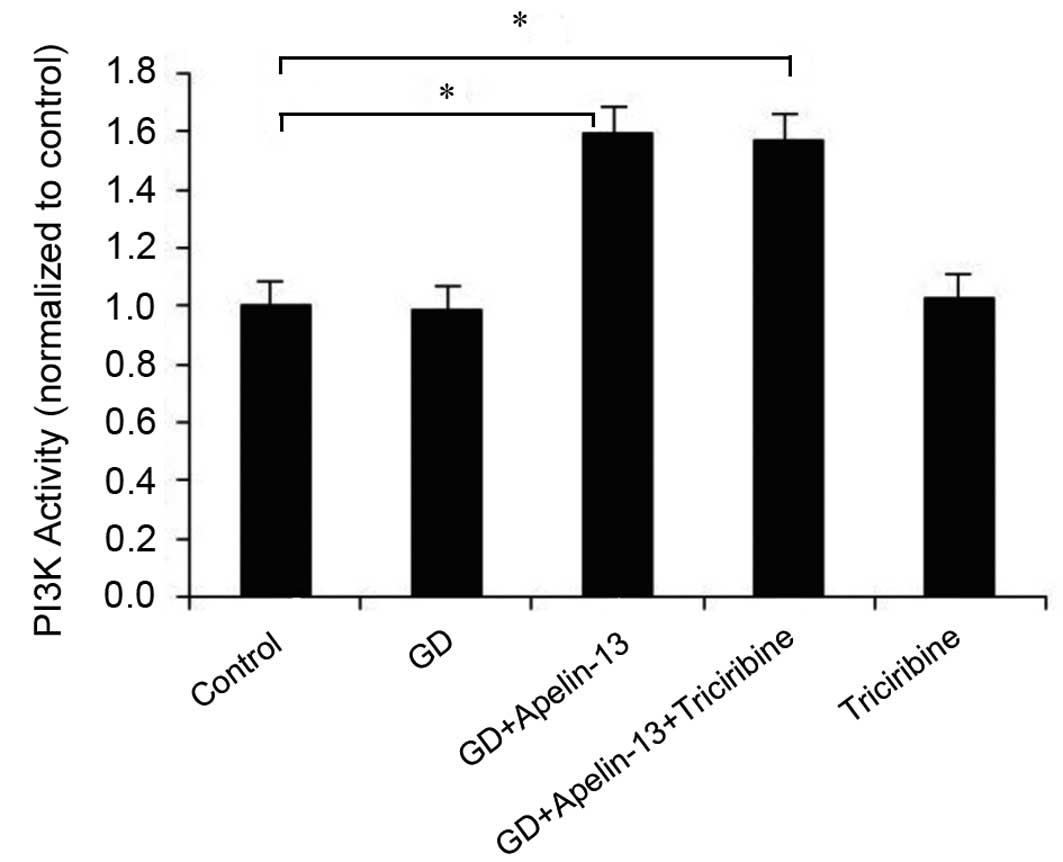
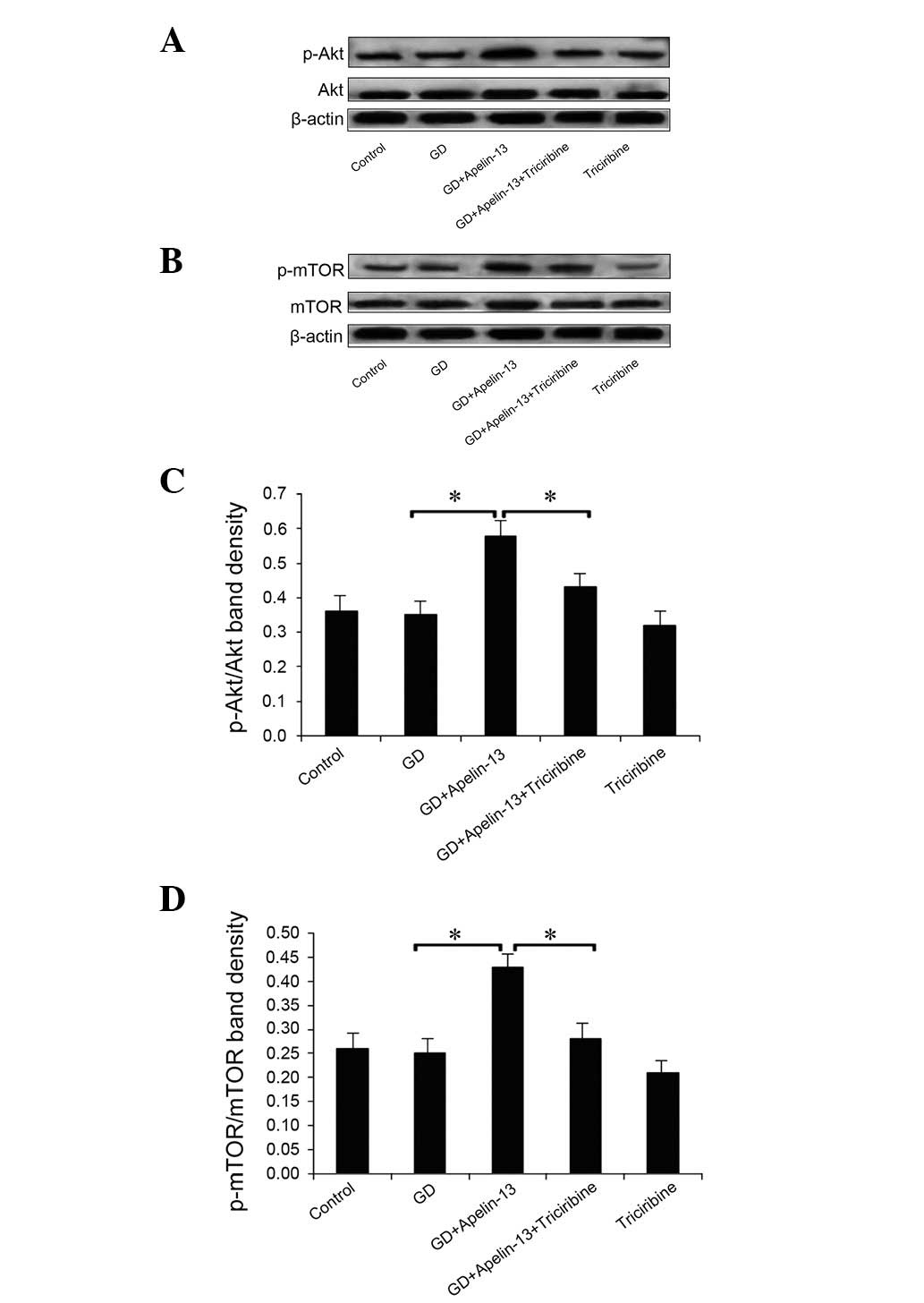
|
1.
|
Matsui Y, Takagi H, Qu X, et al: Distinct
roles of autophagy in the heart during ischemia and reperfusion:
roles of AMP-activated protein kinase and Beclin 1 in mediating
autophagy. Circ Res. 100:914–922. 2007. View Article : Google Scholar
|
|
2.
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sorli SC, van den Berghe L, Masri B,
Knibiehler B and Audigier Y: Therapeutic potential of interfering
with apelin signalling. Drug Discov Today. 11:1100–1106. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Dutta D, Calvani R, Bernabei R,
Leeuwenburgh C and Marzetti E: Contribution of impaired
mitochondrial autophagy to cardiac aging: mechanisms and
therapeutic opportunities. Circ Res. 110:1125–1138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Quazi R, Palaniswamy C and Frishman WH:
The emerging role of apelin in cardiovascular disease and health.
Cardiol Rev. 17:283–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tycinska AM, Lisowska A, Musial WJ and
Sobkowicz B: Apelin in acute myocardial infarction and heart
failure induced by ischemia. Clin Chim Acta. 413:406–410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ren X and Zhang Z: Effect of Apelin-13 on
glucose deprivation-induced rat myocardial cell autophagy.
Guangdong Yi Xue. 32:3176–3177. 2011.(In Chinese).
|
|
8.
|
Zhu S, Sun F, Li W, et al: Apelin
stimulates glucose uptake through the PI3K/Akt pathway and improves
insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem.
353:305–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tatemoto K, Hosoya M, Habata Y, et al:
Isolation and characterization of a novel endogenous peptide ligand
for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhang Z, Yu B and Tao GZ: Apelin protects
against cardiomyocyte apoptosis induced by glucose deprivation.
Chin Med J (Engl). 122:2360–2365. 2009.PubMed/NCBI
|
|
11.
|
Xin Y, Xu X and Huang Y: Isolation,
primary culture and identification of cardiac fibroblasts and
cardiac myocytes of neonatal mouse. Xinjiang Yi Xue Yuan Xue Bao.
28:541–547. 2011.(In Chinese).
|
|
12.
|
Folli F, Saad MJ, Backer JM and Kahn CR:
Insulin stimulation of phosphatidylinositol 3-kinase activity and
association with insulin receptor substrate 1 in liver and muscle
of the intact rat. J Biol Chem. 267:22171–22177. 1992.PubMed/NCBI
|
|
13.
|
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA
and Leeuwenburgh C: Skeletal muscle autophagy and apoptosis during
aging: effects of calorie restriction and life-long exercise. Exp
Gerontol. 45:138–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tatemoto K: Search for an endogenous
ligand of the orphan G protein-coupled receptor - discovery of
apelin, a novel biologically active peptide. Nihon Rinsho.
58:737–746. 2000.(In Japanese).
|
|
15.
|
Galanth C, Hus-Citharel A, Li B and
Llorens-Cortès C: Apelin in the control of body fluid homeostasis
and cardiovascular functions. Curr Pharm Des. 18:789–798. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tempel D, de Boer M, van Deel ED, et al:
Apelin enhances cardiac neovascularization after myocardial
infarction by recruiting aplnr+ circulating cells. Circ Res.
111:585–598. 2012.PubMed/NCBI
|
|
17.
|
Balasis ME, Forinash KD, Chen YA, et al:
Combination of farnesyltransferase and Akt inhibitors is
synergistic in breast cancer cells and causes significant breast
tumor regression in ErbB2 transgenic mice. Clin Cancer Res.
17:2852–2862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dieterle A, Orth R, Daubrawa M, et al: The
Akt inhibitor triciribine sensitizes prostate carcinoma cells to
TRAIL-induced apoptosis. Int J Cancer. 125:932–941. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chen Y, Azad MB and Gibson SB: Methods for
detecting autophagy and determining autophagy-induced cell death.
Can J Physiol Pharmacol. 88:285–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Martinet W, De Meyer GR, Andries L, Herman
AG and Kockx MM: In situ detection of starvation-induced autophagy.
J Histochem Cytochem. 54:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Arad M, Seidman CE and Seidman JG:
AMP-activated protein kinase in the heart: role during health and
disease. Circ Res. 100:474–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hardie DG: AMP-activated/SNF1 protein
kinases: conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yu L, McPhee CK, Zheng L, et al:
Termination of autophagy and reformation of lysosomes regulated by
mTOR. Nature. 465:942–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Gottlieb RA, Finley KD and Mentzer RM Jr:
Cardioprotection requires taking out the trash. Basic Res Cardiol.
104:169–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Cuervo AM: Autophagy: in sickness and in
health. Trends Cell Biol. 14:70–77. 2004. View Article : Google Scholar : PubMed/NCBI
|