Introduction
Biofilms are surface-attached colonies of bacteria
on various biotic and abiotic surfaces, encased in a hydrated
matrix of exopolymeric substances, proteins, polysaccharides and
nucleic acids. Biofilms play a major role in the pathogenesis of
device-associated infections. Biofilm formation is a complex
developmental process (1–4). Surface-attached bacterial biofilms
are considered to be a significant source of nosocomial infections,
which are responsible for intravascular device-associated
bacteremias and ventilator-associated pneumonia (5–7).
Biofilm formation allows for immune evasion and resistance to
antibiotics since the bacteria in a biofilm are protected by a
matrix, and the slow rate of metabolism of cells enables them to
survive 100- to 1000-fold concentrations of antibiotics (8–10).
Heavy antibiotic use has greatly increased antibiotic resistance
due to genetic mutation and this problem is continually increasing
in severity (10,11). Recently, much attention has been
focused on the need for new antimicrobial agents (12–16).
For the majority of pathogens, iron (Fe) is
essential for growth and the functioning of key enzymes, including
those involved in DNA synthesis, electron transport and oxidative
stress defense (17). The
post-transition metal gallium (Ga) has an ionic radius almost
identical to that of Fe, and numerous organisms are unable to
distinguish Ga3+ from Fe3+. Ga is able to
disrupt Fe-dependent processes since, unlike Fe3+,
Ga3+ cannot be reduced under physiological conditions,
and sequential oxidation and reduction are critical for many of
Fe’s biological functions. In vitro and in vivo
studies show that Ga3+ inhibits the growth and biofilm
formation of various pathogens by interfering with Fe signaling
(18).
Numerous polymers, such as polyvinyl chloride (PVC),
are widely used biomaterials for cardiovascular and other
artificial devices. In this study, we developed a new Ga-based
coating material suitable for PVC. We demonstrated that following
the surface treatment of PVC with a Ga coating, biofilm formation
may be inhibited.
Materials and methods
Bacterial strains and culture
Pseudomonas aeruginosa was cultured in LB
liquid medium (consisting of 10.0 g/l peptone, 5.0 g/l yeast
extract and 10.0 g/l NaCl, pH 7.0–7.2) and incubated at 37°C with
shaking (180 rpm) for 24 h. The inoculum was collected and diluted
with normal saline.
Streptococcus pyogenes was cultured in
Müller-Hinton (MH) medium supplemented with 10% (v/v) sheep blood
and incubated at 36°C with shaking (120 rpm) for 6 h. The inoculum
was collected and diluted with normal saline.
Minimum inhibitory concentration (MIC)
determination
Overnight cultures (37°C) of the tested strains were
diluted 10-fold in fresh tryptic soy broth (TSB) and incubated
(37°C) until the exponential growth phase was reached. The inocula
(10 μl) containing 5×106 CFU/ml of each reference
strain were added to each well in the presence or absence of
gallium nitrate at different concentrations. Certain wells were
reserved in each plate to test the sterility control of the medium
(no inoculum added) and inoculum viability (no gallium nitrate
added). The turbidity of the medium was directly proportional to
the growth of the bacteria, which was measured by a microtiter
plate reader (Tecan, Milan, Italy) at 600 nm absorbance. After
incubation for 24 h at 37°C, non-adherent bacteria were washed
three times with 0.9% (w/v) NaCl. The biofilms were stained with
0.1% (w/v) crystal violet solution for 10 min, washed and then
dissolved with 33% (v/v) acetic acid for 10 min. The biofilm mass
was determined by measuring the absorbance at 590 nm using a
microtiter plate reader. The MIC was defined as the concentration
that completely inhibited visible cell growth during a 24-h
incubation period at 37°C.
Surface coating
The gelatin aqueous solution (0.5%) was auto-claved.
After cooling, 50 μl gelatin solution was mixed with gallium
nitrate (Sigma, Bornem, Belgium) and a stock solution of a
chelator. The final concentrations of gallium nitrate and various
chelators are shown in Table I.
The mixture was added to the PVC 96-well plate and left to dry at
37°C in a sterile incubator overnight. The wells were then washed
using saline to remove loosely attached Ga from the surface of the
wells.
 | Table ICompositions of gallium (Ga) and
ligands, and inhibition of biofilm formation. |
Table I
Compositions of gallium (Ga) and
ligands, and inhibition of biofilm formation.
| Ligand | Molar ratio
(ligand:Ga) | Ga remaining (%) | Inhibitory index
(%) |
|---|
| EDTA | 1:1 | 50 | 41 |
| 2:1 | 97 | 99 |
| 3:1 | 100 | 70 |
| 4:1 | 100 | 43 |
| EGTA | 1:1 | 39 | 35 |
| 2:1 | 92 | 87 |
| 3:1 | 100 | 72 |
| 4:1 | 100 | 67 |
| EDTA+EGTA | 1:1:1 | 94 | 89 |
| EDTA+EGTA | 1:2:1 | 100 | 67 |
| EDTA+salicylic
acid | 1:1:1 | 81 | 77 |
| EDTA+salicylic
acid | 1:2:1 | 96 | 81 |
| EDTA+chloride
anion | 1:1:1 | 66 | 68 |
| EDTA+chloride
anion | 1:2:1 | 85 | 70 |
Antimicrobial durability
The antimicrobial efficacy of the Ga-coated PVC chip
was assessed over time in a system that imitated the blood stream
flushing through the surface of the endovascular medical device.
PVC chips of identical surface area were dipped into the
Ga-chelator-gelatin solution and allowed to dry twice. The chips
were then placed in a container through which water flowed at a
speed of 2 cm/sec. After 3 days, the amount of Ga remaining on the
Ga-coated PVC chip was measured and its antimicrobial efficacy was
evaluated.
The inhibitory index of the Ga coat=[1–(absorbance
of the biofilm on the assay chips/absorbance of the biofilm on the
negative control chips)]×100%.
To determine the amount of Ga remaining after
washing, the PVC chips were burned to ash in a crucible. The
content of Ga in the ash was analyzed by back titration with a
standard zinc solution.
Analysis of the Ga cation content by back
titration with a standard zinc solution
The coated PVC chips treated with the flow-erosion
system were burned to ash in a crucible. The ash was dissolved in
aqueous hydrochloric acid solution (2 M) over heat. The solution
was then transferred into a volumetric flask and the volume was
standardized to 50 ml and homogenized well. An excess of
ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA) solution was
added to 10 ml of the solution and the pH was adjusted to 5.8 with
hexamethylenetetramine buffer. Xylenol orange (5 drops) was added
as an indicator.
A zinc standard solution was used for back titration
to determine the Ga content. The PVC chips not subjected to water
erosion were used as a positive control.
Static biofilm formation
A static biofilm formation assay was carried out in
24-well PVC microtiter plates (Falcon; Becton Dickinson Labware,
Oxnard, CA, USA). Briefly, overnight bacteria cultures were diluted
to ∼1×106 CFU/ml with fresh sterile medium. Aliquots
(100 μl) of the diluted cultures were added to each well
pre-coated with gelatin in the presence of different concentrations
of Ga. The plates were incubated at 37°C for 24 h without agitation
for biofilm growth and non-adherent bacteria were washed away three
times with 0.9% (w/v) NaCl. The biofilms were strained with 0.1%
(w/v) crystal violet solution for 10 min and washed three times.
Adherent bacterial cells were observed by phase contrast
microscopy.
Isopropanol (150 μl)-0.04 M HCl and 50 ml of
0.25% SDS were added to each well to resolubilize crystal violet.
The biofilm mass was determined by measuring the absorbance at 590
nm using the microtiter plate reader. Assays were carried out three
times in five replicates.
Statistical analysis
Statistical analysis was performed using a paired
Student’s t-test, with Bonferroni correction, or one sample
Student’s t-test. P<0.05 was considered to indicate a
statistically significant result.
Results
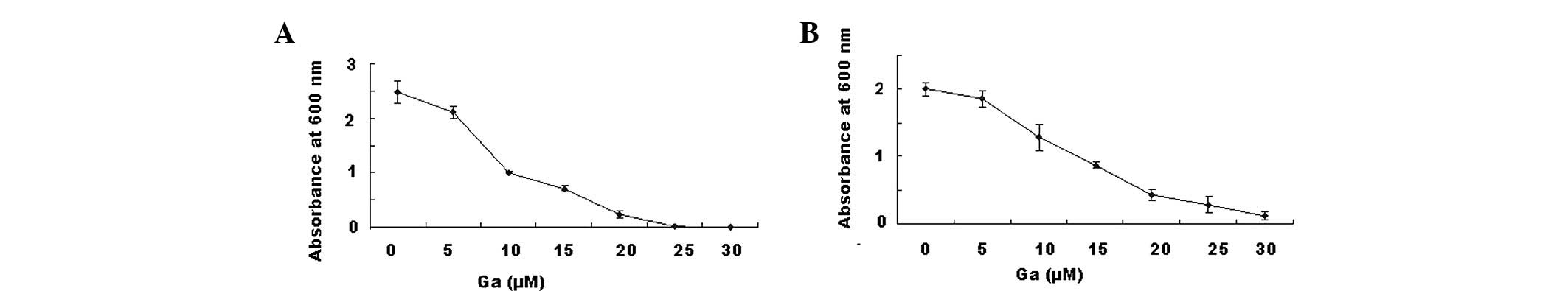
Determination of MIC
Pseudomonas aeruginosa (Fig. 1A) and Streptococcus pyogenes
(Fig. 1B) were grown in 96-well
microplates with medium containing gallium nitrate at
concentrations ranging from 5 to 30 μM. The plates were
incubated at 37°C for 24 h with agitation. The turbidity of the
medium was directly proportional to the growth of the bacteria,
which was measured by a microtiter plate reader at 600 nm
absorbance. As shown in Fig. 1,
gallium nitrate inhibited the growth of bacteria in a
dose-dependent manner, with a MIC of ∼20 μM.
Effects of different ligands on gallium
nitrate adhesion
To select the most effective chelator to prevent Ga
cations on the surface of the medical device from being eroded by
the blood stream, we fixed Ga cations onto the surface with various
ligands. The efficiency was judged by the amount of the Ga
remaining in the coat and the antibacterial activity of the
remaining Ga coat following the erosion of the PVC chip for 3 days.
The Ga coat not subjected to water flow was used as a control to
measure the amount of Ga and the antibacterial activity of the Ga
coat.
We investigated commercial ligands including EDTA,
ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid
(EGTA), salicylic acid and chloride anion in various ratios with
respect to the Ga cation. As demonstrated in Table I, when the concentration was kept
at 20 nM, a mixture of EDTA and Ga at a ratio of 2:1 in the
gelatine coat effectively inhibited bacterial growth. After
flushing for 3 days, 97% of the initial amount of Ga cation
remained on the surface and inhibited biofilm formation by 99%
compared with that observed on a negative control PVC chip coated
with gelatin and EDTA.
When the ratio of EDTA to Ga was >2:1, although
100% Ga remained in the gelatin, the inhibitory index was low.
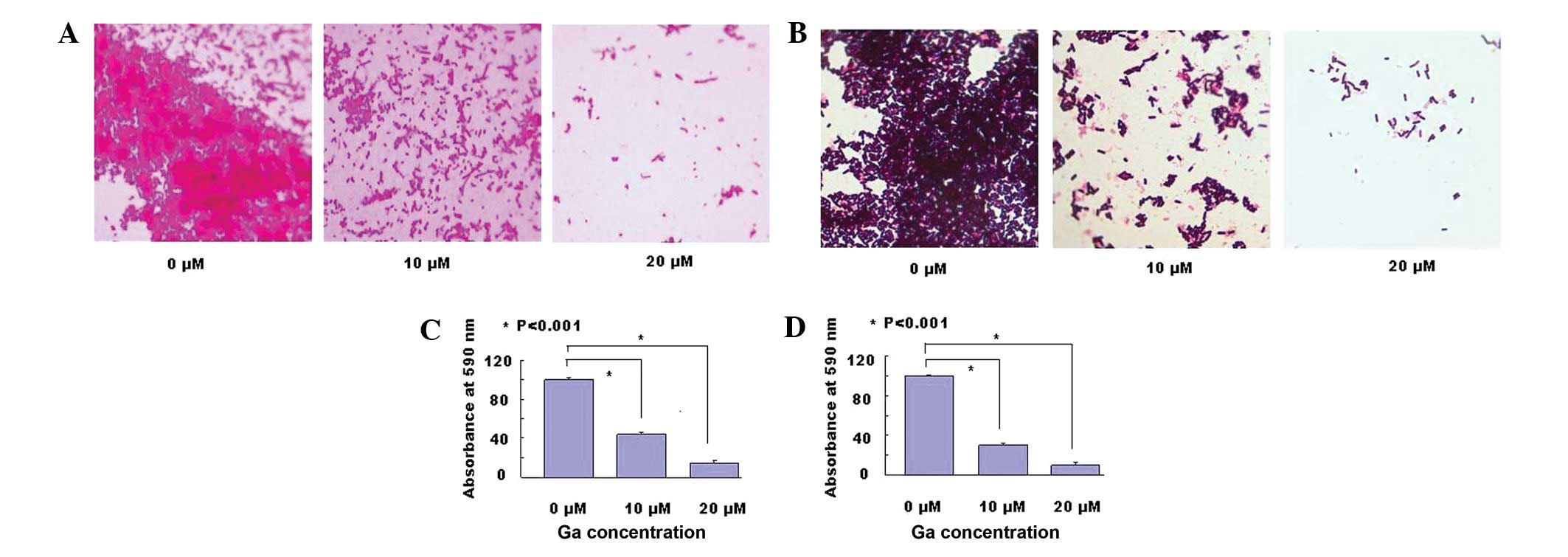
Biofilm inhibition
To assess the ability of surface pre-treatment with
Ga to inhibit bacterial biofilm formation, Pseudomonas
aeruginosa or Streptococcus pyogenes cell suspensions
were placed into Ga-treated plates and cultured at 37°C for 24 h
without agitation. Thereafter, the plates were washed and stained
with crystal violet, before visualization under a phase contrast
microscope. As demonstrated in Fig. 2A
and B, a clear decrease of the biofilm with crystal violet
staining was observed in the wells pre-treated with Ga.
In addition, after the bacteria were cultured, the
unattached bacteria were washed away. The remaining biofilm was
stained by crystal violet and dissolved with acetic acid. The
biofilm mass was determined by measuring the absorbance at 590 nm
using a microtiter plate reader. As Fig. 2C and D demonstrate, the biofilms of
Pseudomonas aeruginosa and Streptococcus pyogenes
were reduced by 85% and 90%, respectively, at 20 μM Ga.
Discussion
An increasing number of clinical procedures require
the use of biomedical devices. These devices, used in either the
short- or long-term, are associated with device-associated
infections due to bacterial colonization and proliferation
(19). It is estimated that ∼half
of the 2 million cases of nosocomial infections that occur each
year in the United States are caused by indwelling devices
(20). These infections result in
morbidity and mortality of the patients and increased costs for the
healthcare system. This is especially true in orthopedic medicine,
where biofilms may result in serious complications involving
prosthetic and other medical device infections, impacting
morbidity, mortality and medical costs (21,22).
The bacteria in a biofilm are much more resistant to antibiotics
than their planktonic form. Implants increase the risk of infection
100,000-fold, possibly due to the impairment of the microbiocidal
activities of granulocytes (23).
Biofilm formation is a complex developmental process
involving attachment and immobilization on a surface, cell-to-cell
interaction, microcolony formation, formation of a confluent
biofilm and development of a three-dimensional biofilm structure.
Bacterial biofilms are able to form on either biotic or abiotic
surfaces (1–4). The removal of biofilms from the
infected site poses a great challenge to the physicians since it
usually requires surgical intervention and radical antimicrobial
therapy (20).
Due to the resistance of biofilms to antibiotics,
new strategies to increase the sensitivity of pathogens in biofilms
or new bacteria-killing agents are needed. The replacement of
Fe3+ with Ga3+ interferes with bacterial DNA
and protein synthesis, and blocks the redox reactions that depend
on Fe electron acquisition. The replacement of Fe has been
demonstrated to inhibit Pseudomonas aeruginosa growth and
biofilm formation and kill planktonic and biofilm bacteria in
vitro. In the present study, we further evaluated the effects
of a Ga-coating on biofilm formation on PVC, a material often used
for medical implants. We identified several ligands that were
suitable for retaining Ga in a gelatin coat on the surface of a PVC
plate. The results indicated that a mixture of EDTA and Ga in a 2:1
ratio in gelatin was the most effective for retaining Ga on the PVC
surface. After flushing for 3 days, 97% Ga remained on the surface
and biofilm formation was almost completely inhibited. The average
velocity of the blood stream in the main veins, including the
inferior vena cava, pulmonary vein, portal vein, right femoral vein
and left femoral vein, is ∼0.35 cm/sec, and the speed may reach up
to 0.8 cm/sec following alcohol consumption. Therefore we used a
water-flow speed of 2 cm/sec to erode the coat of the PVC
chips.
Ga is already FDA-approved for the treatment of
hyper-calcemia of malignancy. As Pseudomonas aeruginosa may
cause acute and chronic lung infections and result in significant
morbidity and mortality in patients with cystic fibrosis, and
Streptococcus pyogenes is one of the main pathogens
responsible for respiratory infections and rheumatic heart disease,
an EDTA-Ga-gelatin coating on implants may be a potential strategy
in the prevention of infections associated with medical
devices.
References
|
1
|
O’Toole G, Kaplan HB and Kolter R: Biofilm
formation as microbial development. Annu Rev Microbiol. 54:49–79.
2000.PubMed/NCBI
|
|
2
|
Costerton JW, Stewart PS and Greenberg EP:
Bacterial biofilms: a common cause of persistent infections.
Science. 284:1318–1322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall-Stoodley L and Stoodley P: Evolving
concepts in biofilm infections. Cellular Microbiol. 11:1034–1043.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart PS and Costerton JW: Antibiotic
resistance of bacteria in biofilms. Lancet. 358:135–138. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adair CG, Gorman SP, Feron BM, et al:
Implications of endotracheal tube biofilm for ventilator-associated
pneumonia. Intensive Care Med. 25:1072–1076. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koerner RJ: Contribution of endotracheal
tubes to the pathogenesis of ventilator-associated pneumonia. J
Hosp Infect. 35:83–89. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campoccia D, Montanaro L and Arciola CR:
The significance of infection related to orthopedic devices and
issues of antibiotic resistance. Biomaterials. 27:2331–2339. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raad II and Hanna HA: Intravascular
catheter-related infections: new horizons and recent advances. Arch
Intern Med. 162:871–878. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donlan RM: Biofilms and device-associated
infections. Emerg Infect Dis. 7:277–281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mah TF and O’Toole GA: Mechanisms of
biofilm resistance to antimicrobial agents. Trends Microbiol.
9:34–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mack D, Nedelmann M, Krokotsch A,
Schwarzkopf A, Heesemann J and Laufs R: Characterization of
transposon mutants of biofilm-producing Staphylococcus
epidermidis impaired in the accumulative phase of biofilm
production: genetic identification of a hexosamine-containing
polysaccharide intercellular adhesin. Infect Immun. 62:3244–3253.
1994.PubMed/NCBI
|
|
12
|
Bavington C and Page C: Stopping bacterial
adhesion: a novel approach to treating infections. Respiration.
72:335–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ofek I, Hasty DL and Sharon N:
Anti-adhesion therapy of bacterial diseases: prospects and
problems. FEMS Immunol Med Microbiol. 38:181–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinberg D, Feldman M, Ofek I and Weiss
EI: Effect of a high-molecular weight component of cranberry on
constituents of dental biofilm. J Antimicrob Chemother. 54:86–89.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beckloff N, Laube D, Castro T, et al:
Activity of an antimicrobial peptide mimetic against planktonic and
biofilm cultures of oral pathogens. Antimicrob Agents Chemother.
51:4125–4132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng X, Kawahara KI, Miyanohara H, et al:
1,5-Anhydro-D-fructose: A natural antibiotic that inhibits the
growth of gram-positive bacteria and microbial biofilm formation to
prevent nosocomial infection. Exp Ther Med. 2:625–628.
2011.PubMed/NCBI
|
|
17
|
Bullen JJ, Rogers HJ, Spalding PB and Ward
CG: Iron and infection: the heart of the matter. FEMS Immunol Med
Microbiol. 43:325–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaneko Y, Thoendel M, Olakanmi O, et al:
The transition metal gallium disrupts Pseudomonas aeruginosa
iron metabolism and has antimicrobial and antibiofilm activity. J
Clin Invest. 117:877–888. 2007.PubMed/NCBI
|
|
19
|
Vasilev K, Cook J and Griesser HJ:
Antibacterial surfaces for biomedical devices. Expert Rev Med
Devices. 6:553–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Darouiche RO: Treatment of infections
associated with surgical implants. N Engl J Med. 350:1422–1429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Busscher HJ, Rinastiti M, Siswomihardjo W
and van der Mei HC: Biofilm formation on dental restorative and
implant materials. J Dent Res. 89:657–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arciola CR, Campoccia D, Speziale P,
Montanaro L and Costerton JW: Biofilm formation in
Staphylococcus implant infections. A review of molecular
mechanisms and implications for biofilm-resistant materials.
Biomaterials. 33:5967–5982. 2012.PubMed/NCBI
|
|
23
|
Jensen PO and Tolker-Nielsen T: Report
from Eurobiofilms 2011. Future Microbiology. 6:1237–1245. 2011.
View Article : Google Scholar
|