Introduction
Choroidal neovascularization (CNV) is the main cause
of macular diseases, including age-related macular degeneration
(AMD), highly myopic maculopathy and impaired vision. The toughness
of new vessels is extremely poor. These vessels usually break
through Bruch’s membrane to enter the subretinal space, thereby
causing exudation or hemorrhage, retinal tissue damage and rapid
loss of vision, as well as inducing pigmentary epithelial
detachment or retinal neuroepithelial detachment. CNV angiopoiesis
is a series of complex pathological processes, including
endothelial cell activation, extracellular matrix changes, basement
membrane degradation, budding, proliferation and migration of
endothelial cells, capillary loop formation and lumen transfixion.
Although the angiopoiesis mechanism remains unclear, its processes
are affected by a number of positive or negative regulation
factors. Among them, the receptor tyrosine kinase (RTK) system is
an important gene family and three vital RTKs are involved in CNV
formation. They are the vascular endothelial growth factor (VEGF)
and VEGF receptor (VEGFR), angiogenin/Tie receptor and Ephrin/Eph
receptor systems. The VEGF/VEGFR system mainly induces vascular
endothelial differentiation and angiopoiesis, whereas the
angiogenin/Tie receptor system mainly regulates vascular maturation
and quiescence in the later angiopoietic stages. The Ephrin/Eph
receptor system affects the developing vascular endothelial cells
and may play an important role in the neovascularization assembly
process by transmitting a bidirectional signal, transferring
position guide information and controlling asymmetric arteriovenous
establishment (1).
The corresponding Ephrin ligand and Eph receptor
expression in the neovascularization of ocular tissues, including
the cornea, retina and choroid, as well as in vitro and
in vivo animal experiments, all suggest that the Ephrin/Eph,
VEGF/VEGFR and angiogenin/Tie receptor systems are all involved in
the development and progression of ocular neovascularization.
Although the VEGF/VEGFR system intervention has achieved a degree
of efficacy in terms of its potential application value in the
clinical treatment of relevant ocular neovascularization diseases
(2,3), the results are not completely
satisfactory. EphrinB2/EphB4 is the specific growth factor of
vascular endothelial cells in the Ephrin/Eph family. EphrinB2
ligand and EphB4 receptor expression is definitely present in the
vasa sanguinea retinae and choroidal vessels of rats, mice
and cattle. Furthermore, EphrinB2/EphB4 expression is present in
the retinal neovascularization model of high oxygen-induced
retinopathy of mouse prematurity and the laser-induced CNV model,
indicating that EphrinB2/EphB4 plays a role in retinal
neovascularization and CNV formation (4,5).
In the present study, we applied argon laser
photocoagulation to establish the experimental CNV model. The EphB4
monoclonal antibody was subsequently injected into the vitreous
bodies in the eyes of experimental animals to observe its effect on
experimental CNV progression and conduct a quantitative analysis
investigating the regulation of the EphrinB2/EphB4 system on
choroidal neovascularization. Thus, this study proposes a new model
for the CNV angiopoiesis mechanism and provides a theoretical basis
for the prevention and control of CNV diseases in the clinical
setting.
Materials and methods
Animals
Healthy 7-week-old C57BL/6J female mice were
provided by the Experimental Animal Center of the Military Medical
Science Academy of the Chinese People’s Liberation Army. This study
was carried out in strict accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee
(IACUC) of the 463rd Hospital of Chinese People’s Liberation Army.
The study was approved by the ethics committee of Shengjing
Hospital of China Medical University, Shenyang, China.
CNV model preparation
The experimental CNV model of C57BL/6J mouse eyes
was established using the argon laser photocoagulation method. The
experimental animals were anesthetized with 10% chloral hydrate
solution (3.5 ml/kg body weight) by intraperitoneal injection.
Mydrin-P and atropine gutta was used for mydriasis. Prior to
surgery, the morphology of the fundus oculi was carefully observed
in all experimental animals to confirm that it was normal. The
argon laser was used with the following parameters: wavelength, 514
nm; light spot diameter, 100 μm; shooting time, 0.1 sec and
energy, 100 mW. In addition, the retinal posterior pole was
arranged at the 9, 12, 3 and 6 o’clock positions around the optic
disc to avoid the large vasa sanguinea retinae and each
position was shot once at Bruch’s membrane breakthrough to induce
experimental CNV. In case of laser excitation, the bubble generated
at the photocoagulation position was regarded as the indicator of
breaking through Bruch’s membrane.
Eighteen mouse eyes were randomly selected for the
experimental group. On days 0, 3, 6 and 9 after CNV model
establishment, 0.1 μg 1 μl EphB4 monoclonal antibody
was injected into the vitreous space. The other 18 mouse eyes were
classified as the control group. At the same time points, an equal
amount of balanced salt solution was injected into the vitreous
space. The specific method of vitreous injection was as follows: an
experimental animal was anesthetized with 10% chloral hydrate
solution (3.5 ml/kg body weight) by intraperitoneal injection and
fixed. The upper and lower eyelids of the mouse were separated with
ophthalmic microforceps. Orbital margin tissues were gently pressed
to take out the eyeball. The needle of a 1 μl microsyringe
(Shanghai Anting Microsyringe Factory, Shanghai, China) was
inserted at the position vertical to the eyeball wall at the rear
of the superior temporal corneoscleral limbus and 1 μl EphB4
monoclonal antibody or balanced salt solution was slowly injected.
After removing the needle, the pinhole was gently and immediately
pressed with a cotton swab to reset the eyeball. The eye was
subsequently coated with erythromycin eye ointment.
On day 10 after CNV model establishment, high
molecular weight fluorescein isothiocyanate (FITC)-dextran
endocardial perfusion and choroidal stretched preparation
examination were conducted on the experimental group and
histopathological examination of the control group was performed to
observe CNV progression. Quantitative analysis and comparison were
conducted to evaluate the inhibition of the EphB4 monoclonal
antibody on experimental CNV progression.
FITC-dextran perfusion and choroidal
stretched preparation examination
Thirty-six light spots of nine mouse eyes were
assessed from the experimental and control groups. FITC-dextran
perfusion and choroidal stretched preparation examination (1) was carried out according to the method
described by Edelman and Castro (6). An experimental animal was
anesthetized with 10% chloral hydrate solution (3.5 ml/kg) by
intraperitoneal injection and fixed; then, the thoracic cavity was
rapidly opened. Subsequently, 50 mg high molecular weight
FITC-dextran (molecular weight, 2×106; Sigma-Aldrich,
St. Louis, MO, USA) was dissolved in 1.0 ml distilled water and
injected into the left ventricle. At the late perfusion stages, the
heart was gently pressed with the forceps to facilitate perfusion
for adequate contrast. Next, the eyeball was removed and fixed in
4% formaldehyde. After 1–2 h, the eyeball was cut open along the
corneoscleral limbus to remove the cornea, lens and retina and
observe them under an operating microscope. The free choroid was
sheared in a radial shape and spread onto a slide. After adding a
small amount of glycerogelatin, the slide was covered with a cover
slip. Finally, the choroidal stretched preparation was observed
under a fluorescence microscope (Leica DM3000, Fukuoka, Japan) and
photographed. At the same time, MacScope software (Mitani Co.,
Fukuoka, Japan) was used to calculate the experimental CNV area at
each light spot and calculate the mean value of each group.
Histopathological examination
Thirty-six light spots from mouse eyes were sampled
from the experimental and control groups. After each animal was
sacrificed, the eyeballs were removed, fixed in 4% formaldehyde for
24 h, conventionally dehydrated and embedded in paraffin wax. The
optic nerve parallel to the sagittal plane at the laser
photocoagulation position was selected and slices with a thickness
of 6.0 μm were prepared continuously. The sections were
stained with hematoxylin-eosin, observed under a light microscope
and photographed. At the same time, MacScope software was used to
calculate the ratio (M/C) of the maximum thickness of experimental
CNV in each light spot, that is, the distance from the choroidal
bottom at the center of the light spot to the top of the
neovascularization membrane (M) to the surrounding normal choroidal
thickness (C). The mean values of the various groups were
calculated.
Results
FITC-dextran perfusion and choroidal
stretched preparation examination
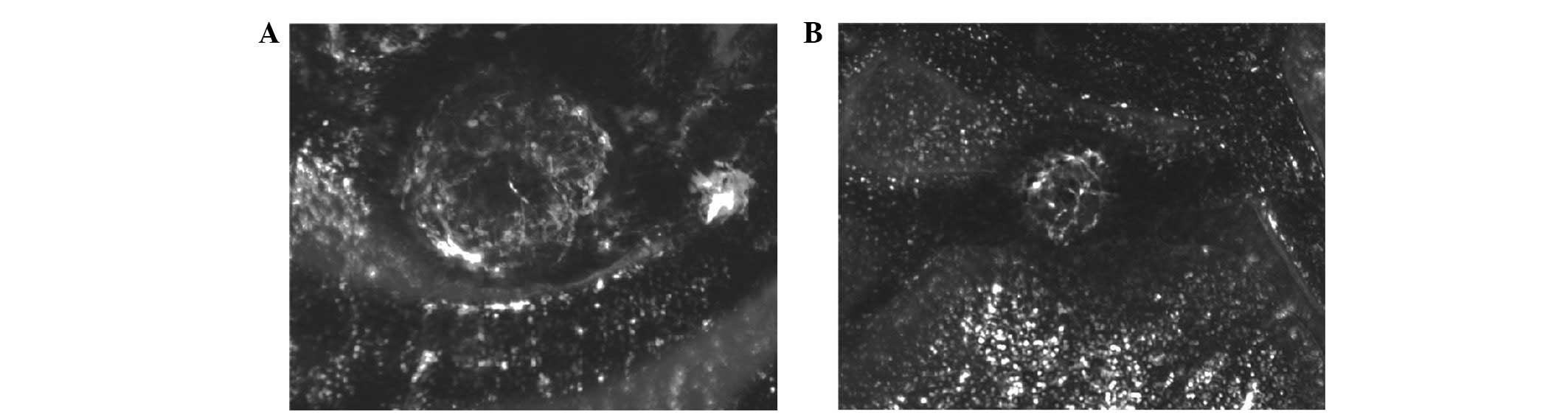
On day 10 following photocoagulation, CNV was
observed in all light spots in the control group, presenting a
larger reticular structure composed of flat vessels. In the
experimental group, neovascularization network areas in the light
spots were smaller, and a number only presented vascular circles.
The quantitative analysis of the CNV area revealed that the mean
CNV area of the control group was (28.12±3.79) ×10−3
mm2, whereas that of the experimental group was
(19.19±2.48) ×10−3 mm2. The CNV of the
experimental group was significantly inhibited (t=11.84, P<0.01;
Fig. 1).
Histopathological examination
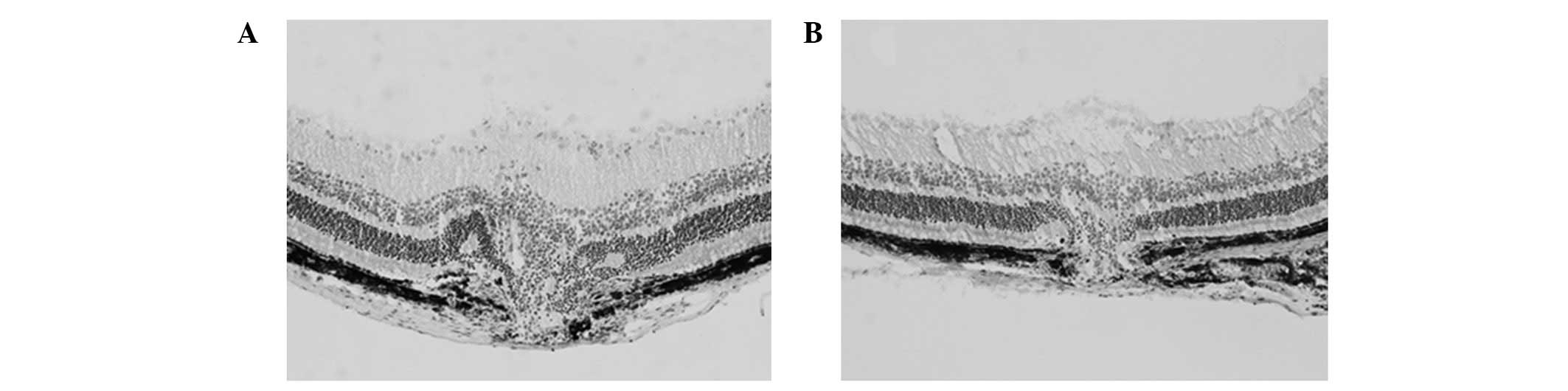
In the experimental group, CNV was thinner and
smaller. Quantitative analysis of the CNV thickness revealed that
the ratio (M/C) of the control group was 2.60±0.63, whereas that of
the experimental group was 1.74±0.28. CNV progression in the
experimental group was significantly inhibited (t=7.45, P<0.01;
Fig. 2).
Discussion
The Eph receptor and Ephrin ligand are divided into
subclasses A and B according to the sequence conservation and their
affinity difference. Nine types of EphA receptors (EphA1 to A9), 6
types of EphrinA ligands (EphrinA1 to A6), 6 types of EphB
receptors (EphB1 to B6) and 3 types of EphrinB ligands (EphrinB1 to
B3) have been identified (7).
EphrinA is attached to the cell membrane via glycosyl-phosphatidyl
inositol (GPI), whereas EphrinB is a transmembrane protein. The
characteristics of Ephrin ligands depend on the requirement to bind
and break through the cell membrane and thereby activate the
function of corresponding receptors. Therefore, the Ephrin/Eph
interaction depends on direct intercellular contact (8). Unlike other RTKs, the Eph receptor
and ligand are subject to phosphorylation, thereby mediating
bidirectional signal transmission.
In cultured endothelial cells, typical positive
signals (EphrinB2 to EphB4) reduce the proliferation and migration
of the EphB4 cell. On the contrary, negative signals (EphB4 to
EphrinB2) increase the proliferation and migration of the EphrinB2
cell (9–16). Such bidirectional effects of
EphrinB2/EphB4 may regulate the endothelial cell guidance and
spatial combination process and thus cause the correct
differentiation of arteriovenous vessels, as well as the reasonable
division of arterial and venous capillaries. EphrinB2 and its
receptor, EphB4, are respectively regarded as relatively specific
molecular markers of the original artery and original vein and they
specifically damage the signal transduction of EphrinB2/EphB4,
which causes remodeling defects and developmental defects in the
primary capillary network of the embryonic vascular system, thereby
resulting in embryonic mortality (17–21).
He et al (5)
observed EphB4 and EphrinB2 expression in the cultured choroidal
capillary endothelial cells of rats and the laser-induced CNV
membrane, in which EphB4 expression was stronger than EphrinB2
expression. EphB4 dominated in physiological or pathological
choroidal vessels. Erber et al (22) identified that the EphB4 pathway
plays an important role in the regulation process of acquired
vascular configuration remodeling, permeability and other aspects,
including tumors and ocular vascular tissues. This function is
independent of EphB4 protein tyrosine kinase receptor activity,
namely the non-positive EphB4 pathway. EphB4 participates in the
above process by binding with the ligand EphrinB2 in the negative
signal pathway. He et al (5) administered an intravitreous injection
of the soluble monomer sEphB4 in vitro and identified that
sEphB4 inhibits laser-induced CNV development in rats and that the
neovascularization area and permeability are greatly reduced.
Another study (4) reported that
the soluble chimera EphB4-Fc (artificially synthesized EphB4
extracellular domain composition bound with an immunoglobulin Fc
fragment to form the chimera with a dimerization effect; used as an
EphrinB2 agonist) also inhibits CNV formation. Contrastingly, the
findings based on tumors revealed that EphB4 is located on the
tumor cell surface. After the EphB4 extracellular domain is bound
with vascular EphrinB2, it promotes angiopoiesis and endothelial
cell migration and proliferation, thereby promoting tumor growth;
whereas soluble sEphB4 inhibits tumor growth and angiopoiesis
(23). These results are contrary
to the negative signal theory; however, the specific mechanism is
unclear. A number of scholars consider that the ‘bidirectional
signal’ theory is based on in vitro cultured cells and is
affected by numerous factors. Therefore, it cannot be simply copied
in vivo.
In the present study, EphB4 monoclonal antibody was
injected into the vitreous space to specifically damage the
EphrinB2/EphB4 signal transduction. The results demonstrated that
EphB4 significantly inhibited argon laser-induced CNV in mouse
eyes. Compared with the control group, the CNV area and the ratio
of maximum thickness of neovascularization to normal choroidal
thickness in the experimental group were significantly reduced. We
consider that after the EphB4 monoclonal antibody specifically
binds with the EphB4 receptor, it blocks the negative signal
pathway to the maximum extent and inhibits the neovascularization
configuration remodeling, thus suppressing vascular development. In
addition, the EphB4 monoclonal antibody interferes with the
functions of other vascular factors by blocking EphrinB2/EphB4
signal transduction.
Similar to other RTKs, including VEGF and basic
fibroblast growth factor (bFGF), Eph-Ephrin has a conserved
tyrosine residue. However, the possibility of crosswise binding and
transmitting signals between them remains unclear. Few reports are
available on the correlation of VEGF with an Eph receptor and its
Ephrin ligand. One study suggested that VEGF induces EphrinB2
expression and activates the potential EphB4 receptor (21). However, after the Eph receptor
binds with the ligand, it weakens the cell growth induced by growth
factors, including VEGF and bFGF. In the present study, CNV growth
was inhibited by blocking endogenous EphrinB2/EphB4 signal
transduction, which was not consistent with the above theory. The
possible reason lies with the involvement of CNV formation in the
joint regulation of a number of pathways and multiple cytokines,
which requires further investigation.
The role of EphrinB2/EphB4 signal transduction in
angiopoiesis has been increasingly studied. One study (24) proposed the feasibility of treating
human malignancy- and angiopoiesis-related diseases by humanized
low-immunity rat EphB4 monoclonal antibody. Another study observed
EphB4 receptor expression in human iris tissue and cultured
vascular endothelial cells of the iris (25). Furthermore, EphB4 receptor
expression exists in human choroidal tissue. Therefore, the EphB4
monoclonal antibody is expected to become a promising treatment in
the intervention treatment of CNV. In the present study, EphB4
monoclonal antibody was unable to inhibit neovascularization
formation completely. Combined with the achieved results for the
interventional treatment of CNV targeting VEGF/VEGFR, the
successful treatment of targeting CNV formation should begin
simultaneously with a number of pathways and multiple
cytokines.
References
|
1
|
Héroult M, Schaffner F and Augustin HG:
Eph receptor and ephrin ligand-mediated interactions during
angiogenesis and tumor progression. Exp Cell Res. 312:642–650.
2006.PubMed/NCBI
|
|
2
|
Kojima T, Chang JH and Azar DT:
Proangiogenic role of ephrinB1/EphB1 in basic fibroblast growth
factor-induced corneal angiogenesis. Am J Pathol. 170:764–773.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Hicks D, Brantley-Sieders D, et
al: Inhibition of retinal neovascularization by soluble EphA2
receptor. Exp Eye Res. 82:664–673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zamora DO, Davies MH, Planck SR, et al:
Soluble forms of EphrinB2 and EphB4 reduce retinal
neovascularization in a model of proliferative retinopathy. Invest
Ophthalmol Vis Sci. 46:2175–2182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He S, Ding Y, Zhou J, et al: Soluble EphB4
regulates choroidal endothelial cell function and inhibits
laser-induced choroidal neovascularization. Invest Ophthalmol Vis
Sci. 46:4772–4779. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edelman JL and Castro MR: Quantitative
image analysis of laser-induced choroidal neovascularization. Exp
Eye Res. 71:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J and Hughes S: Role of the ephrin
and Eph receptor tyrosine kinase families in angiogenesis and
development of the cardiovascular system. J Pathol. 208:453–461.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis S, Gale NW, Aldrich TH, et al:
Ligands for Eph-related receptor tyrosine kinases that require
membrane attachment or clustering for activity. Science.
266:816–819. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamada K, Oike Y, Ito Y, et al: Distinct
roles of ephrin-B2 forward and EphB4 reverse signaling in
endothelial cells. Arterioscler Thromb Vasc Biol. 23:190–197. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Füller T, Korff T, Kilian A, Dandekar G
and Augustin HG: Forward EphB4 signaling in endothelial cells
controls cellular repulsion and segregation from ephrinB2 positive
cells. J Cell Sci. 116:2461–2470. 2003.PubMed/NCBI
|
|
11
|
Maekawa H, Oike Y, Kanda S, et al:
Ephrin-B2 induces migration of endothelial cells through the
phosphatidylinositol-3 kinase pathway and promotes angiogenesis in
adult vasculature. Arterioscler Thromb Vasc Biol. 23:2008–2014.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oike Y, Ito Y, Hamada K, et al: Regulation
of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling
between endothelial cells and surrounding mesenchymal cells. Blood.
100:1326–1333. 2002.PubMed/NCBI
|
|
13
|
Helbling PM, Saulnier DM and Brändli AW:
The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict
angiogenic growth of embryonic veins in Xenopus laevis.
Development. 127:269–278. 2000.PubMed/NCBI
|
|
14
|
Zhang XQ, Takakura N, Oike Y, et al:
Stromal cells expressing ephrin-B2 promote the growth and sprouting
of ephrin-B2(+) endothelial cells. Blood. 98:1028–1037.
2001.PubMed/NCBI
|
|
15
|
Kim I, Ryu YS, Kwak HJ, et al: EphB
ligand, ephrinB2, suppresses the VEGF- and angiopoietin 1-induced
Ras/mitogen-activated protein kinase pathway in venous endothelial
cells. FASEB. 16:1126–1128. 2002.PubMed/NCBI
|
|
16
|
Steinle JJ, Meininger CJ, Forough R, Wu G,
Wu MH and Granger HJ: Eph B4 receptor signaling mediates
endothelial cell migration and proliferation via the
phosphatidylinositol 3-kinase pathway. J Biol Chem.
277:43830–43835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinle JJ, Meininger CJ, Chowdhury U, Wu
G and Granger HJ: Role of ephrinB2 in human retinal endothelial
cell proliferation and migration. Cell Signal. 15:1011–1017. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gale NW and Yancopoulos GD: Growth factors
acting via endothelial cell-specific receptor tyrosine kinases:
VEGFs, angiopoietins and ephrins in vascular development. Genes
Dev. 13:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Flanagan JG and Vanderhaeghen P: The
ephrins and Eph receptors in neural development. Annu Rev Neurosci.
21:309–345. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams RH, Wilkinson GA, Weiss C, et al:
Roles of ephrinB ligands and EphB receptors in cardiovascular
development: demarcation of arterial/venous domains, vascular
morphogenesis and sprouting angiogenesis. Genes Dev. 13:295–306.
1999. View Article : Google Scholar
|
|
21
|
Hamada K, Oike Y, Ito Y, et al: Distinct
roles of ephrin-B2 forward and EphB4 reverse signaling in
endothelial cells. Arterioscler Thromb Vasc Biol. 23:190–197. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erber R, Eichelsbacher U, Powajbo V, et
al: EphB4 controls blood vascular morphogenesis during postnatal
angiogenesis. EMBO J. 25:628–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martiny-Baron G, Korff T, Schaffner F, et
al: Inhibition of tumor growth and angiogenesis by soluble EphB4.
Neoplasia. 6:248–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Z, Jin H and Qian Q: Humanized
anti-EphB4 antibodies for the treatment of carcinomas and
vasculogenesis-related diseases. Expert Opin Ther Pat.
19:1035–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zamora DO, Babra B, Pan Y, Planck SR and
Rosenbaum JT: Human leukocytes express ephrinB2 which activates
microvascular endothelial cells. Cell Immunol. 242:99–109. 2006.
View Article : Google Scholar : PubMed/NCBI
|