Introduction
Mesial temporal lobe epilepsy (MTLE) is by far the
most common focal epilepsy and is often associated with
pharmacoresistance. The hippocampus and amygdala are commonly
involved in the initial phases of electroencephalography (EEG)
discharges of seizures arising from the temporal lobe. Patients
with intractable seizures due to unilateral MTLE are excellent
candidates for surgical treatment and resective surgery achieves a
short-term cure (seizure freedom according to Engel class IA) in up
to 85% of cases and long-term cure in 57–66% of cases (1). Unfortunately, up to 30% of temporal
lobe epilepsy cases are unsuitable for surgery due to the bilateral
nature of the disease or concerns for the risk of memory deficit,
severe amnesia following the removal of the amygdalohippocampal
complex (2–4) and visual field defects, as well as
cognitive impairment (5,6). Thus, surgical resection is not
recommended in patients with bilateral independent temporal foci or
when the eloquent cortex overlaps with the presumed epileptogenic
zone.
Deep brain stimulation (DBS) is being used with
increasing frequency as a treatment for drug-resistant epilepsy.
Various targets are approached, including seizure spread relays and
direct focus stimulation (7–10).
Randomized clinical trials studying open and closed-loop systems
have been reported (11,12). In 2000, Velasco et al
proposed the use of amygdalohippocampal DBS (AH-DBS) to control
MTLE (13). In that study, 10
patients with diagnostic hippocampal electrodes were used for the
trial of subacute hippocampal stimulation prior to temporal
lobectomy. In seven of the cases, seizures stopped and interictal
spikes demonstrated a significant reduction following subacute
stimulation. In the subsequently published case series of chronic
hippocampal stimulation, more than half of the patients experienced
a seizure reduction >50% (9,14–16).
Although the mechanism of action remains unclear, AH-DBS has become
a selective temporal lobe epilepsy treatment.
Here, we report two patients treated with AH-DBS for
drug-resistant temporal lobe epilepsy. The study was carried out
with approval from the Ethics Committee of Beijing Sanbo Brain
Hospital (Beijing, China) and according to the Declaration of
Helsinki. Informed consent was obtained from all patients
involved.
Case reports
Case 1
History
The patient was a 34-year-old male who came to
Beijing Sanbo Brain Hospital with a 31-year history of epileptic
seizures. The history of growth and development were normal. There
was a history of febrile seizures starting at three years old, with
a gradual emergence of repeated seizures. Habitual seizures were
complex partial seizures with behavioral arrest and secondarily
generalized tonic-clonic seizures. Seizure frequency was 1–2 times
every month. The patient received pharmacotherapy with maximally
tolerable doses of carbamazepine for ten years and seizure
frequency was controlled to 1–2 times every year. However, the
seizure frequency of the patient then increased to 2–3 times every
month and the severity increased. Thus, the patient received
presurgical evaluation.
Presurgical evaluation
A comprehensive presurgical workup was performed,
including clinical history, neurological examination, video EEG
(V-EEG), magnetic resonance imaging (MRI, 1.5 Tesla; Siemens,
Germany), magnetoencephalography (MEG) and neuropsychological
assessment. MRI scans revealed left hippocampal sclerosis. V-EEG
monitoring continued for 14 days and recorded three seizures. The
interictal EEG revealed epileptiform discharges in the left
hemisphere; however, the ictal EEG revealed epileptiform discharges
originating in the right temporal lobe. MEG revealed diffuse
epileptiform discharges in the left temporal and central areas.
Neuropsychological assessment revealed that the patient had
moderate cognitive and memory damage. With suspected temporal lobe
epilepsy and to further clarify the side of the onset zone, the
patient underwent invasive monitoring with stereotactic
implantation of depth and strip electrodes (covering mesial and
lateral temporal lobes bilaterally). The interictal EEG of invasive
monitoring revealed asynchronous spikes in the right frontotemporal
and left temporal lobe and the ictal EEG revealed epileptiform
discharges originating in the right hippocampus. The patient was
presented at a multi-disciplinary conference and recommended for
AH-DBS.
Surgical procedure and parameter
settings
The patient was fixed with a stereotactic head frame
(Leksell G frame, Elekta Instruments AB, Stockholm, Sweden) under
local anesthesic and received an MRI scan in stereotactic
conditions. The MRI data were transferred to the surgery planning
system (Elekta Instruments AB). Targeting was performed by direct
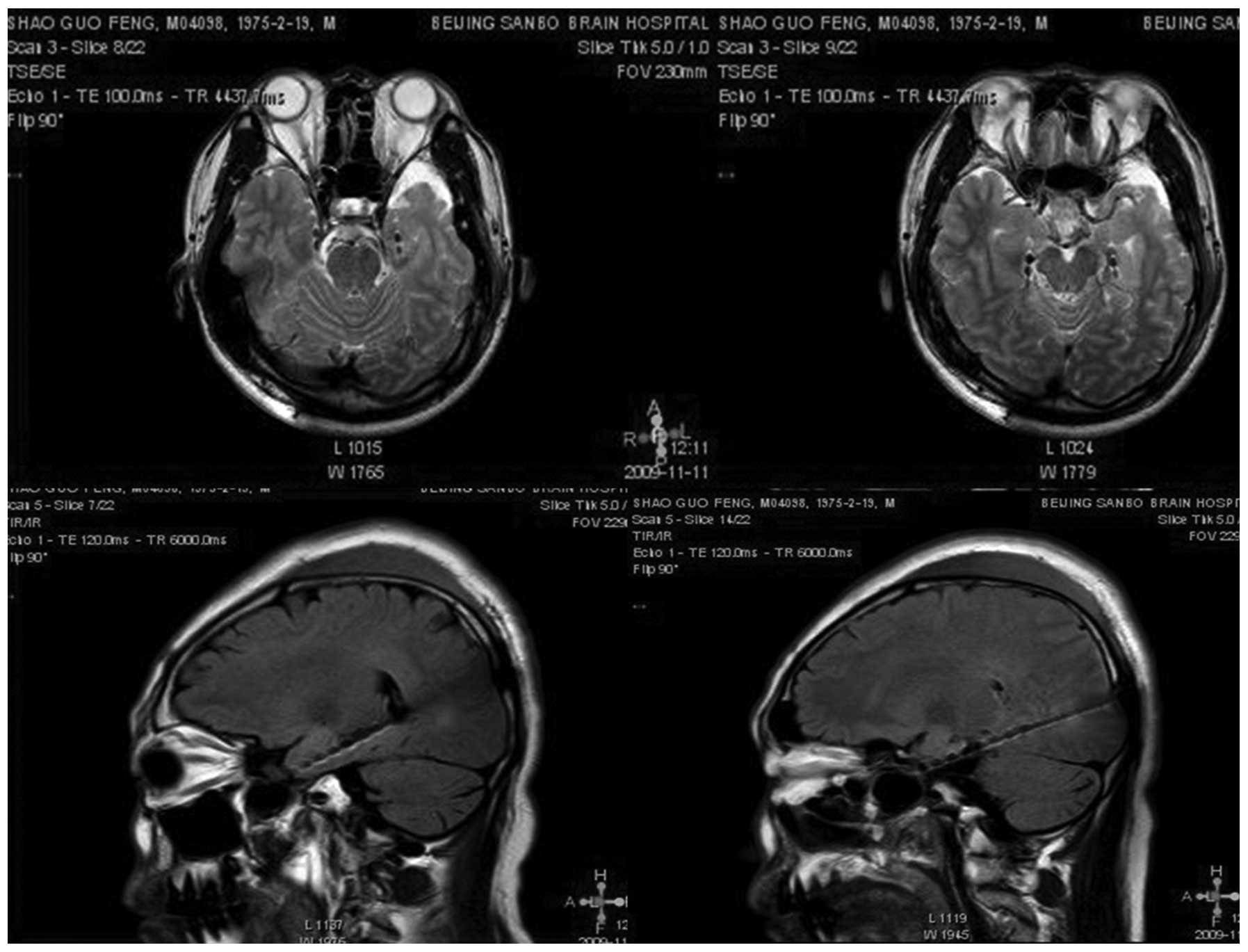
visualization of the amygdalohippocampal junction (Fig. 1) and the lead path through the axis
of the body of the hippocampus. The patient was implanted with a
quad-contact electrode (model 3146, St. Jude Medical, St. Paul, MN,
USA) under the guidance of the stereotactic system to the bilateral
intended target through the posterior occipital approach, under
local anesthesia and in a semi-sitting position. After an X-ray was
used to confirm correct target positioning, a programmable internal
pulse generator (model 3716, St. Jude Medical) was implanted in a
subclavicular subcutaneous pouch and connected with the electrodes
by means of extension wires under general anesthesia (Fig. 2).
The generator was turned on 4 weeks after surgery.
Initial stimulating parameters were as follows: current intensity,
1.5 mA; on-period, 60 sec; off-period, 180 sec; pulse width, 450
μsec and stimulating frequency, 130 Hz. In addition, the first two
contacts were used as cathodes and the case box as an anode. The
current intensity was gradually increased to 2.0 mA in the first
month after surgery and the patient was observed for 3 months.
Medication was maintained at 800 mg/day carbamazepine following
surgery.
Long term follow-up
Follow-up and adjustments of parameters were
conducted by the epileptologist, in a single blind design. The
patient had parameter adjustment and machine tests once every 3
months. The seizure frequency at baseline was determined by
recording the number of monthly seizures and then averaging the
number of monthly seizures relative to the last 3 months before the
implant. The clinical outcome was determined by comparing seizure
frequency (the number of seizures/month) following AH-DBS with the
baseline. The last follow-up was 36 months after surgery and the
final parameter settings were 3.6 mA, 450 μsec, 130 Hz and cycling
with 60 sec on, 180 sec off. The patient experienced a seizure
frequency reduction of 90% in respect to the baseline.
Additionally, seizure duration became shorter and the severity of
attack was reduced. The patient was reported to have improved
quality of life by relatives.
Case 2
History
A 27-year-old male was referred for a 19-year
history of drug-resistant epilepsy. The patient had experienced
stereotypic seizures since childhood and the first seizure occurred
aged 8 years due to fever. Seizure was characterized by a sudden
tonic-clonic seizure with loss of consciousness for ∼5 min, without
aura. After the first seizure, they began to repeatedly appear and
occasionally secondary general tonic-clonic seizures occurred.
Habitual seizures were absent. Pharmacotherapy was administered
with maximally tolerable doses of valproate, carbamazepine and
phenobarbital in mono- and polytherapy; however, seizure frequency
was still 10–15 times every month. Due to the disappointing results
of the drug treatment, the patient received presurgical
evaluation.
Presurgical evaluation
For presurgical workup, neurological examination,
MRI, V-EEG, MEG, fluorodeoxyglucose-positron emission tomography
(FDG-PET) imaging and neuropsychological assessment were performed,
as well as evaluation of the patient’s clinical history. MRI scans
revealed bilateral hippocampal sclerosis. Scalp EEG monitoring
continued for 2 days and recorded three seizures. The interictal
EEG revealed asynchronous epileptiform discharges in the bilateral
temporal lobe and the ictal EEG revealed diffused epileptiform
discharges in the left hemisphere. MEG revealed epileptiform
discharges in the bilateral temporal lobe. FDG-PET identified low
metabolic activity in the left temporal lobe. Neuropsychological
assessment revealed that the patient had moderate cognitive and
memory damage. The patient underwent invasive monitoring with the
stereotactic implantation of depth and strip electrodes. The
interictal EEG of invasive monitoring revealed asynchronous spikes
in the bilateral temporal lobe and the ictal EEG revealed
epileptiform discharges originating in the left hippocampus and the
base of the temporal lobe. The patient was also recommended for
AH-DBS following the multidisciplinary conference.
Surgical procedure and parameter
settings
The surgical procedures and parameter settings were
the same as in case 1. Medication was changed to 900 mg/day
oxcarbazepine following surgery.
Long term follow-up
The last follow-up was 18 months after surgery and
the final parameter settings were 2.6 mA, 450 μsec, 130 Hz and
cycling with 60 sec on, 180 sec off. The patient experienced a
seizure frequency reduction of 65% in respect to the baseline.
Additionally, seizure duration was shorter and the severity of
attack was reduced. The patient was reported to have an improved
quality of life by relatives.
Discussion
DBS has become established as a long-term safe and
effective treatment for movement disorders (17). There is a great interest in the use
of DBS as an innovative treatment for drug-resistant epilepsy. A
number of targets have been attempted, including the centromedian
thalamic nucleus (18,19), the caudate nucleus (20), the locus coeruleus (21), the anterior thalamic nucleus
(22) and the subthalamic nucleus
(23). Electrical stimulation of
these targets aims to activate a postulated anticonvulsant control
system in the brain that restores the imbalance between excitatory
and inhibitory processes that led to the epileptic seizures
(24). These are classified as
indirect electrical stimulation neuromodulation. However, for
patients with MTLE, the treatment results of stimulation of these
targets are not ideal (25).
Electrical seizure onset in the amygdala and
hippocampus is the key feature of MTLE (26). The target of AH-DBS is the primary
epileptogenic focus and is classified as direct stimulation
neuromodulation.
Here, we reported two cases of successful AD-DBS in
patients who were diagnosed with MTLE. For the first patient, the
onset zone was located in the right temporal lobe, as confirmed by
invasive V-EEG. However, due to left hippocampal sclerosis and the
risk of memory dysfunction with resective surgery, as well as the
possibility of not achieving complete seizure treatment due to the
interictal epileptic discharge identified by EEG and MEG, we did
not recommend resective surgery.
Similarly, patient 2 had a seizure onset zone
located at the left temporal lobe and hippocampus; however, due to
the bilateral hippocampal sclerosis and PET results indicating
hypometabolism of the right temporal lobe, removal of the left
hippocampus may have resulted in severe memory dysfunction.
Therefore, this patient was also not a good candidate for resective
surgery.
Prior to the use of neuromodulation, patients that
were excluded as candidates for temporal lobectomy and left with no
other alternative, had to suffer great physical and psychological
damage. With the development of neuromodulation technology, more
and more patients with drug-resistant epilepsy benefit.
AH-DBS as a treatment of MTLE reduces the seizure
frequency in the majority of patients by >50%. In a number of
cases the frequency is reduced by 90% and certain patients become
seizure free (9,14–16,27).
Several cases reported a seizure frequency lower than the baseline
levels once electrical stimulation had ended and this phenomenon
was attributed to residual anticonvulsive effect (28,29).
The main reason for the two patients being accepted for AH-DBS was
out of concern for the potential risk of memory deficit with
temporal lobectomy. Memory decline following temporal lobectomy has
been documented in several studies; however, no AH-DBS patient
demonstrated such a decline, not even with bilateral stimulation
(27). Seizure reduction occurred
in all patients in our case series who accepted AH-DBS and in one
patient the seizure frequency was reduced by up to 95%.
Additionally, neither of the patients demonstrated a memory
deficit.
Currently, there is no consensus on the most
appropriate choice of stimulus parameters. Experimental evidence in
animals indicates that prolonged low frequency stimulation (1 Hz
applied for 10–15 min) inhibits the development and expression of
amygdala-kindled seizures (30);
however, this has not been confirmed in human trials. A number of
researchers select low-frequency electrical stimulation (0.1–25
Hz), while others select high-frequency electrical stimulation
(90–130 Hz). In the present cases, we selected the high-frequency
electrical stimulation of 90 Hz.
One study considered that patients with hippocampal
sclerosis require a strong stimulation (high stimulus amplitude, at
≥1 V and/or multipolar configuration) in order to decrease the
seizure frequency. Furthermore, when the amplitude of the bipolar
stimulation is <1 V, the seizure frequency increases (31). The impedance of case 1 was 450
(left) and 380 Ohms (right) and the set current was 3.6 mA.
According to Ohm’s law (I=U/R), the voltage was ∼1.6 (left) and 1.4
V (right). The voltage in case 2 was ∼1.3 (left) and 1.1 V
(right).
We identified that although the generators were not
turned on in the first month after implantation, the seizure
frequency reduction was observed in the two cases. Conversely, the
seizure frequency increased in the first month after the generator
was turned on. In one case, the seizure frequency even increased
beyond the baseline and then gradually decreased with the strength
of the current. The decrease of seizure frequency in the first
month after implantation may be related to microlesional effects, a
phenomenon that was reported by Schulze-Bonhage et al
(32). As the microlesional zone
gradually repaired, the current intensity remained at a low level
(<1 V). At that time, the seizure frequency increased again
until the current was gradually increased. After that, the seizure
frequency decreased until it reached a steady state. Therefore,
higher currents may be more effective at controling seizure
attacks, so the current should be strengthened to the effective
level necessary. Early control of seizures is likely to enhance the
patients’ confidence in the treatment. A clear limitation of this
study is the small sample size. It should be considered that only
two subjects were included, leading to the possibility of selection
bias.
The choice of pulse width and stimulus contacts may
change the stimulus range. A larger stimulus range is considered to
have a better control effect; however, it also involves greater
stimulus-related discomfort, faster battery consumption and in
certain cases, possible seizure increase. In addition, it remains
inconclusive whether continuous stimulation or intermittent
stimulation is more effective.
AH-DBS proved to be safe with no side-effects.
Similar to the use of DBS in the treatment of other diseases, the
most significant potential complication is hemorrhage, reported in
∼5% of patients (33). Hemorrhage
usually does not require surgical treatment. Improved surgical
planning, careful surgery and reduced repeated puncture help to
avoid the chance of this complication. In addition, skin erosions
and infection are the other main complications of the placement of
stimulation devices, particularly in children or thin patients
(27). Although all precautions
are taken to avoid skin erosion and infection, it is difficult to
completely avoid them. It may be of value to improve the
stimulation system design to make it smaller and more compatible.
In our cases, none of these side-effects were observed.
One study demonstrated that the reduction of seizure
frequency following hippocampal stimulation is <50% (34). Other studies demonstrated prolonged
seizure control in patients who underwent invasive recording with
conventional electrodes. A number of studies support the hypothesis
that actual stimulation is not necessary to achieve efficacy and
claim that efficacy is based on the electrode microthalamotomy
effect that is provoked by the insertion of the electrodes
(35,36). The indications and results are not
yet fully validated and the therapeutic objective should remain
palliative. In addition, the mechanism of action of DBS in reducing
seizures remains unclear; however, it should be recognized that, at
this point in time, DBS is a promising treatment option for a
subgroup of carefully selected patients with MTLE who are not
suitable candidates for resective surgery. Furthermore, more cases
and control studies are required to refine the patient selection
criteria, anatomical targets and ideal stimulation parameters. With
more extensive trials, AH-DBS in MTLE is likely to become a
valuable alternative.
AH-DBS is a safe, micro-invasive alternative in
patients with MTLE who are not suitable candidates for resective
surgery. It effectively reduces seizures without a negative effect
on memory performance. Currently, the most appropriate choice of
stimulus parameters remains unclear. A larger sample size and
well-designed randomized control studies are required to elucidate
the impact of this treatment.
References
|
1
|
Tellez-Zenteno JF, Dhar R and Wiebe S:
Long-term seizure outcomes following epilepsy surgery: a systematic
review and meta-analysis. Brain. 128:1188–1198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwan P and Brodie MJ: Early identification
of refractory epilepsy. N Engl J Med. 342:314–319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helmstaedter C, Kurthen M, Lux S, Reuber M
and Elger CE: Chronic epilepsy and cognition: a longitudinal study
in temporal lobe epilepsy. Ann Neurol. 54:425–432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapur N and Prevett M: Unexpected amnesia:
are there lessons to be learned from cases of amnesia following
unilateral temporal lobe surgery? Brain. 126:2573–2585. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gleissner U, Helmstaedter C, Schramm J and
Elger CE: Memory outcome after selective amygdalohippocampectomy: a
study in 140 patients with temporal lobe epilepsy. Epilepsia.
43:87–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Helmstaedter C, Van Roost D, Clusmann H,
Urbach H, Elger CE and Schramm J: Collateral brain damage, a
potential source of cognitive impairment after selective surgery
for control of mesial temporal lobe epilepsy. J Neurol Neurosurg
Psychiatry. 75:323–326. 2004.PubMed/NCBI
|
|
7
|
Velasco F, Velasco AL, Velasco M, et al:
Central nervous system neuromodulation for the treatment of
epilepsy. I. Efficiency and safety of the method. Neurochirurgie.
54:418–427. 2008.
|
|
8
|
Halpern CH, Amadani U, Litt B, Jaggi J and
Baltuc G: Deep brain stimulation for epilepsy. Neurotherapeutics.
5:59–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boon P, Vonck K, De H, et al: Deep brain
stimulation in patients with refractory temporal lobe epilepsy.
Epilepsia. 48:1551–1560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andrade DM, Zumsteg D, Hamani C, et al:
Long-term follow-up of patients with thalamic deep brain
stimulation for epilepsy. Neurology. 66:1571–1573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morrell M: Results of a multicenter double
blinded randomized controlled pivotal investigation of the RNS
system for treatment of intractable partial epilepsy in adults.
Epilepsia. 50(Suppl 11): S4902010.
|
|
12
|
Fisher R, Salanova V, Witt T, et al:
Electrical stimulation of the anterior nucleus of thalamus for
treatment of refractory epilepsy. Epilepsia. 51:899–908. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Velasco M, Velasco F, Velasco AL, et al:
Subacute electrical stimulation of the hippocampus blocks
intractable temporal lobe seizures and paroxysmal EEG activities.
Epilepsia. 41:158–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vonck K, Boon P, Claeys P, Dedeurwaerdere
S, Achten E and Van Roost D: Long-term deep brain stimulation for
refractory temporal lobe epilepsy. Epilepsia. 46(Suppl 5): 98–99.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vonck K, Boon P, Achten E, De RJ and
Caemaert J: Long-term amygdalohippocampal stimulation for
refractory temporal lobe epilepsy. Ann Neurol. 52:556–565. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tellez-Zenteno JF, McLachlan RS, Parrent
A, Kubu CS and Wiebe S: Hippocampal electrical stimulation in
mesial temporal lobe epilepsy. Neurology. 66:1490–1494. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wojtecki L, Südmeyer M and Schnitzler A:
The current treatment of Parkinson’s disease. Dtsch Arztebl.
104:2513–2522. 2007.(In German).
|
|
18
|
Velasco F, Velasco M, Velasco AL and
Jimenez F: Effect of chronic electrical stimulation of the
centromedian thalamic nuclei on various intractable seizure
patterns: I. Clinical seizures and paroxysmal EEG activity.
Epilepsia. 34:1052–1064. 1993. View Article : Google Scholar
|
|
19
|
Velasco F, Velasco M, Jimenez F, et al:
Predictors in the treatment of difficult to control seizures by
electrical stimulation of the centromedian thalamic nucleus.
Neurosurgery. 47:295–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chkhenkeli SA and Chkhenkeli IS: Effects
of therapeutic stimulation of nucleus caudatus on epileptic
electrical activity of brain in patients with intractable epilepsy.
Stereotact Funct Neurosurg. 69:221–224. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feinstein B, Gleason CA and Libet B:
Stimulation of locus coeruleus in man. Preliminary trials for
spasticity and epilepsy. Stereotact Funct Neurosurg. 52:26–41.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hodaie M, Wennberg RA, Dostrovsky JO and
Lozano AM: Chronic anterior thalamus stimulation for intractable
epilepsy. Epilepsia. 43:603–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benabid AL, Koudsie A, Benazzouz A, et al:
Deep brain stimulation of the corpus luysi (subthalamic nucleus)
and other targets in Parkinson’s disease. Extension to new
indications such as dystonia and epilepsy. J Neurol. 248(Suppl 3):
37–47. 2001.
|
|
24
|
Chkhenkeli SA, Sramka M, Lortkipanidze GS,
et al: Electrophysiological effects and clinical results of direct
brain stimulation for intractable epilepsy. Clin Neurol Neurosurg.
106:318–329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Velasco AL, Velasco F, Velasco M, Jiménez
F, Carrillo-Ruiz JD and Castro G: The role of neuromodulation of
the hippocampus in the treatment of intractable complex partial
seizures of the temporal lobe. Acta Neurochir. 97:329–332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spencer SS, Guimaraes P, Katz A, Kim J and
Spencer D: Morphological patterns of seizures recorded
intracranially. Epilepsia. 33:537–545. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Velasco AL, Velasco F, Velasco M, et al:
Electrical stimulation of the hippocampal epileptic foci for
seizure control: a double-blind, long-term follow-up study.
Epilepsia. 48:1895–1903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Velasco AL, Velasco F, Jimenez F and
Velasco M: Interfering with the genesis and propagation of
epileptic seizures by neuromodulation. Biomedical Engineering
Fundamentals. Bronzino JD: 3rd edition. CRC Press; Boca Raton, FL:
pp. 36.1–36.12. 2006
|
|
29
|
Velasco AL, Velasco F, Jimenez F, et al:
Neuromodulation of the centromedian thalamic nuclei in the
treatment of generalized seizures and the improvement of the
quality of life in patients with lennox-gastaut syndrome.
Epilepsia. 47:1203–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weiss SR, Li XL, Rosen JB, Li H, Heynen T
and Post RM: Quenching: inhibition of development and expression of
amygdale kindled seizures with low frequency stimulation.
Neuroreport. 6:2171–2176. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boëx C, Seeck M, Vulliémoz S, et al:
Chronic deep brain stimulation in mesial temporal lobe epilepsy.
Seizure. 20:485–490. 2011.PubMed/NCBI
|
|
32
|
Schulze-Bonhage A, Dennig D, Wagner K, et
al: Seizure control resulting from intrahippocampal depth electrode
insertion. J Neurol Neurosurg Psychiatry. 81:352–353. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fisher RS: Anterior thalamic nucleus
stimulation: issues in study design. Deep Brain Stimulation and
Epilepsy. Luders H: Martin Dunitz Inc; New York, NY: pp. 307–322.
2003
|
|
34
|
McLachlan RS, Pigott S, Tellez-Zenteno JF,
Wiebe S and Parrent A: Bilateral hippocampal stimulation for
intractable temporal lobe epilepsy: Impact on seizures and memory.
Epilepsia. 51:304–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodaie M, Wennberg RA, Dostrovsky J and
Lozano A: Chronic anterior thalamic stimulation for intractable
epilepsy. Epilepsia. 43:603–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Katariwala NM, Bakay RA, Pennel PB, Olson
LD, Henry TR and Epstein CM: Remission of intractable epilepsy
following implantation of intracranial electrodes. Neurol.
57:1505–1507. 2001. View Article : Google Scholar : PubMed/NCBI
|