Introduction
Perinatal hypoxic ischemic encephalopathy (HIE) is a
major cause of neonatal mortality and long-term neurological
disorders, including cerebral palsy, mental retardation, epilepsy
and learning disabilities (1). In
developed and non-developed countries, 2–5 of every 1,000 infants
develop perinatal HIE, with 20–40% of these infants suffering from
major neurological sequelae and growth retardation (2).
Currently, therapeutic hypothermia is commonly
administered to infants with moderate to severe asphyxia (3). While these practices yield promising
results, abnormal outcomes have been observed in almost half of the
infants treated with therapeutic hypothermia and infants with
severe damage have not been saved (4). Previous experimental data have
demonstrated that hypothermia extends the duration of the
therapeutic window (5,6) and neuroprotection may be reinforced
with certain drugs administered during this period (6–8). The
therapeutic window following HIE (i.e., the interval during which
intervention may be efficacious in preventing ongoing brain injury
and/or reducing the severity of ultimate brain injury) is short and
considered to be <6 hours (4–6).
Currently, research is focused on pre-clinical studies of agents
that may exert synergistic activity with hypothermia, with the aim
that sequelae-free survival may be improved with a combination
(8,9).
Several agents have been shown to be neuroprotective
in experimental neonatal HI models, although extremely few agents
have been used successfully in clinical practice (9). Cytokine-associated brain injury in HI
brain damage is gaining increasing attention. Several cells of the
brain (microglia, astrocytes, endothelial cells and neurons) are
known to secrete cytokines. Environmental mononuclear phagocytes, T
lymphocytes, natural killer cells and polymorphonuclear cells with
cytokine synthesizing and releasing capabilities are considered to
be capable of crossing the blood-brain barrier, contributing to
inflammation and gliosis in the brain (10).
Inflammatory mediators (cytokines and chemokines)
have been implicated in the pathogenesis of HIE and may represent a
final common pathway of brain injury (11). Animal studies indicate that
cytokines, particularly interleukin (IL)-1β, contribute to HI
damage. The exact mechanisms by which inflammatory mediators
contribute to this process remain unclear (10). Of the proinflammatory cytokines,
tumor necrosis factor-α (TNF-α), IL-6 and IL-1β have extremely
significant roles in the cytokine cascade. Primary effects of
cytokines include endothelial cell activation, leukocyte
endothelial adhesion, chemotaxis of leukocytes to inflammation
sites, secretion of free oxygen radicals, nitric oxide (NO)
synthesis, degranulation, intracellular access of sodium,
phagocytosis and procoagulant activity (12).
Although the mechanism of action of immunoglobulin
(Ig) is not yet completely understood, Ig is known to be involved
in the regulation of Fc receptor expression and function,
complement activation and cytokine network activity. In addition,
Ig is involved in the provision of anti-idiotypic antibodies, as
well as the activation, differentiation and functional regulation
of T and B cells (13).
Administration of Ig to mice in an induced stroke model has been
shown to reduce the infarct volume by decreasing C3b expression at
the ischemic site and apoptosis (14). In addition, Ig is involved in
reducing post-stroke complement-mediated cell damage and
suppressing the activation of microglia and astrocytes, which
reduces the release of proinflammatory cytokines and suppresses
caspase-3 activation. This in turn reduces the activation of
endothelial cells and thus protects the integrity of the
blood-brain barrier (15).
The objective of the present study was to evaluate
the neuroprotective effects of Ig, an antibody with activity at
various stages of the HIE process, which is used in clinical
practice for the treatment of several disorders of neonates, in a
neonatal HI rat model.
Materials and methods
Animals
Seven-day-old Wistar rats (n=40) of either gender,
which were delivered spontaneously, were used in this experimental
study. Rat pups were obtained from Experimental Animal Unit of
Akdeniz University (Antalya, Turkey). The study was performed with
the approval of the Ethics Committee of Akdeniz University (Faculty
of Medicine, Antalya, Turkey). Data for the control and hypoxia
groups were obtained from our recent study (16).
Animal preparation and surgical
procedure
Rat pups were anaesthetized by ether inhalation and
the duration of anaesthesia was <5 min. HI brain injury was
induced according to the Levine-Rice model (17). Briefly, a median incision was made
in the neck and, under microscopic magnification, the left common
carotid artery was dissected and ligated with a 6–0 silk suture.
Following the suturing of the wound, the animals were allowed a 3-h
recovery and feeding period. In control group animals, neither
ligation, nor hypoxia was performed (sham surgery). With the
exception of the control group, rats were then placed in a plastic
chamber and exposed to a continuous flow of 8% oxygen and 92%
nitrogen for 2 h. Following the hypoxic period, the rats had a 2-h
recovery period in an open chamber without any supplemental oxygen.
The animals in the control group were placed in an open chamber for
the same intervals. The chambers were partially submerged in a
water bath at 37ºC to maintain a constant thermal environment.
Following these procedures, all pups were sacrificed by
decapitation.
Seven-day-old pups were randomly divided into three
groups: Control (n=8), following the median neck incision, neither
ligation nor hypoxia was performed; hypoxia (n=16), 0.5 ml saline
was injected intraperitoneally immediately following hypoxia; and
Ig + hypoxia (n=16; Octagam®; Octapharma, Vienna,
Austria), rat pups were intraperitoneally administered 1 g/kg Ig
immediately following hypoxia. Eight rats from each of the hypoxia
and Ig + hypoxia groups were sacrificed 4 and 24 h following drug
administration. The rats in the control group were decapitated at
the 4 h time-point. Caspase-3 activity and IL-1β, IL-6 and TNF-α
mRNA expression levels were studied in the left half of the
brain.
Determination of TNF-α, IL-6 and IL-1β
mRNA expression levels by qPCR
Total RNA was isolated from the tissues of each
group using a Purelink RNA mini kit (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
High capacity RNA to cDNA master mix (Applied Biosystems, Foster
City, CA, USA) was used for cDNA synthesis in each sample. TaqMan
gene expression master mix (Applied Biosystems), TNF-α, IL-6 and
IL-1β primers and probes (Taqman Gene Expression Assay numbers:
Rn00562055_m1, Rn01410330_m1 and Rn00580432_m1; Applied Biosystems)
were used for PCR amplification using the Light Cycler 480 II
Real-Time PCR System (Roche Applied Science, Mannheim, Germany).
TaqMan PCR was carried out according to the manufacturer's
instructions. GAPDH (Taqman Gene Expression Assay number:
Rn01775763_g1; Applied Biosystems) was used as a housekeeping gene
for this experiment. In order to identify the relative TNF-α, IL-6
and IL-1β mRNA expression levels in the hypoxia and Ig + hypoxia
groups, 2−ΔΔCt (fold change) values were calculated, as
described previously (18).
Caspase-3 colorimetric assay
Active caspase-3 levels were measured using a
commercial kit (Caspase-3/CPP32 Colorimetric Assay kit; BioVision,
Milpitas, CA, USA) according to the manufacturer's instructions.
The assay was based on spectrophotometric detection of the
chromophore p-nitroaniline (pNA) following cleavage from the
labeled substrate, Asp-Glu-Val-Asp-pNA. The pNA light emission was
quantified using a microtiter plate reader (Multiskan Spectrum,
Thermo Labsystems, Franklin, MA, USA) at 405 nm. Values were
expressed as arbitrary unit/μg protein.
Statistical analysis
Data were analyzed using the statistical package
program SPSS for Windows version 18.0 (SPSS, Inc., Chicago, IL,
USA). Descriptive statistics, including frequency distribution,
mean and SD, were used to describe the sample. Kruskal-Wallis
variance analysis was utilized to determine inter-group
differences; paired groups were compared using a Mann-Whitney U
test for analyses yielding significant results; time-dependent
variables were analyzed by Wilcoxon test and the correlations among
the variables were examined using Spearman's correlation. P<0.05
was considered to indicate a statistically significant
difference.
Results
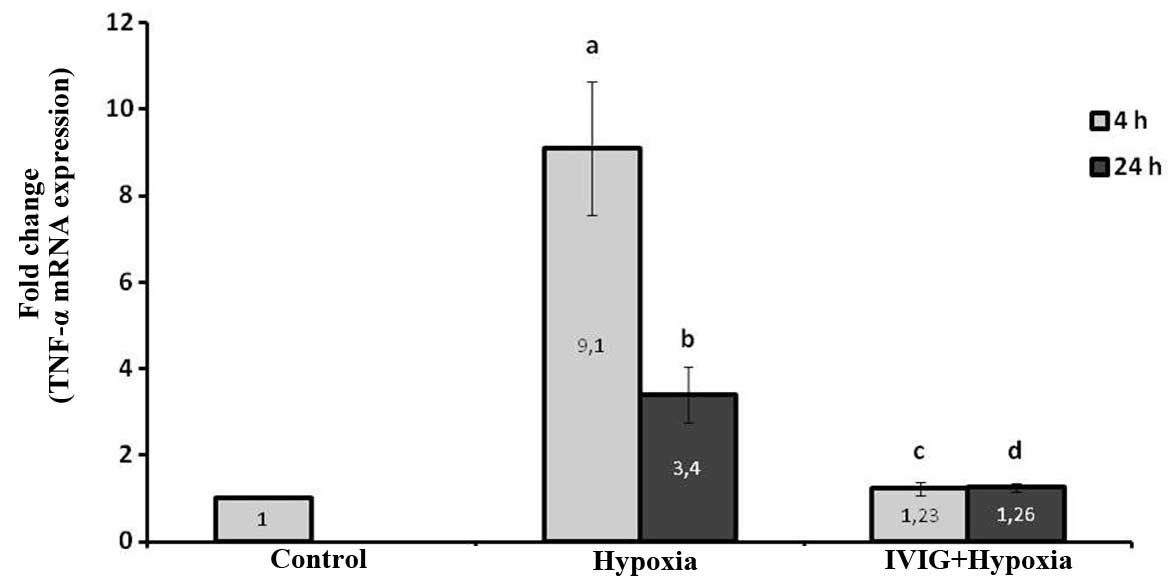
Effect of Ig on TNF-α mRNA expression
levels in ischemic left hemispheres
Induction of cerebral ischemia increased TNF-α mRNA
expression levels significantly at 4 and 24 h, following ischemia
in the left ischemic hemispheres in the hypoxia group compared with
those in the control (P=0.002 for 4 and 24 h; Fig. 1).
The systemic administration of Ig significantly
reduced the TNF-α mRNA expression levels in ischemic tissue at 4
and 24 h following HIE compared with those in the hypoxia group
(P=0.004 for 4 and 24 h; Fig. 1).
These results indicate that Ig reduced TNF-α production in cortical
ischemic tissue following hypoxia.
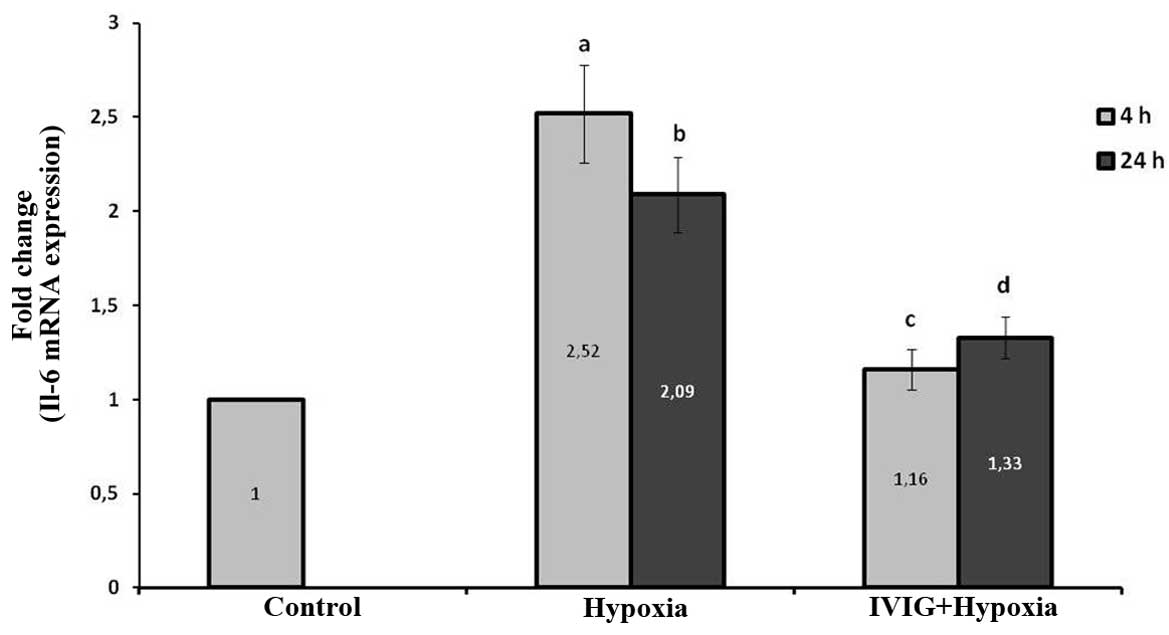
Effect of Ig on IL-6 mRNA expression
levels in ischemic left hemispheres
The induction of cerebral ischemia increased IL-6
mRNA expression levels significantly at 4 and 24 h following
ischemia in the left ischemic hemispheres in the hypoxia group
compared with those in the control group (P=0.002 for 4 and 24 h;
Fig. 2).
Systemic administration of Ig significantly reduced
the IL-6 mRNA expression levels in ischemic tissue at 4 and 24 h
following HIE compared with those in the hypoxia group (P=0.004 for
4 and 24 h; Fig. 2). These results
indicate that Ig reduced IL-6 production in cortical ischemic
tissue following hypoxia.
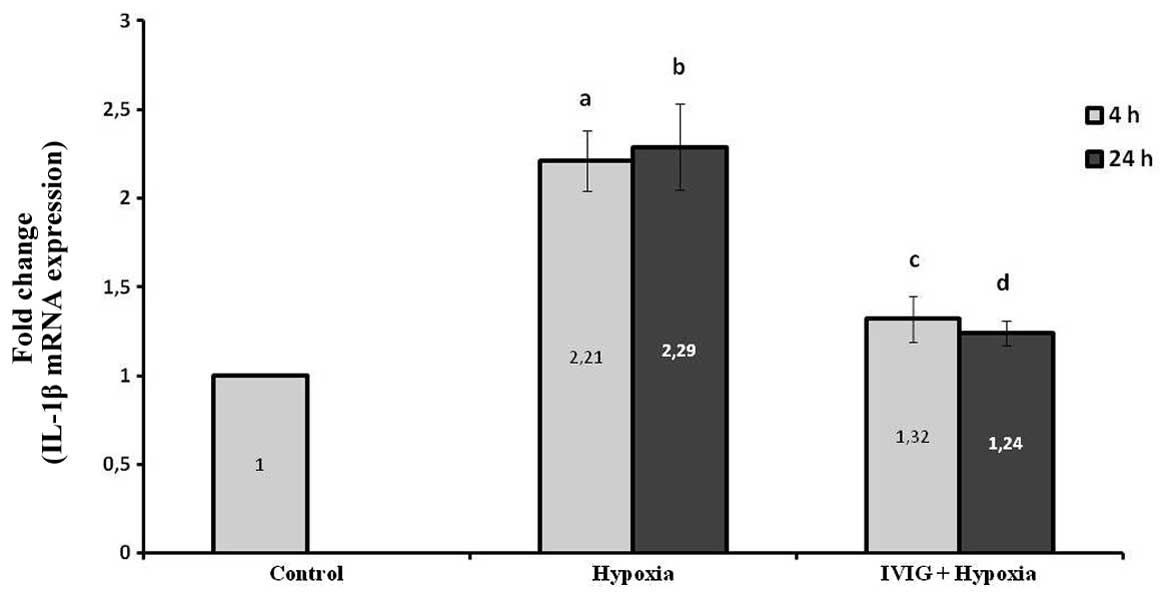
Effect of Ig on IL-1β mRNA expression
levels in ischemic left hemispheres
The induction of cerebral ischemia increased IL-1β
mRNA expression levels significantly at 4 and 24 h following
ischemia in the left ischemic hemispheres in the hypoxia group
compared with those in the control (P=0.002 for 4 and 24 h;
Fig. 2).
Systemic administration of Ig significantly reduced
the IL-1β mRNA expression levels in ischemic tissue at 4 and 24 h
following HIE compared with those in the hypoxia group (P=0.01 for
4 h and P=0.005 for 24 h; Fig. 3).
These results indicate that Ig reduced IL-1β production in cortical
ischemic tissue following hypoxia.
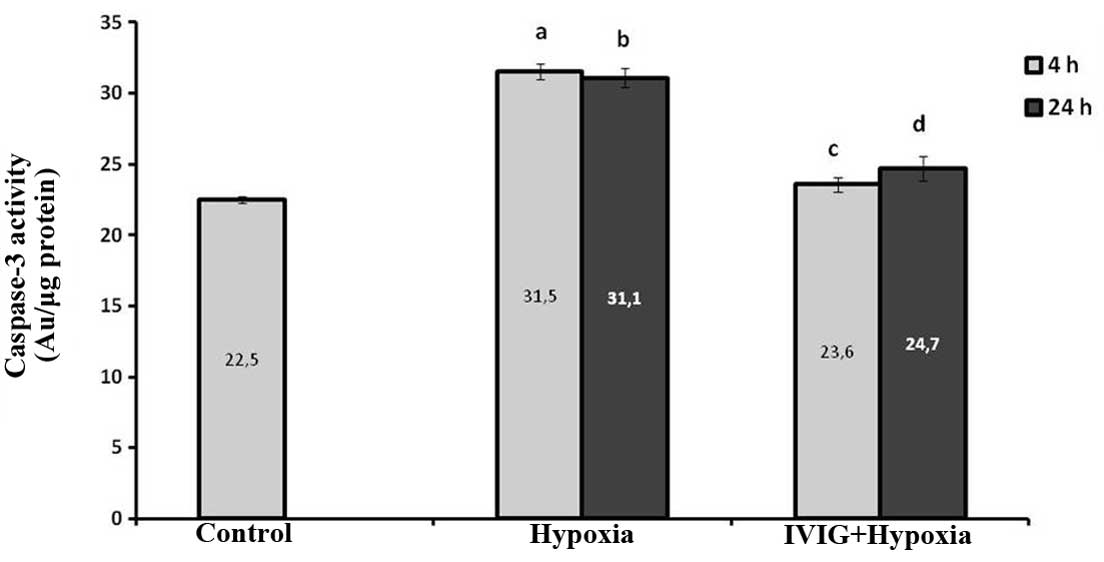
Effect of Ig on caspase-3 activities in
ischemic left hemispheres
As shown in Fig. 4,
caspase-3 activity in the left half of the brains in the hypoxia
group were found to have increased significantly compared with
those in the control group (P=0.004 for 4 and 24 h). Caspase-3
activities in the brains of the Ig + hypoxia group were
significantly lower than those of the hypoxia group (P=0.004 for 4
and 24 h; Fig. 4).
Discussion
In previous studies, the latent phase between the HI
event and cell death was found to be extremely critical in HI
injury and this period was named the ‘therapeutic window’ (4–8).
This period may range between 6 and 12 h in a neonate who has
suffered from hypoxia and ischemia. Terminating the cascade of
molecular events that develop during the therapeutic window phase
and lead to cell death or limiting the cascade at a certain point
is extremely critical in reducing or preventing the sequelae
(9).
It is well established that excitotoxicity and free
radical production have negative contributions to the early
ischemic response. However, significant functional improvements
have not been achieved in clinical and experimental studies where
various agents were administered selectively for treatment or
stopping progression during the stages of this mechanism. A second
wave of necrosis occurs in response to an ischemic attack, which is
mediated by the neuroinflammatory response to the damage (19). Accumulated evidence demonstrates
that targeting the late neuroinflammatory response with the
treatment may be a promising approach for therapeutic intervention
(19,20).
Rat models of HI injury provide a convenient method
for understanding histopathological and biochemical outcomes, as
well as the long-term neurological impacts of HIE (21). Rats aged 7 days were used in the
present study. The brains of a 7-day-old rat litter were considered
to be relevant for the perinatal period in humans, particularly
with regard to brain development in the latter species (22).
Previous studies have shown increased numbers of
apoptotic neurons in both brain hemispheres, with a more pronounced
increase in the hemisphere with carotid artery blockage, following
ischemia and 1–3 h hypoxia in neonatal rats. These studies
indicated that the acute effects of HIE may be managed with agents
that reduce apoptosis (23,24).
In the present study, a significant increase was observed in
caspase-3 levels in the hypoxia group compared with those in the
control at 4 and 24 h following HI induction.
The pathophysiological role of inflammatory
cytokines in the development of HI brain damage has been studied in
clinical and experimental studies. Overall, IL-1β has been shown to
potentiate ischemic brain damage. Rapid elevations of IL-1β levels
in the brain tissue have been demonstrated following HI injury or
transient mid-cerebral artery occlusion in neonatal rats (25,26).
In the present study, increased IL-1β mRNA expression was observed
following 4 and 24 h in the hypoxia group compared with the
control.
In addition, TNF-α, IL-6 and IL-1β are known to be
the major inflammatory mediators with increased levels in HIE.
These cytokines are released by peripheral monocytes and
macrophages, as well as by astrocytes and microglia of the central
nervous system (26,27). The primary effects of cytokines
include endothelial cell activation, leukocyte endothelial
adhesion, chemotaxis of leukocytes to an inflammation site,
secretion of free oxygen radicals, NO synthesis, degranulation,
intracellular sodium access, phagocytosis and procoagulant activity
(28).
TNF-α is known to be an apoptosis activator that
potentiates Fas expression and potentially results in neuronal
apoptosis (22). An experimental
model has also shown that there is a transient increase in IL-1β
and TNF-α levels, peaking 6 h following hypoxia and that brain
damage was prevented with TNF-α inhibition (29). Similarly, in the present study,
increased TNF-α mRNA expression levels were observed at 4 and 24 h
in the hypoxia group compared with those in the control group and
apoptosis was reduced by the suppression of elevations in TNF-α
mRNA expression in the treatment groups.
Given the aforementioned benefits of Ig treatment,
studies have investigated its effects on the inflammatory cascade
and apoptosis by inducing stroke in adult rats. Arugman et
al (14) reported almost
complete elimination of mortality and a 50–60% reduction in infarct
size with Ig administration in adult rats exposed to experimental
stroke.
To the best of our knowledge, there are no
experimental studies in the literature examining the effects of Ig
on neonatal HIE and there are only two clinical studies. Chen et
al (30) compared the efficacy
of Ig with that of routine treatment in neonates with HIE. The
authors reported improvements in abnormal primitive reflex duration
and muscle tone, the elimination of convulsions and a shorter
duration of hospitalized care for the group treated with Ig
compared with the group receiving routine treatment. The authors
also concluded that Ig alleviated brain damage and multi-organ
dysfunction and that HIE duration was shortened by the inhibition
of IL-6 and TNF-α production. In a similar study by Dong et
al (31), levels of IL-6, 8
and 10 decreased significantly on day 3 relative to those on day 0
in neonates with HIE treated with Ig. Decreased levels were not
observed in the hypoxic group without Ig treatment. The authors
therefore hypothesized that Ig treatment may provide a short-term
improvement of brain damage in neonates with HIE.
In the present experimental model, Ig was selected
as an anti-inflammatory agent to prevent cerebral apoptosis by
reducing or preventing an inflammatory response. The effects of Ig
on cerebral apoptosis in a neonatal HI rat model were evaluating in
this novel study. The observations indicate that Ig administration
may be an efficient treatment approach for reducing cerebral
apoptosis, based on significantly lower IL-6, IL-1β and TNF-α mRNA
expression levels and caspase-3 activity in the animals treated
with Ig, as measured at 4 and 24 h following HI injury with a
colorimetric method. Ig contains high-affinity neutralizing
antibodies against IL-1β, IL-6 and TNF-α in quantities that are
sufficient to suppress circulating proinflammatory pathogenic
cytokines or downregulate the synthesis of cytokines by T cells
(32). The modulation of cytokines
and cytokine antagonists by Ig is another major mechanism by which
Ig exerts its anti-inflammatory effects. Ig has been shown to
selectively trigger the production of IL-1 receptor antagonist, the
natural antagonist of IL-1 (33).
In the present study, a correlation analysis between
TNF-α/IL-1β mRNA expression levels and infarct size was not
performed as the volumes of the infarct sizes were not measured.
The aim was to ascertain cytokine gene expression and caspase-3
activation in the area of infarction; therefore no examinations
were performed on the contralateral hemisphere.
In conclusion, the experimental model of the present
study indicated that Ig therapy reduced caspase-3 activity, thereby
reducing apoptosis to a significant extent. Ig also reduced TNF-α,
IL-6 and IL-1β expression. Ig may also provide therapeutic effects
in stroke through the inhibition of cytokines and the subsequent
infiltration of inflammatory cells, thus reducing inflammation in
the region of infarction. Traditional treatment of HIE is
supportive care. Therapeutic hypothermia has become common practice
in a number of institutions since a benefit in moderate to severe
encephalopathic newborns has been observed; however, it does not
completely protect or repair an injured brain; therefore, the
search for adjuvant therapies continues. Ig may be a candidate drug
for combining with therapeutic hypothermia in the treatment of HIE.
However, further studies are required to investigate this.
References
|
1
|
Volpe JJ: Hypoxic-ischemic encephalopathy:
clinical aspects. Neurology of the Newborn. 5th edition. WB
Saunders; Philadelphia, PA: pp. 400–480. 2008
|
|
2
|
Perlman JM: Pathogenesis of
hypoxic-ischemic brain injury. J Perinatol. 27:S39–S46. 2007.
View Article : Google Scholar
|
|
3
|
Kapetanakis A, Azzopardi D, Wyatt J and
Robertson NJ: Therapeutic hypothermia for neonatal encephalopathy:
a UK survey of opinion, practice and neuroinvestigation at the end
of 2007. Acta Paediatr. 98:631–635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gluckman PD, Wyatt JS, Azzopardi D,
Ballard R, Edwards AD, Ferriero DM, et al: Selective head cooling
with mild systemic hypothermia after neonatal encephalopathy:
multicentre randomised trial. Lancet. 365:663–670. 2005. View Article : Google Scholar
|
|
5
|
O'Brien FE, Iwata O, Thornton JS, De Vita
E, Sellwood MW, Iwata S, et al: Delayed whole-body cooling to 33 or
35 degrees C and the development of impaired energy generation
consequential to transient cerebral hypoxia-ischemia in the newborn
piglet. Pediatrics. 117:1549–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Barks JD, Xu G and Silverstein FS:
Topiramate extends the therapeutic window for hypothermia-mediated
neuroprotection after stroke in neonatal rats. Stroke.
35:1460–1465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma D, Hossain M, Chow A, Arshad M, Battson
RM, Sanders RD, et al: Xenon and hypothermia combine to provide
neuroprotection from neonatal asphyxia. Ann Neurol. 58:182–193.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jatana M, Singh I, Singh AK and Jenkins D:
Combination of systemic hypothermia and N-acetylcysteine attenuates
hypoxic-ischemic brain injury in neonatal rats. Pediatr Res.
59:684–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelen D and Robertson NJ: Experimental
treatments for hypoxic ischaemic encephalopathy. Early Hum Dev.
86:369–377. 2010. View Article : Google Scholar
|
|
10
|
Dammann O and O'Shea TM: Cytokines and
perinatal brain damage. Clin Perinatol. 35:643–663. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McAdams RM and Juul SE: The role of
cytokines and inflammatory cells in perinatal brain injury. Neurol
Res Int. 2012:5614942012.PubMed/NCBI
|
|
12
|
Lakhan SE, Kirchgessner A and Hofer M:
Inflammatory mechanisms in ischemic stroke: therapeutic approaches.
J Transl Med. 7:972009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durandy A, Kaveri SV, Kuijpers TW, Basta
M, Miescher S, Ravetch JV and Rieben R: Intravenous immunoglobulins
- understanding properties and mechanisms. Clin Exp Immunol.
158(Suppl 1): 2–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arumugam TV, Tang SC, Lathia JD, Cheng A,
Mughal MR, Chigurupati S, et al: Intravenous immunoglobulin (IVIG)
protects the brain against experimental stroke by preventing
complement-mediated neuronal cell death. Proc Natl Acad Sci USA.
104:14104–14109. 2007. View Article : Google Scholar
|
|
15
|
Arumugam TV, Woodruff TM, Lathia JD,
Selvaraj PK, Mattson MP and Taylor SM: Neuroprotection in stroke by
complement inhibition and immunoglobulin therapy. Neuroscience.
158:1074–1089. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalay S, Oztekin O, Tezel G, et al: The
effects of intraperitoneal pentoxifylline treatment in rat pups
with hypoxic ischemic encephalopathy. Pediatr Neurol. 49:319–323.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rice JE III, Vannucci RC and Brierley JB:
The influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 9:131–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
VanGuilder HD, Vrana KE and Freeman WM:
Twenty-five years of quantitative PCR for gene expression analysis.
Biotechniques. 44:619–626. 2008.PubMed/NCBI
|
|
19
|
Leonardo CC and Pennypacker KR:
Neuroinflammation and MMPs: potential therapeutic targets in
neonatal hypoxic-ischemic injury. J Neuroinflammation. 6:132009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lai AY and Todd KG: Microglia in cerebral
ischemia: molecular actions and interactions. Can J Physiol
Pharmacol. 84:49–59. 2006.PubMed/NCBI
|
|
21
|
Yager JY: Animals models of
hypoxic-ischemic brain damage in the newborn. Semin Pediatr Neurol.
11:31–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Northington FJ, Graham EM and Martin LJ:
Apoptosis in perinatal hypoxic-ischemic brain injury: how important
is it and should it be inhibited? Brain Res Rev. 50:244–257. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atici A, Bozlu G, Turhan AH, Polat A,
Nayci A, Okuyaz C and Taskinlar H: The role of trapidil on neuronal
apoptosis in neonatal rat model of hypoxic ischemic brain injury.
Early Hum Dev. 84:243–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bozlu G, Atici A, Turhan AH, Polat A,
Nayci A, Okuyaz C and Taskinlar H: Platelet-activating factor
antagonist (ABT-491) decreases neuronal apoptosis in neonatal rat
model of hypoxic ischemic brain injury. Brain Res. 1143:193–198.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Denker SP, Ji S, Dingman A, Lee SY,
Derugin N, Wendland MF and Vexler ZS: Macrophages are comprised of
resident brain microglia not infiltrating peripheral monocytes
acutely after neonatal stroke. J Neurochem. 100:893–904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oygür N, Sönmez O, Saka O and Yeǧin O:
Predictive value of plasma and cerebrospinal fluid tumour necrosis
factor-alpha and interleukin-1 beta concentrations on outcome of
full term infants with hypoxic-ischemic encephalopathy. Arch Dis
Child Fetal Neonatal Ed. 79:F190–F193. 1998.PubMed/NCBI
|
|
27
|
Foster-Barber A, Dickens B and Ferriero
DM: Human perinatal asphyxia: correlation of neonatal cytokines
with MRI and outcome. Dev Neurosci. 23:213–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kilpatrick L and Harris MC: Cytokines and
the inflammatory response. Fetal and Neonatal Physiology. Polin RA,
Fox WW and Fletcher J: 2nd edition. WB Saunders; Philadelphia, PA:
pp. 1967–79. 1998
|
|
29
|
Galasso JM, Wang P, Martin D and
Silverstein FS: Inhibition of TNF-alpha can attenuate or exacerbate
excitotoxic injury in neonatal rat brain. Neuroreport. 11:231–235.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen S, Yang X and Li CR: Immunoglobulin
therapy in neonates with hypoxic ischemic encephalopathy. Chin J
Pediatr. 3:38–42. 2000.(In Chinese).
|
|
31
|
Dong YN, Zhang QY, Sui ZG, et al: Short
term effects of high-dose intravenous immunoglobulin therapy in
neonates with hypoxic ischemic encephalopathy. Med J Qilu. 1:23–27.
2006.
|
|
32
|
Toungouz M, Denys CH, De Groote D and
Dupont E: In vitro inhibition of tumour necrosis factor-alpha and
interleukin-6 production by intravenous immunoglobulins. Br J
Haematol. 89:698–703. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crow AR, Song S, Semple JW, Freedman J and
Lazarus AH: A role for IL-1 receptor antagonist or other cytokines
in the acute therapeutic effects of IVIg? Blood. 109:155–158. 2007.
View Article : Google Scholar : PubMed/NCBI
|