Introduction
Cerebral ischemic stroke has a high incidence of
morbidity and mortality and results in a severe disability with a
complex pathogenesis, which is unclear. Thus, this condition
threatens the safety and quality of life of humans. Although the
treatment for cerebral ischemic stroke has significantly improved
recently, none of the available drugs for its treatment and
prevention are considered to be optimal. The establishment of an
optimal animal model, which simulates human cerebral ischemia may
allow future investigations into the pathogenesis of, and
prevention measures for, cerebral ischemia in humans.
Rats are selected as experimental animals due to
their similarities to humans with regard to cerebrovascular anatomy
and function. Furthermore, rats are of a standard species or
strain, exhibit good intraspecific homozygosity and experimental
repeatability and routine monitoring can be conveniently performed.
There are numerous factors that influence the selection of rats as
models; for example, oestrogen protects female rats from cerebral
injury, therefore, male rats are included in investigations
(1). Elderly rats are excluded due
to the anatomical and pathological changes, which occur in their
middle cerebral artery (MCA) and common carotid artery (CCA), such
as tortuosity, luminal stenosis and arteriosclerosis (2). Furthermore, model establishment using
adult rats leads to a relatively high success rate (3). Moreover, certain species of rats
affect the model; for example, a small variation in the MCA of
Fischer-344 rats results in a good consistency of cerebral infarct
volume in the MCA occlusion (MCAO) model, whereas Wistar-Kyoto rats
exhibit the smallest cerebral infarct volume and exhibit the
largest variation. Characteristics of Sprague-Dawley (SD) rats fall
between the abovementioned rat species; therefore, Fischer-344 rats
are optimal for model establishment, however, they are rarely used
as they are difficult to obtain. SD rats differ marginally from
Wistar rats; however, intraoperative bleeding is minimal in SD
rats, which makes surgery less complex. SD rats grow at a faster
rate, are milder and less expensive; thus, they are often selected
as experimental animals (4).
MCAO is the predominant cause of cerebral ischemic
stroke (5) and the suture-occluded
method is extensively used to establish MCAO models for performing
clinical research (6). The
conventional suture-occluded method established by Longa et
al (7) is the prevailing
method for the focal cerebral infarction model. However, it is
complicated, time consuming and inevitably results in ischemic
infarction of tissues (such as the temporalis, lingualis and
pharyngeal muscles), to which the external carotid artery (ECA)
supplies blood, which leads to further complications. To provide an
optimal, reliable and simple animal model for FCIR injury research,
a modified suture-occluded method of Longa et al was used.
The experience of establishing a model using a modified process
through anatomical observation of rats and an analysis of the key
steps in establishing the model was also summarised.
Materials and methods
Animals
In total, 24 healthy, male SD rats (weight, ~220–260
g) were obtained from the Laboratory Animal Center of Southern
Medical University (Guangdong, China) and were randomised into the
sham-surgery (n=12) and surgery (n=12) groups. In each group, six
rats were used for tetrazolium (TTC) staining and six to obtain
tissue sections. The present study was conducted in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of the First Affiliated Hospital of
Xinxiang Medical University (Henan, China).
Suture preparation
Nylon sutures (diameter, ~0.2–0.25 mm) were cut to a
60 mm length. One end of the suture was heated to form a smooth,
spherical shape (diameter, ~0.26–0.3 mm), which was observed under
a BH2 microscope (Olympus Corporation, Tokyo, Japan); the texture
of the opposite end was rough. The suture was labelled with the
required length, sterilised with alcohol and stored in heparin
saline solution.
Establishment of the experimental
model
The rats were fasted for 24 h prior to the
experimental procedures, anaesthetised intraperitoneally using 10%
chloral hydrate (3 ml/kg), fixed in a supine position and incised
at the midline of the neck, (incision length, 20 mm). The left CCA,
internal carotid artery (ICA) and external CA (ECA) were exposed.
The proximal CCA was ligated and the suture was suspended around
the distal CCA for subsequent use, then the ECA was ligated at the
bifurcation of the CCA. The proximal ICA was tied with a slipknot
and the distal ICA was clamped using an artery clamp. A nylon
suture was introduced into the ICA lumen through a puncture wound
from a micro-incision, which was 5-mm distal to the CCA
bifurcation. The slipknot was tightened and the artery clamp was
removed. Insertion ceased once ~19 mm of nylon suture had been
inserted or until resistance was felt (where the tip of the suture
reached the origin of the MCA), which resulted in the occlusion.
The prepared suture was tied and fixed with a subcutaneously
embedded thread residue; subsequently, the incision was closed.
After 2 h, the rats were anaesthetised with diethyl ether, all the
occlusions were removed (2 needles) and the 10 mm nylon suture was
withdrawn gently to reperfuse blood flow. In the sham-surgery
group, 10 mm of nylon suture was inserted into the ICA lumen, and
the remaining procedures were performed as described above. The
intraoperative room temperature was maintained at ~20–30°C and the
postoperative anal temperature of the rats was 37±0.5°C.
Neural function deficit score (NFDS)
The neurological status of the rats was assessed 24
h following ischemia-reperfusion (I/R) according to the method
described by Longa et al (7). The rats were classified into five
grades: Grade 0, no neurological impairment; grade 1, mild
neurological impairment, failure to fully stretch the contralateral
forelimb; grade 2, moderate neurological impairment, rotation to
the contralateral side; grade 3, severe focal neurological
impairment, toppling to the contralateral side; and grade 4, unable
to walk spontaneously, exhibiting conscious disturbance.
Tetrazolium (TTC) staining. The rats were
intraperitoneally overdosed with 10% chloral hydrate and were
sacrificed by decapitation 72 h following I/R. Their brains were
immediately harvested and frozen at −20°C. After 20 min, five
coronal sections (2 μm) were incised from the upper forehead using
a tissue slicer and incubated in TCC staining solution at 37°C for
30 min. The sections were then fixed in fresh 10% formaldehyde for
≥24 h.
Nissl staining
The rats were intraperitoneally overdosed with 10%
chloral hydrate and sacrificed by decapitation 72 h after I/R. The
chests were opened and the left ventricle (LV) was intubated; the
blood was flushed with 4°C normal saline, followed by fixation of
the LV with 4% paraformaldehyde phosphate buffer, overnight, at
4°C. The LV was stored in 20–30% sucrose phosphate buffer at 4°C
until the tissue sank to the bottom of the solution. The temporal
lobe was frozen and serially cut. The section was stained in
Toluidine blue (Wuhan Boster Biotechnology Company, Wuhan, China)
at 37°C for 5 min, washed in distilled water for 3 min.
Conventional dehydration was then conducted using gradient alcohol
and the lobe sections were mounted using a neutral balsam.
Results
Neurobehavioral observation
In the sham-surgery group, no neurobehavioral
abnormality of the contralateral limb was observed: the rats were
active and walked and drank well. Neurobehavioral impairments were
evident in the surgery group: i) The rats exhibited hydroadipsia
and paralysis of the contralateral limb, turned or rotated to the
contralateral side, limped, and appeared dispirited and inactive
(Fig. 1). ii) The results of the
tail suspension test were positive, the rats failed to bend their
contralateral forelimb when their tails were held, or were not able
to independently move their contralateral limbs, and demonstrated
spontaneous rotation to the contralateral side (Fig. 2). iii) The rats exhibited a
decreased ipsilateral palpebral fissure and corestenoma (Fig. 3). A score of 1–3 indicated that the
model had passed the test.
TTC staining
In the sham-surgery group, no infarcts were observed
72 h following I/R, whereas the surgery group demonstrated large
areas of infarct, which were predominantly located in the parietal
cortex and lateral striatum, or at the hippocampus and cerebral
hemisphere. The left middle cerebral artery appeared white in
colour, indicating that there was no haemorrhaging (Fig. 4). Furthermore, no apparent
haemorrhaging was observed in the infarcts.
Nissl staining
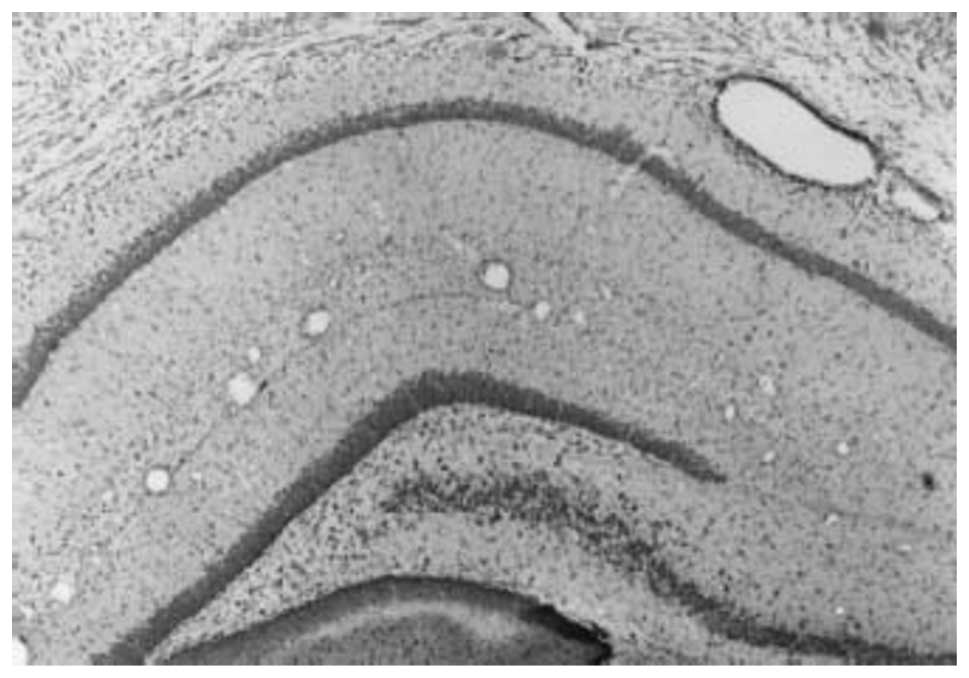
The Nissl-stained histological sections were
observed under a BH2 microscope. In the sham-surgery group, each
layer of neurons in the cerebral cortex was densely arranged,
specifically in layers II to VI. Occasional neuron distribution in
layer I was observed. In layer V, the pyramidal cells were closely
arranged and had larger cell bodies. A dense distribution of
pyramidal cells was observed in the hippocampal CA1 region, which
was divided into 2–5 layers. The cell contour was clear, with
root-like apical dendrites that were apparent towards the radial
layer (Fig. 5). In the surgery
group, the ischemic injury involved the cerebral cortex,
hippocampus, dentate gyrus and additional brain regions. The
cerebral cortex presented six layers and the neurons were
predominantly distributed in layers II to IV and exhibited a
decreased density. The number of pyramidal cells in layer V was
markedly decreased. In the hippocampal CA1 region, the pyramidal
cell layer was thin, with a sparse distribution (Fig. 6).
In the sham-surgery group, the neurons in the
cerebral cortex demonstrated intact morphologies. The Nissl bodies
appeared purple-blue in color, whereas the nucleus was light blue.
The cells were plump, with evenly distributed Nissl bodies observed
in the cytoplasm (Fig. 7). In the
surgery group, the neurons in the cerebral cortex demonstrated
ischemic changes, including decreased neuron density, shortened
apical dendrites, swollen cells (which appeared to be ruptured),
broadened intercellular spaces and neuron disintegration (Fig. 8).
Discussion
Ischemic cerebrovascular disease is a predominant
human cerebrovascular disease and >60% of cases frequently occur
in the MCA. The cerebrovascular structure of rats is similar to
that of humans in that the ICA and vertebrobasilar system form the
Circle of Willis, which supplies blood to the brain through the
arterial branches. Thus, rats are recognised as the optimal animal
for establishing a cerebral ischemic model. The model is divided
into global and focal cerebral I/R, according to the ischemic
scope, or persistent and transient ischemia, according to the
ischemia time. Among all of the animal models pertaining to
strokes, the predominant model is MCAO, which is induced by focal
cerebral ischemia in rats (8).
MCAO can result in dysneuria, such as, typical limb hemiplegia and
cerebral infarcts, which facilitate the observation and assessment
of cerebral ischemia. Various methods of establishing a rat model
of ischemia have been proposed including electrocoagulation, the
suture-occluded method, cerebral embolisation, photochemically
induced thrombosis and mechanical occlusion with craniectomy. The
suture-occluded method is the commonly used approach to establish a
cerebral infarction rat model (9–12),
as demonstrated by Koizumi et al (10) in 1986 and improved by Longa et
al in 1989 (7). The
suture-occluded method without craniotomy is specifically suitable
for investigations into FCIR injury due to the minor trauma area
and the constant ischemic area (13). However, Longa’s method requires
separation and ligation of the ECA and the extracranial branch of
the ICA-pterygopalatine artery as well as insertion of the suture
into the ECA, which is complex to perform in rats due to the small
surgical field, difficulty of separation, tendency for bleeding and
the involvement of microinstruments; thus, its application is
limited.
The suture-occluded method of Zea-Longa (7) was used in the present study to
establish the FCIR model, with certain modifications: i) Incisions
were made at the bifurcation of CCA to insert the suture from the
CCA to the ICA, which avoided the insertion into the ECA and
bypassed the bend at the CCA bifurcation; thus, suturing was
straightforward. ii) Cerebral infarction severity was not affected
by ligation of the pterygopalatine artery. The suture was
mistakenly inserted into the pterygopalatine artery, however, the
resistance was felt after inserting 10 mm of suture; thus, it was
withdrawn and adjusted to a different angle, which was consistent
with the study (8). iii) The
suture was subcutaneously embedded following establishment of the
models; reperfusion was conducted under rapid anaesthesia by
unpicking one to two stitches, withdrawing the suture to the CCA,
cutting the suture near to the skin and closing the incision. This
generally avoided vessel injury, on removal of the suture, as well
as movement and slippage of the suture that resulted from movement
of the rat following revival, which enhanced the success rate and
reliability of the model. iv) Additional surgery did not influence
the healing; rapid anaesthesia with diethyl ether enabled the
reperfusion process to be completed in 5 min without altering the
experimental conditions, which ensured homogeneity of the model.
However, there were numerous factors that affected the model: the
surgical process was standardised as it was performed at the same
time each day, the rats were randomised into different groups, the
age, gender and body weight of the rats was consistent, in addition
to maintaining a constant diameter of the nylon suture and the
spherical shape at the ends.
In vivo evaluation of a successful rat model
includes monitoring the MCA blood flow and electroencephalograph,
distinction between cortical and subcortical infarction by positron
emission tomography and magnetic resonance imaging (MRI), detection
of the cerebral ischemic core and determination of the ischemic
penumbra (14,15). Instrument monitoring is accurate,
however, it is expensive and limited by the experimental
conditions; specifically when a large quantity of animals are
investigated. An optimal NFDS system should be non-invasive, low
cost, reliable, time saving and straightforward to perform. The
symptoms of focal cerebral ischemia are characterised by
contralateral limb dysfunction; therefore, the neuropathy symptom
score is internationally regarded as a general criteria. Numerous
scoring methods exist including, the Bederson method (16), the Nagaoka method (17), the Longa method (7), the Tatlisumak method (18) as well as the Hattori method
(19). Previous studies have
confirmed that the NFDS is able to evaluate MCA infarction due to
the close association between them, moreover, behavioural scores
have been demonstrated to correlate with the cerebral infarct
volume (20). During the
establishment of the MCAO model using numerous SD rats, Boyko et
al (21) identified the
association of the behavioural score with the infarct size and
demonstrated the correlation between the predicted and actual
values using MRI. Neurobehavioral analysis, independent of any
devices, is a valuable, effective, reliable and sensitive method of
evaluating the nervous system. Numerous scholars have suggested
increasingly comprehensive evaluation methods, including the
scoring of neurological and vestibular function in ischemic
animals, which results in an improved assessment. The NFDS by Longa
et al, was used in the present study and the model was
labelled as successful if the rats accorded with any indicators of
the scoring system. However, ipsilateral Horner’s syndrome resulted
from the intraoperative injury of the superior cervical sympathetic
nerve, which resulted in a decreased palpebral fissure and
corestenoma and, therefore, failed to serve as an individual sign
of a successful model.
The surgical principle of the FCIR model was: The
suture (length, 19 mm) in the ICA simultaneously blocked the two
sources of arterial blood in the Circle of Willis; the ipsilateral
ICA and the posterior communicating artery that connects with the
vertebral artery, while the ipsilateral anterior CA maintained the
ability to obtain blood from the contralateral ICA. On withdrawal
of the suture to the ICA, the ischemic area was reperfused through
the contralateral ICA and vertebrobasilar artery of the Circle of
Willis. The ischemia time was ~2–6 h in the rat cerebral I/R injury
model. The interaction between the four physiopathologic
mechanisms, including toxicity of excitatory amino acids,
peri-infarct depolarisation, inflammation and cell apoptosis
following I/R (22), further
aggravating the symptoms of ischemia and reaches a steady state
within ~3–6 h. Kawamura et al (23) demonstrated that ischemia time,
which was >3 h, resulted in the reperfusion rats exhibiting
comparable neurologic manifestations, cerebral infarct volume and
encephaledema to those rats without reperfusion following 24 h of
ischemia. Ischemia duration <1 h, led to reperfusion resulting
in reduced infarcts and greater variation, an ischemia duration of
2 h falls between the abovementioned conditions. Therefore,
reperfusion was performed following an ischemia duration of 2 h. In
addition, cerebral edema peaked three days after the clinical
cerebral infarction; thus, TTC staining was conducted on the third
day.
In conclusion, the results of the present study
revealed that the modified rat MCAO model exhibited evident
indicators of neurologic deficit and resulted in constant infarct
locations. The sham-surgery group demonstrated integral brain
structure without ischemic variation, and no infarcts were observed
following TTC staining, which further demonstrated the reliability
of the model.
References
|
1
|
Simpkins JW, Wen Y, Perez E, Yang S and
Wang X: Role of nonfeminizing estrogens in brain protection from
cerebral ischemia: an animal model of Alzheirmer’s disease
neuropathology. Ann N Y Acad Sci. 1052:233–242. 2005.PubMed/NCBI
|
|
2
|
Buga AM, Bălşeanu A, Popa-Wagner A and
Mogoantă L: Strategies to improve post-stroke behavioral recovery
in aged subjects. Rom J Morphol Embryol. 50:559–582.
2009.PubMed/NCBI
|
|
3
|
Fisher M, Feuerstein G, Howells DW, et al;
STAIR Group. Update of the stroke therapy academic industry
roundtable preclinical recommendations. Stroke. 40:2244–2250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsui T, Arai H, Yuzuriha T, et al:
Elevated plasma homocysteine levels and risk of silent brain
infarction in elderly people. Stroke. 32:1116–1119. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lloyd-Jones D, Adams R, Carnethon M, et
al; American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Heart disease and stroke statistics - 2009
update: a report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
119:480–486. 2009. View Article : Google Scholar
|
|
6
|
Dittmar M, Spruss T, Schuierer G and Horn
M: External carotid artery territory ischemia impairs outcome in
the endovascular filament model of middle cerebral artery occlusion
in rats. Stroke. 34:2252–2257. 2003. View Article : Google Scholar
|
|
7
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyko M, Zlotnik A, Gruenbaum BF, et al:
An experimental model of focal ischemia using an internal carotid
artery approach. J Neurosci Methods. 193:246–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koizumi J, Yoshida Y, Nakazawa T and
Ooneda G: Experimental studies of ischemic brain edema. I: a new
experimental model of cerebral embolism in rats in which
recirculation can be introduced in the ischemic area. Jpn J Stroke.
8:1–8. 1986.
|
|
11
|
Ma J, Zhao L and Nowak TS Jr: Selective,
reversible occlusion of the middle cerebral artery in rats by an
intraluminal approach. Optimized filament design and methodology. J
Neurosci Methods. 156:76–83. 2006. View Article : Google Scholar
|
|
12
|
Durukan A, Strbian D and Tatlisumak T:
Rodent models of ischemic stroke: a useful tool for stroke drug
development. Curr Pharm Des. 14:359–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prieto R, Carceller F, Roda JM and
Avendaño C: The intraluminal thread model revisited: rat stain
differences in local cerebral blood flow. Neurol Res. 27:47–52.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung JS, Wang E, Lo EH and Sun PZ:
Stratification of heterogeneous diffusion MRI ischemic lesion with
kurtosis imaging evaluation of mean diffusion and kurtosis MRI
mismatch in an animal model of transient focal ischemia. Stroke.
43:2252–2254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chauveau F, Cho TH, Riou A, et al: Does
acute behavioral testing reflect successful ischemia in rats with
transient middle cerebral artery occlusion? Int J Stroke.
7:465–472. 2012.PubMed/NCBI
|
|
16
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagaoka A, Suno M, Shibota M and Kakihana
M: Effects of idebenone (CV-2619) on neurological deficits, local
cerebral blood flow, and energy metabolism in rats with
experimental cerebral isehemia. Nihon Yakurigaku Zasshi.
84:303–309. 1984.(In Japanese).
|
|
18
|
Tatlisumak T, Takano K, Carano RA, Miller
LP, Foster AC and Fisher M: Delayed treatment with an adenosine
kinase inhibitor, GP683, attenuates infarct size in rats with
temporary middle cerebral artery occlusion. Stroke. 29:1952–1958.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hattori K, Lee H, Hurn PD, Crain BJ,
Traystman RJ and DeVries AC: Cognitive deficits after focal
cerebral ischemia in mice. Stroke. 3l:1939–1944. 2000. View Article : Google Scholar
|
|
20
|
Airavaara M, Shen H, Kuo CC, et al:
Mesencephalic astrocyte-derived neurotrophic factor reduces
ischemic brain injury and promotes behavioral recovery in rats. J
Comp Neurol. 515:116–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boyko M, Ohayon S, Goldsmith T, et al:
Morphological and neuro-behavioral parallels in the rat model of
stroke. Behav Brain Res. 223:17–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White BC, Sullivan JM, DeGracia DJ, et al:
Brain ischemia and reperfusion: molecular mechanisms of neuronal
injury. J Neurol Sci. 179:1–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawamura S, Li Y, Shirasawa M, Yasui N and
Fukasawa H: Reversible middle cerebral artery occlusion in rats
using an intraluminal thread technique. Surg Neurol. 41:368–373.
1994. View Article : Google Scholar : PubMed/NCBI
|