Introduction
Traditional bone tissue engineering technology is
widely used in the preparation of tissue-engineered bone. The
traditional method extracts inoculated cells for transfer by
trypsin digestion, which results in the loss of a large number of
cells and a reduction in cell activity. Thus, dense bone tissue is
rarely formed by the traditional method. Therefore, issues related
to the preparation and transplantation of cells must be resolved so
that cells with higher biological activities are obtained.
Cell sheet technology is a novel technology that is
used in tissue engineering to prepare and transfer seed cells. In
cell sheet technology, specific temperature-responsive polymers
covalently bind to the surface of a Petri dish to provide a
temperature-responsive Petri dish (1,2). The
surface of the Petri dish is hydrophobic at 37°C, enabling cells to
attach and proliferate. When the temperature is decreased to
<32°C, the polymer surface is hydrophilic and a hydration layer
is formed between the dish surface and the cells. Without using
trypsin digestion, cells are automatically separated from the dish
by cell sheet technology. Cells harvested by cell sheet technology
contain extracellular matrix (ECM) and these ECM cells form a
complete layer of the sheet structure with ion channels, growth
factor receptors and connexins (3,4).
Therefore, ECM cell layers made by cell sheet technology are more
similar to normal tissue than the cells made by the traditional
technology. Previously, cell sheet technology has been applied to
construct various tissues and organs, including periodontal
ligaments, skin, corneal epithelium and bladder epithelial tissue
(5), as well as three-dimensional
structures including cardiac muscle, smooth muscle, glomeruli and
the hepatic lobule (6).
In the present study, canine bone mesenchymal stem
cell (BMSC) sheets were prepared by cell sheet technology. In
combination with canine demineralized bone matrix (DBM) and
platelet-rich plasma (PRP), BMSC sheets were implanted into the
latissimus dorsi of dogs and functional tissue-engineered bone was
obtained. Immunoblot assays were performed to study the efficacy of
the implantation by comparing the levels of the growth factors
platelet-derived growth factor (PDGF) and vascular endothelial
growth factor (VEGF) in the experimental group with those in a
control group implanted with a DBM/PRP/BMSC complex.
Materials and methods
Animals and reagents
In total, 12 dogs weighing between 20 and 25 kg were
provided by the Experimental Animal Center, Medical College
Hospital of Qingdao University (Qingdao, China). DMEM low-glucose
culture medium was purchased from Gibco-BRL (Carlsbad, CA, USA),
the alkaline phosphatase kit was purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China) and temperature reactive
dishes were purchased from Nalge Nunc International (Tokyo, Japan).
Femurs from the dogs were prepared by the Department of Oral and
Maxillofacial Surgery. A scanning electron microscope (JEOL
JSM-840) was purchased from Jeol Ltd. (Tokyo, Japan). All animal
experiments were conducted according to the ethical guidelines of
Qingdao University.
Separation, culture and induction of
BMSCs
Canine bone marrow (10 ml) was extracted and BMSCs
were separated by gradient centrifugation (600 × g for 20 min) and
placed in a flask at a density of 1×106 cells/ml. Next,
10 ml low sugar DMEM culture medium was added and the flask was
placed in a humidified incubator at a constant temperature of 37°C
with 5% CO2 for culture and subculture. The second
generation of BMSCs was inoculated in high-glucose DMEM osteogenic
induction medium, containing 15% fetal bovine serum, 5 μg/ml
vitamin C, 10 nM dexamethasone and 10 mM β-glycerophosphate.
Alizarin red staining
Calcium nodules were stained with Alizarin red.
Samples in the Petri dishes were washed twice with PBS, fixed with
95% ethanol for 10 min and then washed three times with water. The
samples were stained with 0.1% Alizarin red-Tris-HCl (pH 8.3) at
37°C for 30 min. Samples were rinsed with distilled water, dried
and then mounted for observation under an inverted light microscope
(Olympus optical Co., Ltd., Tokyo, Japan).
von Kossa staining
Samples in the Petri dishes were washed with PBS
twice, fixed with 4% paraformaldehyde for 5 min and then washed
three times with water. Next, 1 ml silver nitrate solution (5%) was
added to the dishes and the samples were irradiated for 1 h under
UV light. The silver nitrate solution was then removed and the
samples were washed with distilled water three times. Next, 1 ml
sodium thiosulfate solution (5%) was added to the dishes. After 1
min, the sodium thiosulfate solution was removed and the samples
were dried at room temperature, followed by observation under a
microscope.
Preparation of the BMSC cell sheet
The fourth generation of BMSCs, with a density of
1×106 cells/ml, was inoculated into a
temperature-responsive dish with a diameter of 3.5 cm. The dish was
placed in an incubator at 37°C with 5% CO2 and saturated
humidity. When proliferative fusion of BMSCs reached 90%, the dish
was placed in an incubator at 20°C with 5% CO2 and
saturated humidity for 20 min. Following the shedding of the cell
sheet, it was observed under an inverted microscope.
Preparation of canine DBM
Soft tissue and periosteum were removed from the
femurs of the dogs. The bones were preserved at −80°C for 6 months.
Processes, including degreasing, decalcification and the removal of
non-collagen protein, were performed. Canine allogeneic DBM with a
size of 2×2×0.5 cm was formed following freeze-drying.
Preparation and activation of PRP
PRP was prepared during surgery. In total, 30 ml
blood was obtained from the femoral vein, using a sterile needle
containing 4.2 ml anticoagulant. Following centrifugation at 4°C
(160 × g for 20 min), a small number of red blood cells had
precipitated at the bottom of the tube. The majority of the
supernatant was discarded. The residual liquid (~1 ml) was the PRP,
which was activated by the addition of 200 μl thrombin. The PRP was
gently agitated for ~10 sec, until it formed a jelly, for wrapping
the DBM/MSC complex.
Construction of the BMSC/BMSC cell sheet
and DBM/PRP complex
At 24 h prior to implantation, the BMSC suspension
was slowly transferred on to the DBM until the DBM was completely
soaked. Next, the samples were incubated at 37°C with 5%
CO2 for 24 h. The PRP and the prepared cell sheet were
gently placed on the surface of the DBM for implantation.
Implantation
For anesthesia, 20 mg/kg ketamine was
intramuscularly administered to the dogs. Back hair was removed
bilaterally for skin preparation and disinfection. The skin and
superficial fascia was dissected. When the flap was retracted, the
edge of the latissimus dorsi was identified. The thoracodorsal
artery and vein were dissected in the deep surface of the
latissimus dorsi. The DBM/PRP/BMSCs/BMSC cell sheets were implanted
in the left side and the DBM/PRP/BMSC complexes were implanted in
the right side, to serve as a control in each dog. Penicillin
(2,000,000 U/day) was intramuscularly injected every day for 1 week
following surgery. The stitches were removed 14 days after the
surgery. Two dogs were sacrificed at 4, 8 and 12 weeks following
surgery, for gross observation and histological examination.
Immunoblot assays
Total proteins were harvested from the tissues of
the control and experimental implantation sites of the dogs. The
proteins were separated on SDS-PAGE gels and subjected to
immunoblot analyses. The primary antibodies anti-PDGF (sc-128;
1:200) anti-VEGF (sc-7269; 1:200) and anti-β-actin (sc-130301;
1:10,000) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Secondary antibodies were
horseradish-peroxidase-conjugated secondary anti-mouse IgG (31430;
1:10,000; Pierce Biotechnology, Inc., Rockford, IL, USA). Bound
antibodies were detected using an electrochemiluminescence (ECL)
system (Pierce Biotechnology, Inc.). Experiments were repeated
>3 times. The developed film was scanned using the AlphaImager
gel imaging systems (AlphaImager, Santa Clara, CA, USA). The
scanned immunoblot images were analyzed using Quantity One software
(Bio-Rad Laboratories, Hercules, CA, USA). β-actin was used as an
internal control. The relative expression level of PDGF and VEGF
was calculated based on the gray value of β-actin.
Statistical analysis
SPSS 18.0 statistical software was used for
statistical analysis (SPSS Inc., Chicago, IL, USA). Data were
presented as mean ± standard deviation (SD). The difference between
the control group and experimental group was analyzed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant result.
Results
Cell morphology of the prepared BMSC cell
sheet
During the preparation of the BMSC cell sheet, small
numbers of primary cultured BMSCs were observed to be adherent
within the first 24 h, which were in a polygonal, oval or
pleomorphic growth state. At 72 h, the majority of cells were
spreading and adherent. On day 7, visible colonies had formed,
which grew in swirling or radial shapes (Fig. 1A). On day 12, cells were completely
fused in a swirling shape, with the majority in long spindle shapes
(Fig. 1B).
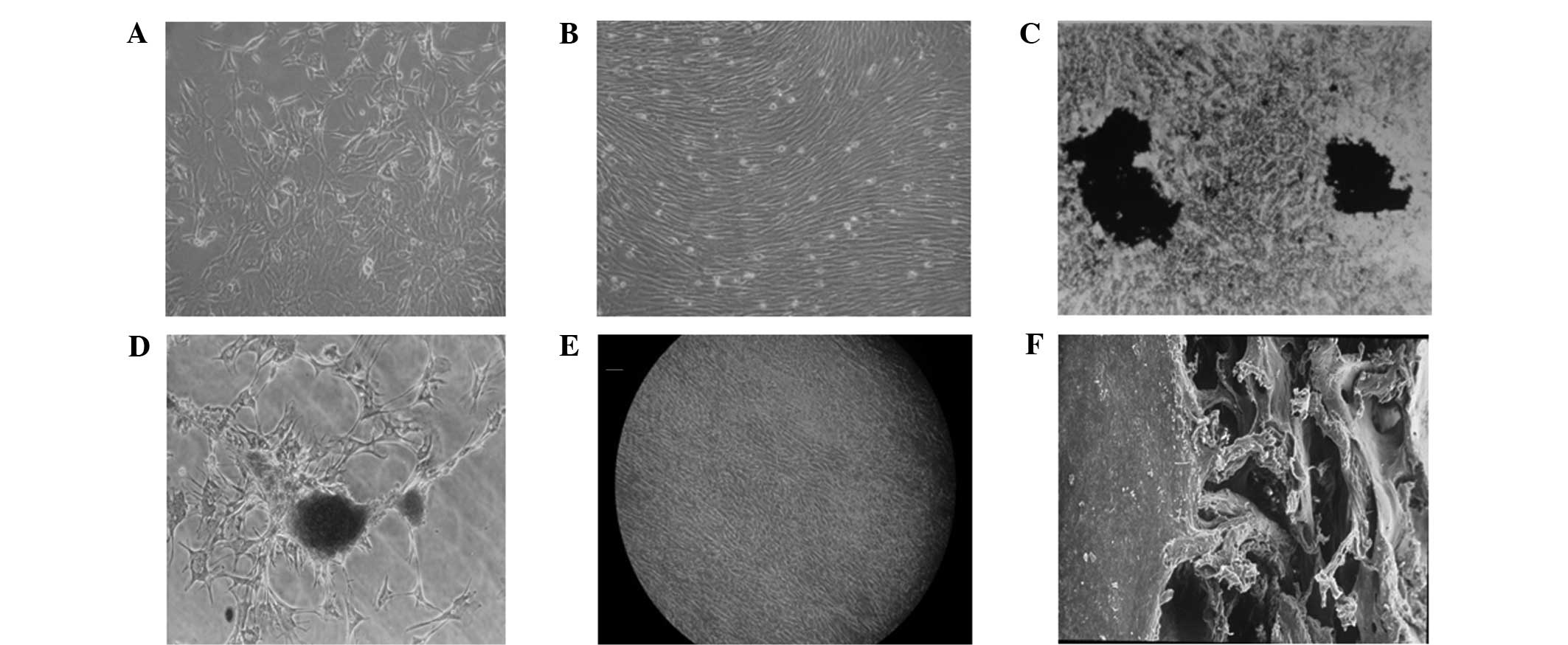 | Figure 1Cell morphology of the prepared BMSC
cell sheet. (A) BMSCs following 7 days of primary culture
(magnification, ×100). (B) BMSCs following 12 days of primary
culture demonstrated whirlpool growth (magnification, ×100). (C)
von Kossa staining showed two large black stained areas in a cell
density zone (magnification, ×250). (D) Alizarin red staining of
nodules (magnification, ×250). (E) Formation of the BMSC cell
sheet, as observed under an inverted microscope, with cells in
short spindle or pleomorphic shapes with unclearly defined
boundaries (magnification, ×100). (F) The cell sheet was inoculated
with good adhesion to the DBM, as observed under an electron
microscope (magnification, ×100). BMSCs, bone mesenchymal stem
cells; DBM, demineralized bone matrix. |
Following osteogenic induction, the proliferation of
the BMSCs became significantly slower. On day 7, the shapes of the
cells changed from long fusiforms to polygonal and square shapes.
Between days 21 and 28, calcified nodules formed. The nodules were
stained black by von Kossa staining and orange by Alizarin red
staining. Black and white images of the staining are shown in
Fig. 1C and D.
On day 7 after induction, the temperature-responsive
dish containing the BMSCs was placed in an incubator at 20°C with
5% CO2. The cells gradually separated from the bottom of
the dish. After 20 min, the cells and ECM were removed to form a
complete cell sheet. Under a light microscope, the morphology of
the cells was observed to have changed to a short spindle or
pleomorphic shape, with unclearly defined boundaries (Fig. 1E). The cells gradually shrank to
form a dense cell sheet detached from the bottom of the dish. One
week following the inoculation of the cell sheet to the surface of
the DBM, the DBM was wrapped by the cell sheet, as observed under a
scanning electron microscope (Fig.
1F). These results indicate that at 20°C, BMSCs detached
automatically from the temperature-responsive culture dishes to
form an intact cell sheet.
Histological analyses of osteoblasts
To determine the osteogenic effectiveness of the
BMSC cell sheets, implantation into canine latissimus dorsi was
performed. The dogs were implanted with the DBM/PRP/BMSC cell
sheet/BMSC (the experimental group) and DBM/PRP/BMSC complexes (the
control group). Stitches were removed from the implantation sites
14 days after surgery. Two dogs were euthanized at 4, 8 and 12
weeks following surgery for gross observation and histological
examination.
Four weeks following implantation in the canine
latissimus dorsi, active osteogenesis was observed in the
experimental group, with considerable numbers of osteoblasts and
capillaries detected around the trabecular bone (Fig. 2A). In the control group, the
osteogenesis and vascular density were less evident, and trabecular
bone fibrosis was visible (Fig.
2B). After 8 weeks, the trabecular bone was coarse, osteoblasts
were active, capillaries were abundant between the trabeculae and
fresh fibrosis was observed in the experimental group, whereas in
the control group, the trabecular bone was coarse and the
trabeculae had abundant vascularity and marked fibrosis (data not
shown). After 12 weeks, the trabecular bone was regular and thick
with a high density, fibrous tissue was present in the mature
trabecular bone and the blood vessel density was reduced in the
experimental group (Fig. 2C). In
the control group, there was less trabecular bone and numerous
blood vessels and marked fibrosis were observed (Fig. 2D). These results indicate that
osteogenesis in the DBM/PRP/BMSC cell sheet/BMSC group was
significantly more effective than that in the DBM/PRP/BMSCs
group.
PDGF and VEGF growth factor levels in the
control and experimental groups
To further determine the effectiveness of the BMSC
cell sheets on implantation efficiency, total proteins were
harvested from tissues in the control and experimental implantation
sites of the dogs. The proteins were separated on SDS-PAGE gels and
subjected to immunoblot analyses of the levels of the growth
factors PDGF and VEGF. The cellular protein, β-actin, served as a
loading control in the immunoblot analyses. The mean normalized OD
of the protein bands, relative to the OD of the β-actin band, was
calculated from each condition and subjected to statistical
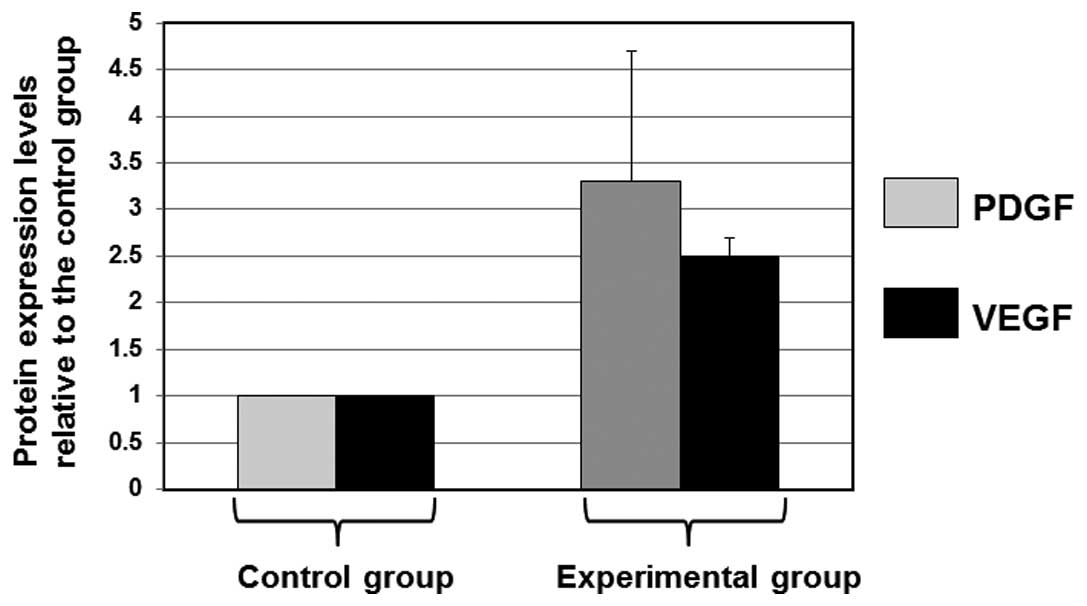
analysis. Error bars show the SD of the mean (Fig. 3). As shown in Fig. 3, the mean levels of PDGF and VEGF
in the experimental group were 3.2- and 2.5-fold higher than the
mean expression levels in the control group, respectively. These
results indicate that BMSC cell sheets are functional and more
effective than the control cell complexes.
Discussion
In the present study, cell sheet technology was used
to construct tissue-engineered bone. BMSCs were induced to
differentiate into osteoblasts for BMSC cell sheet preparation.
Using a scanning electron microscope, the prepared BMSCs were shown
to be in a complete sheet structure, containing a layer of ECM.
BMSC sheet layers retain cell surface proteins, including ion
channels and connexin, which enable the effective transmission of
signals and the maintenance of a coherent function (3). The BMSCs were arranged in a dense
sheet layer, which was similar to natural bone during the formation
of osteoblasts. The shrinkage-generated stress was due to a
specific regulation effect on the polarization of BMSCs (7). A lamellar bone structure was formed
that was similar to that of the surrounding mineral with regard to
deposition and calcification. Single- or multi-layer BMSC-wrapped
biodegradable scaffolds were implanted into the recipient area,
which was expected to form a functional tissue-engineered bone
having a lamellar bone structure comparable to that of normal
bone.
The DBM prepared in the present study was
constructed as a collagen grid with a consistent gap size. Scanning
electron microscopy showed that the DBM had a three-dimensional
mesh structure with a porosity of ~70%. Such three-dimensional mesh
structures provide a surface area for cell adhesion and
proliferation, which aids the nutrition, metabolism and
angiogenesis of bone tissue. PRP is a platelet concentrate formed
by the centrifugation of autologous whole blood. Following
activation by thrombin and calcium chloride, the platelet α
granules release PDGF, transforming growth factor (TGF)-β, VEGF,
epidermal growth factor (EGF), insulin-like growth factor (IGF) and
other growth factors (8–10). With the exception of IGF, the PRP
concentrations of the remaining four types of growth factor were
3–8 times the concentrations in whole blood (11). In addition, PRP preparation is
simple, fast, inexpensive and minimally invasive, and does not
cause immune rejection or the spread of disease. A variety of
growth factors in PRP are known to be important for the promotion
of bone regeneration and angiogenesis (12).
In the present study, BMSCs were prepared by cell
sheet technology, which avoided the use of trypsin digestion. By
this method, cell damage was reduced and therefore, a considerable
amount of ECM was retained, which greatly improved cell utilization
and the biological activity of the transferred cells. Immunoblot
assays were performed to study the effects of the two implant types
by comparing the levels of growth factors PDGF and VEGF. The mean
levels of PDGF and VEGF in the experimental group were 3.2- and
2.5-fold higher than the mean expression levels in the control
group, respectively. The results obtained indicate that BMSC cell
sheets are functional and more effective than the control cell
complexes. Thus, cell sheet technology may be useful in
constructing functional tissue-engineered bones.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation (no. 30872896) and the Natural Science
Foundation of Shandong Province (no. Y2008C77).
References
|
1
|
Yan H and Tsujii K: Thermo-responsive
poly(N-isopropylacrylamide) gel containing polymeric surfactant
poly(2-(methacryloyloxyl)decylphosphate): correlation between rapid
collapsing characters and micelles of polymeric surfactant. J Oleo
Sci. 57:401–405. 2008. View Article : Google Scholar
|
|
2
|
Yamada N, Okano T, Sakai H, et al:
Thermo-responsive polymeric surfaces; control of attachment and
detachment of cultured cells. Makromol Chem Rapid Commun.
11:571–576. 1990. View Article : Google Scholar
|
|
3
|
Shimizu T, Yamato M, Kikuchi A and Okano
T: Cell sheet engineering for myocardial tissue reconstruction.
Biomaterials. 24:2309–2316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jing H, Li NY, Tan S, et al: In vitro
culture and cell sheet preparation of bone marrow mesenchymal
cells. Guo Ji Kou Qiang Yi Xue Za Zhi. 37:272–274. 2010.(In
Chinese).
|
|
5
|
Nishida K, Yamato M, Hayashida Y, et al:
Functional bioengineered corneal epithelial sheet grafts from
corneal stem cells expanded ex vivo on a temperature-responsive
cell culture surface. Transplantation. 77:379–385. 2004. View Article : Google Scholar
|
|
6
|
Gao Z, Chen F, Zhang J, et al:
Vitalisation of tubular coral scaffolds with cell sheets for
regeneration of long bones: a preliminary study in nude mice. Br J
Oral Maxillofac Surg. 47:116–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Yamato M, Nishida K, et al: Cell
delivery in regenerative medicine: the cell sheet engineering
approach. J Control Release. 116:193–203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weibrich G, Kleis WK and Hafner G: Growth
factor levels in the platelet rich plasma produced by 2 different
methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral
Maxillofac Implants. 17:184–190. 2002.PubMed/NCBI
|
|
9
|
Schmitz JP and Hollinger JO: The biology
of platelet-rich plasma. J Oral Maxillofac Surg. 59:1119–1121.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weibrich G, Kleis WK, Hafner G and Hitzler
WE: Growth factor levels in platelet-rich plasma and correlations
with donor age, sex, and platelet count. J Craniomaxillofac Surg.
30:97–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eppley BL, Woodell JE and Higgins J:
Platelet quantification and growth factor analysis from
platelet-rich plasma: implications for wound healing. Plast
Reconstr Surg. 114:1502–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li NY, Yuan RT, Chen T, et al: Effect of
platelet-rich plasma and latissimus dorsi muscle flap on
osteogenesis and vascularization of tissue-engineered bone in dogs.
J Oral Maxillofac Surg. 67:1850–1858. 2009. View Article : Google Scholar : PubMed/NCBI
|