Introduction
Acute lung injury (ALI) and its more severe form,
acute respiratory distress syndrome (ARDS), are clinical syndromes
of acute hypoxic respiratory failure resulting from a variety of
direct and indirect injuries to the parenchyma of the lungs
(1–3). The mortality rate in patients with
ALI/ARDS is ~40% due to slow progress in understanding the
mechanisms responsible for disease pathogenesis (4–6).
Lipopolysaccharide (LPS) induces symptoms in animal models that
closely resemble ALI/ARDS in humans, highlighting strategies to
explore the pathogenesis of ALI/ARDS (7–9).
A reliable experimental animal model is necessary to
elucidate the cellular and molecular pathogenesis of ALI/ARDS
(10–12). In a previous study of mouse models
of LPS-induced ALI, two methods of intratracheal instillation were
compared (13). LPS-induced
pulmonary inflammatory responses were more severe following
trans-tracheal intratracheal instillation relative to those
following trans-oral intratracheal instillation, which may be due
to more effective delivery of LPS to the lungs by trans-tracheal
intratracheal instillation. Of note, delivery of LPS with air from
a prefilled syringe was associated with rapid instillation into the
lung when using the modified procedure of trans-tracheal
intratracheal instillation. Therefore, it is possible that
instilled air may promote delivery of LPS into the alveolar spaces,
resulting in different pulmonary responses. Instilling air from a
prefilled syringe has been used to investigate the pulmonary
toxicity of nanoparticles (14).
However, the influence of intratracheal air instillation on
experimental animal models of ALI has not been assessed.
In the present study, the influence of
trans-tracheal intratracheal air instillation on a mouse model of
LPS-induced ALI was investigated. The aim of the study was to
reveal the role of intratracheal air instillation in LPS-induced
experimental animal models of ALI.
Materials and methods
Animals
Male C57BL/6 mice (n=75; weight, 20±2 g) were
purchased from the Jilin University Animal Center (Changchun,
China). The animals were housed in a room at 22°C with a 12-h
light/dark cycle (6:00 a.m.–6:00 p.m. light). Mice were fed
standard mouse chow and provided water ad libitum. All
animal experiments were approved by the Animal Care Committee of
Jilin University.
Intratracheal instillation
The procedure for trans-tracheal intratracheal
instillation was modified from a previous experiment (15). Briefly, mice were anesthetized by
intraperitoneal injection of 0.1 ml pentobarbital sodium (50 mg/kg;
Sigma, St. Louis, MO, USA) and placed in a supine position head-up
on a board. The board was tilted at a 50-degree angle. A midline
incision was made in the neck to expose the trachea. LPS (Sigma)
was dissolved in 0.9% normal saline (NS) at a concentration of 1
mg/ml. LPS at a dosage of 5 mg/kg or the same volume of NS was
drawn into a sterile plastic catheter (up to a premarked level)
through a 29-gauge needle and rapidly instilled into the lung with
a 1-ml syringe. Following intratracheal instillation, the mice were
placed vertically and rotated for 0.5–1 min to ensure even
distribution of the instillation within the lungs.
In vivo experimental protocol
Mice (n=75) were randomly divided into five groups
(n=15/group) as follows: Control, NS, NS plus air, LPS and LPS plus
air. Mice in the LPS and NS groups were instilled intratracheally
with LPS or NS, respectively, as described in the previous section.
In the LPS plus air and NS plus air groups, LPS or NS,
respectively, was instilled intratracheally with a 1-ml syringe
prefilled with 0.1 ml air. Mice in the control group did not
undergo any treatment. All mice were sacrificed 24 h subsequent to
intratracheal instillation of LPS or NS.
Bronchoalveolar lavage (BAL)
Mice (n=5/group) were exsanguinated and sacrificed
by removal of the eyeballs following anesthesia with an
intraperitoneal injection of 0.1 ml pentobarbital sodium (50
mg/kg). Following surgical isolation of the trachea, mice were
intubated with a 24-gauge cannula. The lungs were flushed with 0.9%
NS in 0.2-ml increments. Recovery of BAL was identical for all
experimental groups and the recovery rate was 87±2%. The BAL fluid
was centrifuged for 5 min at 300 × g, and the supernatant was
analyzed to obtain the biochemical index. Levels of lactate
dehydrogenase (LDH), alkaline phosphatase (ALP) and total protein
were assessed with commercial reagent kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) and interleukin-8 (IL-8)
levels were determined with a commercial, specific ELISA kit
(R&D Systems, Minneapolis, MN, USA), used in accordance with
the manufacturer’s instructions. The pellet was resuspended in 1 ml
phosphate-buffered saline with 1% bovine serum albumin and 0.1%
sodium azide, and a 10-μl aliquot was used to count cells. Cell
viability was assessed by trypan blue staining (Sigma). In
addition, cytospun cells were prepared for Wright’s staining and
differential cell counting using a cytocentrifuge (Academy of
Military Medical Sciences, Beijing, China).
Lung wet/dry weight ratio
Mice (n=5/group) were exsanguinated and sacrificed
by removal of the eyeballs. A median sternotomy was performed, and
the lungs of each mouse were excised. The wet weight of the
exsanguinated whole lungs was measured with an electronic balance,
and the lungs were then oven-dried at 60°C for 72 h prior to the
dry weight being recorded. The wet-to-dry weight ratio was then
calculated.
Lung histology
Mice (n=5/group) were exsanguinated and sacrificed
by removal of the eyeballs. The tracheas of the mice were exposed
and cannulated with PE-90 tubing, the chests were opened and the
lungs were removed and filled with 10% buffered formalin at an
airway pressure of 20 cm H2O for 30 min. Paraffin
embedding was performed with the lungs oriented in a prone
position, and 5-μm sections were cut for hematoxylin and eosin
staining. A lung injury scoring method was utilized to quantify
changes in lung architecture, as evidenced by light microscopy. The
following variables were used to assess the degree of microscopic
injury: alveolar and interstitial edema, neutrophil infiltration
and hemorrhage. Each variable was graded according to the severity
of injury: no injury, 0; injury to 25% of the field, 1; injury to
50% of the field, 2; injury to 75% of the field, 3; and diffuse
injury, 4 (16). The samples were
analyzed based on a scaled grading system by a pathologist who was
blinded to the experimental protocol and the sampling region. Three
slides from each lung sample were randomly screened and the mean
was taken as the representative value of the sample.
Statistical analysis
Statistical analysis was conducted using the SPSS
predictive analytics software 18.0 package (SPSS, Inc., Chicago,
IL, USA), and all data are expressed as the mean ± standard error
of the mean. One-way analysis of variance followed by Bonferroni
(equal variances) or Dunnett’s T3 (heteroscedasticity) post hoc
test were performed to determine the statistical significance
between indicated groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
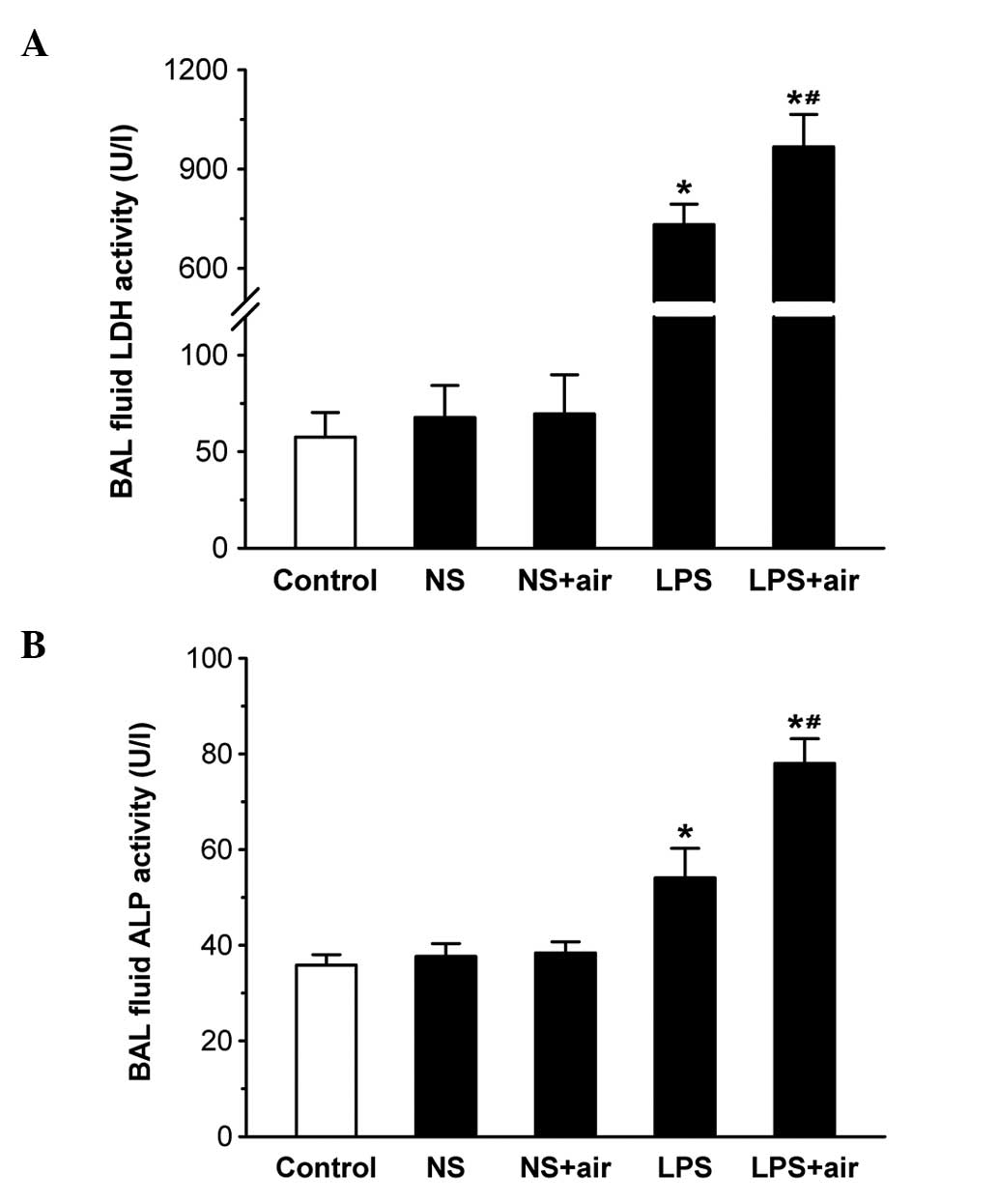
BAL fluid biochemical analysis
LDH and ALP activities and total protein
concentration in the BAL fluid were used as the indicators of cell
injury (Fig. 1 and 2). BAL fluid LDH and ALP activities were
generally consistent with the degree of cell injury: Cell injury
enhances the permeability of the alveolar-capillary barrier,
leading to an increased protein concentration in BAL fluid. No
significant differences were observed in BAL fluid LDH and ALP
activities and total protein concentrations between the
sham-treated (NS plus air and NS) and control groups. BAL fluid LDH
and ALP activities and total protein concentrations were
significantly increased in the LPS plus air and LPS groups compared
with the control group (all P<0.05). These three indices were
significantly increased in the LPS plus air group compared with the
LPS group (all P<0.05). The results indicated that the instilled
air aggravated LPS-induced cell injury.
BAL fluid differential cell counting
BAL fluid differential cell counting was used to
evaluate the number and types of migrated cells, and to further
indicate the category and extent of pulmonary inflammation
(Fig. 3). No significant
differences were observed in the total cell and neutrophil numbers
between the sham-treated (NS plus air and NS) and control groups.
The total cell and neutrophil numbers were significantly increased
in the LPS plus air and LPS groups compared with the control group
(all P<0.05). Furthermore, the total cell and neutrophil numbers
were significantly increased in the LPS plus air group compared
with the LPS group (all P<0.05). These results indicated that
the instilled air promoted LPS-induced neutrophil infiltration into
the lungs.
Lung wet/dry weight ratio
Lung wet/dry weight ratio is used as an indicator of
pulmonary edema (Fig. 4). No
significant differences were observed in the lung wet/dry weight
ratios between the sham-treated (NS plus air and NS) and control
groups. The lung wet/dry weight ratios were significantly increased
in the LPS plus air and LPS groups compared with the control group
(all P<0.05). The lung wet/dry weight ratio was significantly
increased in the LPS plus air group compared with the LPS group
(P<0.05). These results indicated that the instilled air
exacerbated LPS-induced pulmonary edema.
Lung histology
No marked differences were observed in the lung
histology between the sham-treated (NS plus air and NS) and control
groups, indicating that intratracheal instillation of NS with or
without air did not result in acute lung inflammation (Fig. 5A–C). There were different degrees
of fluid accumulation, neutrophil infiltration, congestion and
hemorrhage in the LPS plus air and LPS groups compared with the
control group. There was more protein-rich fluid, and a greater
number of neutrophils and erythrocytes in the alveoli of mice in
the LPS plus air group compared with the LPS group (Fig. 5D and E). Edema, neutrophil
infiltration and hemorrhage scores were significantly increased in
the LPS plus air group compared with the LPS group (all P<0.05;
Fig. 5F). These results indicated
that the instilled air aggravated LPS-induced pathological
changes.
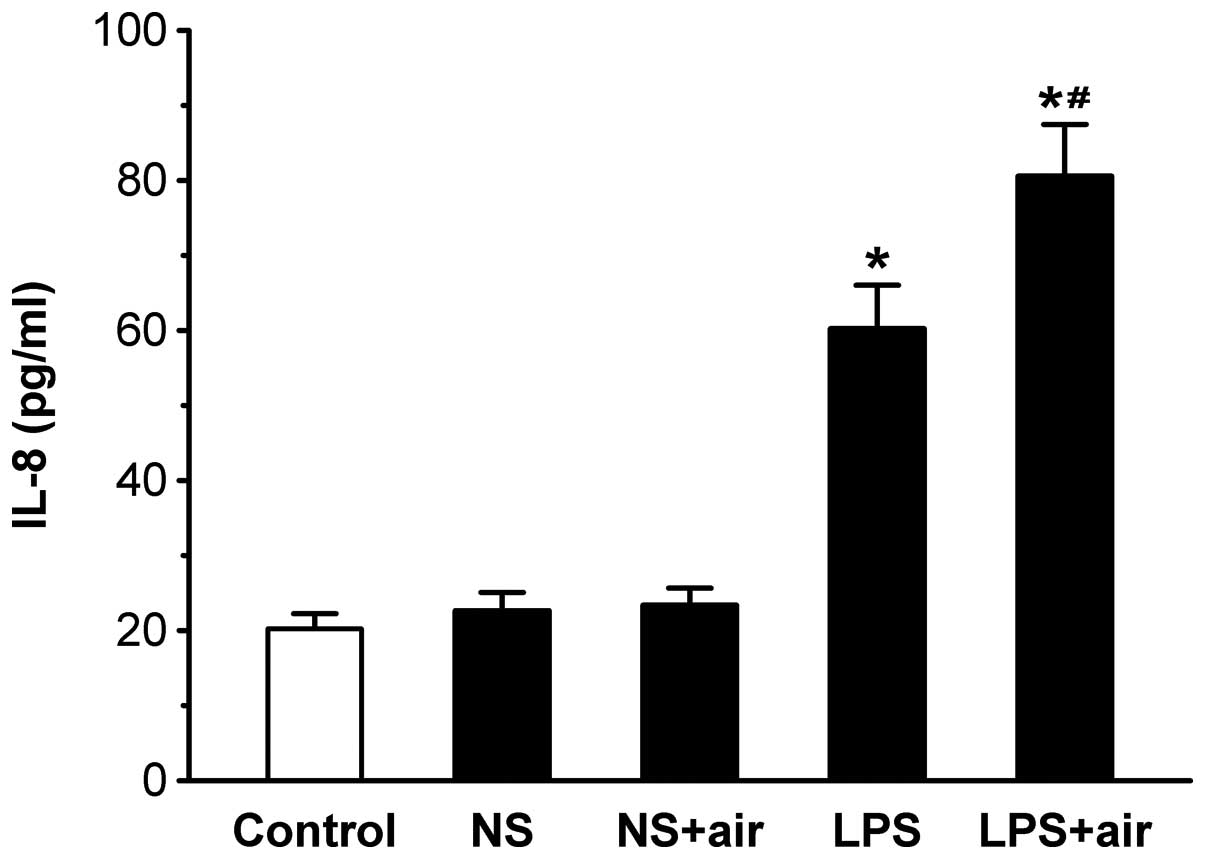
BAL fluid IL-8 concentration
BAL fluid IL-8 concentration was assessed due to its
important role in the pathogenesis of ALI (Fig. 6). No significant differences were
observed in the IL-8 levels between the sham-treated (NS plus air
and NS) and control groups. Levels of IL-8 were significantly
increased in the LPS plus air and LPS groups compared with the
control group (all P<0.05). IL-8 levels were significantly
increased in the LPS plus air group compared with the LPS group
(P<0.05). These results indicated that instilled air increased
LPS-induced IL-8 release.
Discussion
The improvement of intratracheal instillation
procedures may facilitate the establishment of an optimal animal
model of ALI in order to further reveal the cellular and molecular
pathogenesis of ALI. In the present study, the effect of instilling
air by trans-tracheal intratracheal instillation on an LPS-induced
murine model of ALI was investigated. The results demonstrated that
trans-tracheal intratracheal air instillation promoted LPS-induced
ALI, as shown by the more severe acute pulmonary inflammation and
increased IL-8 release.
Alveolar epithelial and vascular endothelial injury,
neutrophil infiltration and increased membrane permeability,
leading to pulmonary edema, are involved in the pathogenesis of
ALI/ARDS (1). In ALI, binding of
LPS to Toll-like receptors on lung cells initiates acute lung
inflammation (17). Chemokines are
secreted from the stimulated pulmonary epithelium and alveolar
macrophages recruit neutrophils into airspaces via the
alveolar-capillary barrier (18–20).
Activated neutrophils release a variety of mediators, including
proteases, reactive oxygen species, histones and peptides, which
cause vascular endothelial and alveolar epithelial injuries
(21). The subsequent increase in
the permeability of the alveolar-capillary barrier leads to the
extravascular accumulation of protein-rich edema fluid (22). Disruption of the capacity for fluid
clearance and surfactant production due to pulmonary epithelial
injury also aggravates pulmonary edema (23). In the present study, instilled air
promoted LPS-induced ALI, as evidenced by the more severe acute
pulmonary inflammation, including cell injury, neutrophil
infiltration and permeability pulmonary edema.
Neutrophils have been indicated to be involved in
the pathogenesis of ALI/ARDS, and IL-8 has been identified as the
main chemotactic factor for neutrophils in the lung fluid of
patients with ALI/ARDS (24–26).
IL-8 may enhance the migratory activity of neutrophils and induce
migration through the alveolar-capillary barrier, resulting in an
accumulation of neutrophils in the alveolar spaces. Therefore, IL-8
levels reflect the severity of ALI in animals and humans. In this
study, BAL fluid IL-8 levels were used to further evaluate the
influence of instilled air on LPS-induced ALI. Instilled air
increased LPS-induced IL-8 release, indicating that instilled air
promotes LPS-induced ALI by increasing IL-8 levels. It has been
demonstrated that alveolar epithelia are capable of producing a
higher level of IL-8 than bronchial epithelia under the stimulation
of LPS (13). However, it is
possible that the instilled air resulted in delivery of LPS to the
alveolar and bronchial epithelial cells in different proportions.
Instilled air may drive the discharge of LPS from the syringe,
delivering an enhanced level of LPS into the alveolar spaces and
resulting in greater LPS exposure in the alveolar epithelia,
increased IL-8 release and more severe acute pulmonary
inflammation.
This study demonstrates that instilled air promotes
LPS-induced ALI. To the best of our knowledge, this is the first
study to reveal the role of intratracheal air instillation in an
LPS-induced experimental animal model of ALI. Instilled air may be
used to improve the method of intratracheal instillation and
establish a more reliable experimental animal model of ALI. This
may enable the further elucidation of the molecular pathogenesis of
ALI/ARDS. The instilled air may deliver more LPS into the alveolar
spaces, leading to more severe acute pulmonary inflammation. The
results of this study may provide guidance for the establishment of
other animal models and for approaches to improve drug
delivery.
Acknowledgements
The authors would like to thank Dr Chunling Dong for
expert assistance throughout this study. Financial support was
provided by the National Natural Science Foundation of China (grant
no. 81100030), the Development and Planning Program of Jilin
Provincial Science and Technology Department (grant no.
20130522022JH), the Administration of Traditional Chinese Medicine
of Jilin Province (grant no. 2012-135), the Jilin University
Scientific Frontier and Interdisciplinary Innovative Program (grant
no. 450060491515) and Jilin University Innovative Training Program
(grant nos. 2012A71198 and 2012C71312).
References
|
1
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li B, Yang J, Huang Q, et al:
Biodistribution and pulmonary toxicity of intratracheally instilled
graphene oxide in mice. NPG Asia Materials. 5:e442013. View Article : Google Scholar
|
|
4
|
Spragg RG, Bernard GR, Checkley W, et al:
Beyond mortality: future clinical research in acute lung injury. Am
J Respir Crit Care Med. 181:1121–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phua J, Badia JR, Adhikari NK, et al: Has
mortality from acute respiratory distress syndrome decreased over
time?: A systematic review. Am J Respir Crit Care Med. 179:220–227.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubenfeld GD, Caldwell E, Peabody E, et
al: Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muñoz NM, Meliton AY, Meliton LN, Dudek SM
and Leff AR: Secretory group V phospholipase A2 regulates acute
lung injury and neutrophilic inflammation caused by LPS in mice. Am
J Physiol Lung Cell Mol Physiol. 296:L879–L887. 2009.PubMed/NCBI
|
|
8
|
Xu XL, Xie QM, Shen YH, et al: Mannose
prevents lipopolysaccharide-induced acute lung injury in rats.
Inflamm Res. 57:104–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HA, Park JH, Lee S, Choi JS, Rhim T
and Lee M: Combined delivery of dexamethasone and plasmid DNA in an
animal model of LPS-induced acute lung injury. J Control Release.
156:60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin TR and Matute-Bello G: Experimental
models and emerging hypotheses for acute lung injury. Crit Care
Clin. 27:735–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reiss LK, Uhlig U and Uhlig S: Models and
mechanisms of acute lung injury caused by direct insults. Eur J
Cell Biol. 91:590–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Gao Z, Xia C, et al: Comparative
study of trans-oral and trans-tracheal intratracheal instillations
in a murine model of acute lung injury. Anat Rec (Hoboken).
295:1513–1519. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lam CW, James JT, McCluskey R and Hunter
RL: Pulmonary toxicity of single-wall carbon nanotubes in mice 7
and 90 days after intratracheal instillation. Toxicol Sci.
77:126–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song YL, Fukuda N, Bai CX, Ma TH, Matthay
MA and Verkman AS: Role of aquaporins in alveolar fluid clearance
in neonatal and adult lung, and in oedema formation following acute
lung injury: studies in transgenic aquaporin null mice. J Physiol.
525:771–779. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su X, Bai CX, Hong QY, et al: Effect of
continuous hemofiltration on hemodynamics, lung inflammation and
pulmonary edema in a canine model of acute lung injury. Intensive
Care Med. 29:2034–2042. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Dong C, Wang G, Zheng H, Wang X and
Bai C: Pulmonary epithelial CCR3 promotes LPS-induced lung
inflammation by mediating release of IL-8. J Cell Physiol.
226:2398–2405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012.PubMed/NCBI
|
|
20
|
Thorley AJ, Ford PA, Giembycz MA,
Goldstraw P, Young A and Tetley TD: Differential regulation of
cytokine release and leukocyte migration by
lipopolysaccharide-stimulated primary human lung alveolar type II
epithelial cells and macrophages. J Immunol. 178:463–473. 2007.
View Article : Google Scholar
|
|
21
|
Grommes J and Soehnlein O: Contribution of
neutrophils to acute lung injury. Mol Med. 17:293–307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kropski JA, Fremont RD, Calfee CS and Ware
LB: Clara cell protein (cc16), a marker of lung epithelial injury,
is decreased in plasma and pulmonary edema fluid from patients with
acute lung injury. Chest. 135:1440–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ware LB and Matthay MA: Alveolar fluid
clearance is impaired in the majority of patients with acute lung
injury and the acute respiratory distress syndrome. Am J Respir
Crit Care Med. 163:1376–1383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pallister I, Dent C and Topley N:
Increased neutrophil migratory activity after major trauma: a
factor in the etiology of acute respiratory distress syndrome? Crit
Care Med. 30:1717–1721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao ZY, Ye QW, Gong WH, Xiang Y and Wan
HY: Humanized monoclonal antibody against the chemokine CXCL-8
(IL-8) effectively prevents acute lung injury. Int Immunopharmacol.
10:259–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azoulay E, Darmon M, Delclaux C, et al:
Deterioration of previous acute lung injury during neutropenia
recovery. Crit Care Med. 30:781–786. 2002. View Article : Google Scholar : PubMed/NCBI
|