Introduction
Benign prostatic hyperplasia (BPH), a condition
characterized by excessive and uncontrolled growth of the prostate
gland, affects ~85% of males over 50 years of age (1). Considering the high incidence of BPH
and the effect this condition has on the quality of life, treatment
of this disease is a priority for public health (2). The aetiology of BPH is complicated
and remains unclear; however, recent novel observations highlight
the key role of aging (3),
hormonal alterations (4),
metabolic syndrome (5) and
inflammation (6).
At present, pharmacotherapy remains the modality of
choice for BPH treatment and may be roughly divided into three
groups: α-blockers, 5α-reductase inhibitors and alternative
therapies (7). However, these
prescription medications may have adverse side-effects, including
orthostatic hypotension, decreased libido and ejaculatory or
erectile dysfunction (8). Due to
these risks, natural products that appear to have limited adverse
events are becoming increasingly important in the treatment of BPH
(9). Previous studies have shown
that a number of natural products, including saw palmetto (10), Sphaeranthus indicus,
Pygeum africanum and Hypoxis rooperi, possess
anti-BPH potential (11).
Bee-collected pollen is an apicultural product that
is composed of nutritionally valuable substances and considerable
amounts of biologically active substances (12). Rape (Brassica campestris L.
var. oleifera DC.) is planted in the majority of regions worldwide.
In China, the bee pollen of this plant is widely used as a natural
supplement to everyday meals and as an herbal medicine to
strengthen the resistance of the body to diseases. This is due to
the abundant nutrient properties, including sugars, proteins,
lipids, vitamins, carbohydrates and phenolic compounds (13–15).
The use of supercritical fluid extracts (SFEs) has been
increasingly studied due to their unique properties, versatile
applications and changes in environmental regulations that foster
the utilization of green solvents. In this field, CO2
has been particularly studied since it is essentially non-toxic,
non-flammable, inexpensive, recyclable, totally dissipated from
extracts at atmospheric pressure and has easily accessible critical
conditions. The aims of the present study were to investigate the
effects of rape pollen SFE-CO2 on testosterone-induced
BPH in rats and the underlying molecular mechanism.
SFE-CO2 was selected since it is known to be rich in
fatty acids and their derivatives and steroids. In addition, pollen
extract contains a complex mixture of compounds that function in
concert to exert a specific bioactivity more effectively than
individual compounds.
Materials and methods
SFE-CO2 extraction
Pollen from Brassica campestris L. var.
oleifera DC. was collected from Inner Mongolia (China) in July 2008
and was identified by Professor Xu Feng (Jiangsu Institute of
Botany, Nanjing, China). A voucher specimen (PN-2008-01) was
deposited in the Herbarium of Shanghai Institute of Pharmaceutical
Industry (Shanghai, China). Two 1,000-g samples of dried pollen, of
which the cell walls were lysed by zymolysis, were extracted by
SFE-CO2 at 40 MPa and 50°C. The combined extract was
evaporated under a reduced pressure to produce a yellow gum (yield,
83 g).
Animals
Specific pathogen-free male Sprague-Dawley rats with
an initial body weight of 230–250 g were purchased from Shanghai
Xipuer - Bi Kai Experimental Animals Ltd. (Shanghai, China). The
rats were housed in clean pathogen-free rooms in an environment
with controlled temperature (22°C), humidity and a 12 h light/dark
cycle. Rats had free access to water and a standard laboratory
diet. All animal procedures were conducted strictly in accordance
with the International Ethical Guidelines and the guide for the
Care and Use of Laboratory Animals. Experiments were approved by
the Institutional Animal Care and Use Committee of Shanghai
Institute of Pharmaceutical Industry.
Construction of the rat BPH model and
drug administration
A rat model of BPH was induced by subcutaneous (sc)
injections of testosterone propionate following castration. One
week following surgery, the rats were randomly divided into five
groups (n=7): Castration (saline 10 ml/kg), model (saline 10
ml/kg), finasteride (5 mg/kg) and two rape pollen
SFE-CO2 extract groups (21.3 or 88.7 mg/kg). Rats in the
model and treatment groups received saline or drug via
gastrogavage, in combination with sc injection of 5 mg/kg
testosterone propionate daily for 30 days, while those in the
castration group received saline by gastrogavage and 1 ml corn oil
by sc injection. The body weight of each rat was measured once a
week.
Animals were anesthetized with pentobarbital (100
mg/kg body weight; i.p.) following final treatment and overnight
fasting. Blood samples were collected from the caudal vena cava.
Serum was separated by centrifugation and stored at −80°C. Whole
prostates were immediately removed and weighed and relative organ
weights were calculated as the ratio of organ weight to body
weight. Sections of the ventral prostate lobe were fixed with 10%
neutral buffered formalin and embedded in paraffin for histological
analysis. The remaining prostate samples were stored at −80°C.
Prostate index
The prostate index of each rat was the ratio of
prostate weight to body weight (mg/g) (16).
Determination of testosterone and
dihydrotestosterone (DHT) levels in the serum and prostate
Prostate tissue was homogenized (1/10, w/v) using a
homogenizer in a tissue lysis/extraction reagent containing a
protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA).
Homogenates were centrifuged at 12,000 × g for 25 min at 4°C and
the protein concentration in the supernatant fractions was
determined using a bicinchoninic acid protein assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA), according to the
manufacturer’s instructions.
Testosterone and DHT levels in the serum and
prostate were measured using an enzyme-linked-immunosorbent assay.
DHT and testosterone kits were purchased from Bio-Rad Laboratories,
Inc. (Hercules, CA, USA).
Histopathological examination
To assess morphological changes in the prostate,
tissues were embedded in paraffin, cut into sections of 4 μm
thickness and stained with hematoxylin and eosin (MHS-16 and
HT110-1-32; Sigma-Aldrich). Tissues were subsequently mounted and
coverslipped, using mounting medium, for microscopic examination
(Nikon, Tokyo, Japan).
Immunohistochemical detection of
5α-reductase and cyclooxgenase-2 (COX-2)
Paraffin-embedded tissue sections of 3 μm thickness,
collected from three rats per group, were deparaffinized with
xylene, hydrated using an ethanol series and heated in citrate
buffer (pH 6.0) for 5 min. Next, the sections were blocked with 5%
bovine serum albumin (BSA) in Tris-buffered saline (TBS) for 2 h.
This was followed by incubation at a concentration of 1 μg/ml with
anti-5α-reductase or anti-COX-2 rabbit monoclonal antibodies (AbD
Serotec, Oxford, UK) with 5% BSA in TBS overnight at 4°C. After
washing the slides with TBS, the sections were incubated with the
corresponding secondary antibody (Abcam, Cambridge, MA, USA).
Sections were then washed with TBS and incubated for 10 min in a
solution of 0.02% diaminobenzidine containing 0.01%
H2O2. Counterstaining was performed using
hematoxylin and the slides were visualized under a light
microscope. At least three sections per rat were investigated and
immunohistochemical quantification was conducted using image
analysis software (Optimas 6.5, Bothell, WA, USA).
Statistical analysis
Measurement data are expressed as the mean ± SD.
Statistically significant differences between treated and control
groups were determined using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference. These results were analyzed with SPSS 16.0 statistical
software (SPSS Inc., Chicago, IL, USA)
Results
Effect of rape pollen SFE-CO2
on the prostatic index
Prostatic index is an important indicator in BPH. As
shown in Table I, the
testosterone-induced BPH group exhibited a significant increase in
prostatic index compared with the vehicle-treated group. By
contrast, the finasteride-treated group demonstrated a significant
reduction in prostatic index compared with the testosterone-induced
BPH group. The rape pollen SFE-CO2 groups showed
significant reductions in prostatic index compared with the
testosterone-induced BPH group.
 | Table IEffect of rape pollen extract on the
prostatic index. |
Table I
Effect of rape pollen extract on the
prostatic index.
| Group | Treatment | Prostatic index
×10−3 |
|---|
| Castration |
Vehicle-treated | 2.124±0.075 |
| BPH model | Testosterone | 4.166±0.070a |
| Finasteride | Testosterone + 5
mg/kg finasteride | 3.287±0.122b |
| Pollen extract
(low) | Testosterone + 21.3
mg/kg pollen extract | 3.890±0.103b |
| Pollen extract
(high) | Testosterone + 88.7
mg/kg pollen extract | 3.469±0.144b |
Effect of rape pollen SFE-CO2
on testosterone and DHT levels in the serum
As shown in Fig. 1A and
B, the testosterone-induced BPH group had significantly
increased serum testosterone levels compared with those in the
castration group. However, the finasteride- and pollen-treated
groups had significantly decreased serum testosterone levels
compared with those in the testosterone-induced BPH group. Serum
DHT levels in the testosterone-induced BPH group were significantly
increased compared with those in the castration group. However, the
serum DHT levels in the finasteride- and pollen-treated groups were
significantly decreased compared with those in the
testosterone-induced BPH group.
 | Figure 1Effects of rape pollen
SFE-CO2 on (A and C) testosterone and (B and D) DHT
levels in (A and B) serum and (C and D) prostate. The rape pollen
SFE-CO2 treatment groups exhibited significantly
decreased testosterone and DHT levels in the serum and prostate
compared with the BPH group. Rape pollen SFE-CO2 or
finasteride treatment was administered 1 h prior to testosterone
injection. #P<0.01, vs. castration;
*P<0.05, vs. BPH. Castration, corn oil injection (sc)
+ PBS (p.o.); BPH, testosterone (sc) + PBS (p.o.); finasteride, 5
mg/kg finasteride (p.o.) + testosterone (sc); pollen (low), 21.3
mg/kg rape pollen SFE-CO2 (p.o.) + testosterone (sc);
pollen (high), 88.7 mg/kg rape pollen SFE-CO2 (p.o.) +
testosterone (sc); SFE, supercritical fluid extract; DHT,
dihydrotestosterone; BPH, benign prostatic hyperplasia; PBS,
phosphate-buffered saline. |
Effects of rape pollen SFE-CO2
on testosterone and DHT levels in the prostate
In the prostate, while the testosterone-induced BPH
group exhibited increased levels of testosterone and DHT compared
with those in the castration group, the finasteride-treated group
had markedly decreased testosterone and DHT levels compared with
those in the BPH group. Similarly, the pollen-treated group
exhibited significantly reduced testosterone and DHT levels
compared with those in the BPH group (Fig. 1C and D).
Effect of rape pollen SFE-CO2
on prostate tissue by histopathological examination
As shown in Fig. 2,
epithelial cell layers and stromal spaces in the prostate were
larger in the testosterone-induced BPH group compared with those in
the castration group. The finasteride-treated group exhibited mild
glandular hyperplasia compared with the testosterone-induced BPH
group. Pollen-treated animals also exhibited a reduction in
epithelial cell layers and stromal spaces compared with the BPH
group, which was similar to the finasteride-treated group.
 | Figure 2Effects of rape pollen
SFE-CO2 on prostate hyperplasia in (A) castration, (B)
BPH, (C) finasteride, (D) pollen (low) and (E) pollen (high)
groups. Histological examination of the prostate tissue was
performed 24 h after the final testosterone injection. Prostate
tissues were fixed, sectioned at 4 μm thickness and stained with
hematoxylin and eosin solution (magnification, ×1,200). Castration,
corn oil injection (sc) + PBS (p.o.); BPH, testosterone (sc) + PBS
(p.o.); finasteride, 5 mg/kg finasteride (p.o.) + testosterone
(sc); pollen (low), 21.3 mg/kg rape pollen SFE-CO2
(p.o.) + testosterone (sc); pollen (high), 88.7 mg/kg rape pollen
SFE-CO2 (p.o.) + testosterone (sc); SFE, supercritical
fluid extract; BPH, benign prostatic hyperplasia; PBS,
phosphate-buffered saline. |
Effect of rape pollen SFE-CO2
on 5α-reductase expression
Expression levels of 5α-reductase I and II were
detected immunohistochemically. As shown in Figs. 3 and 4, the testosterone-induced BPH group had
significantly increased 5α-reductase I and II expression levels
compared with those in the castration group. However, the
finasteride- and pollen-treated groups had significantly decreased
5α-reductase I and II expression levels compared with those in the
testosterone-induced BPH group.
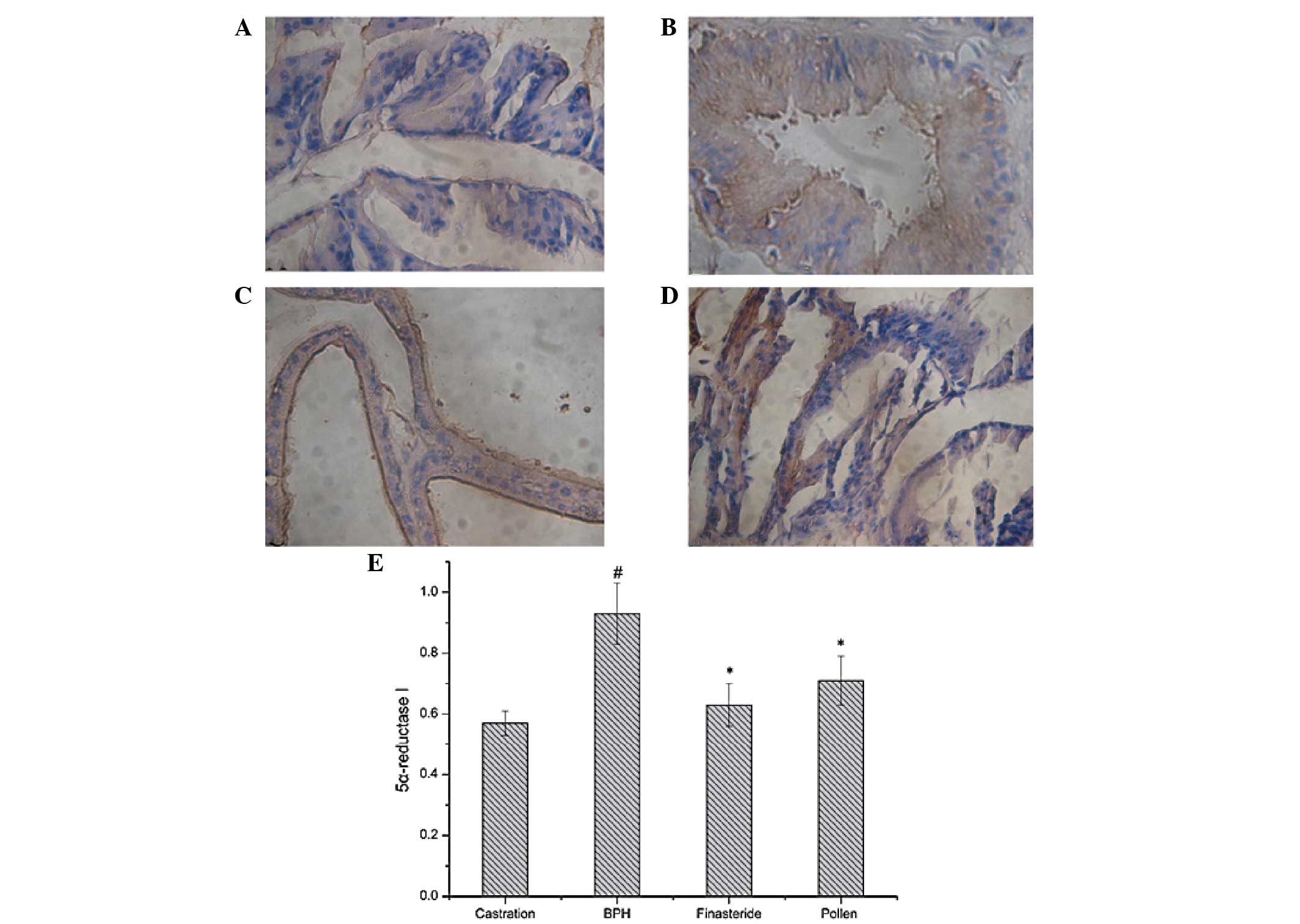 | Figure 3Immunohistochemical staining of
5α-reductase type I in the prostate tissues of rats in the (A)
castration, (B) BPH, (C) finasteride and (D) pollen groups. (E)
Quantitative image analysis of the immunohistochemical staining
expressed as optical densities across 10 fields for each rat
section. #P<0.05, vs. castration;
*P<0.05, vs. BPH. Castration, corn oil injection (sc)
+ PBS (p.o.); BPH, testosterone (sc) + PBS (p.o.); finasteride, 5
mg/kg finasteride (p.o.) + testosterone (sc); pollen, 88.7 mg/kg
rape pollen SFE-CO2 (p.o.) + testosterone (sc); SFE,
supercritical fluid extract; BPH, benign prostatic hyperplasia;
PBS, phosphate-buffered saline. Magnification, ×100. |
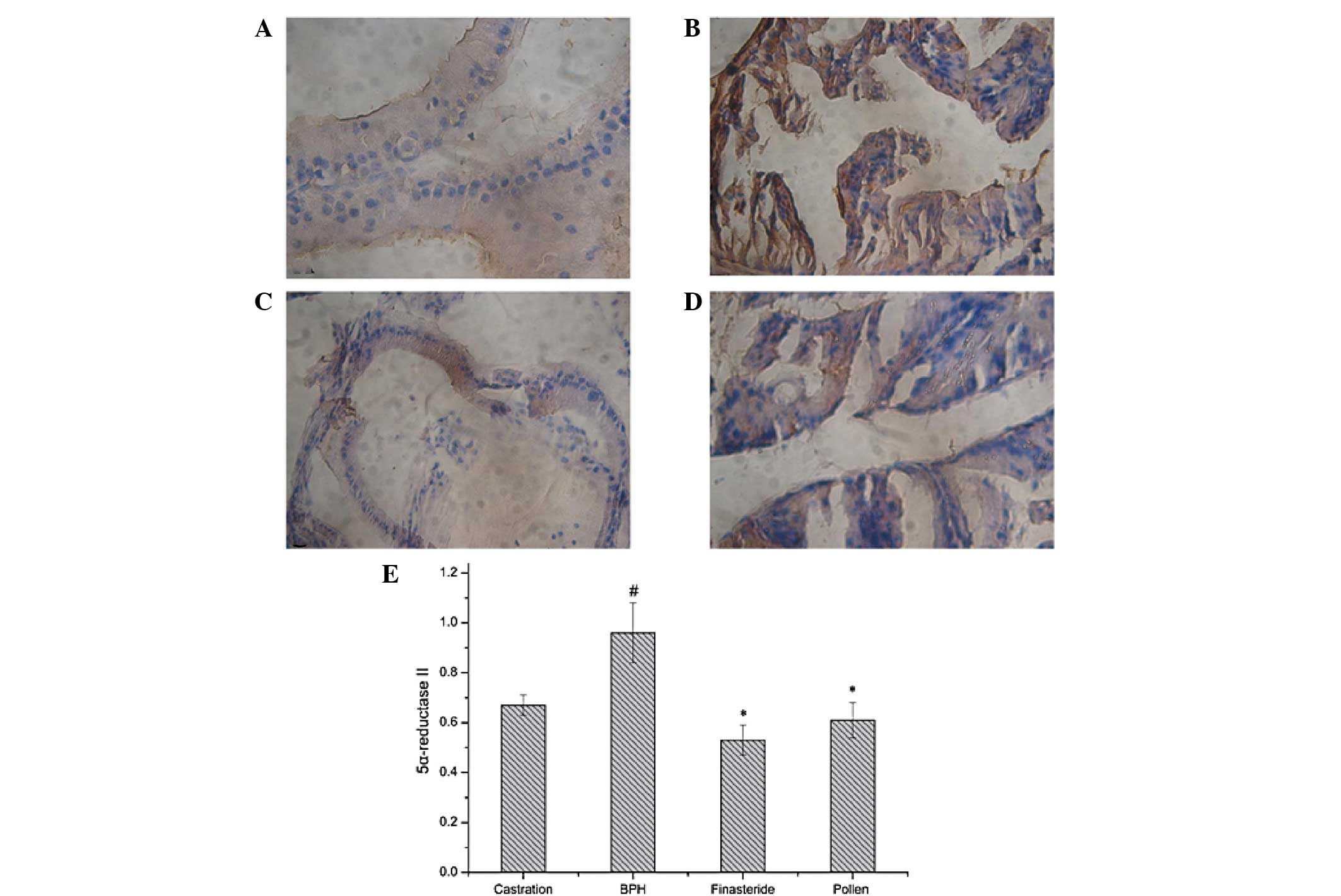 | Figure 4Immunohistochemical staining of
5α-reductase type II in the prostate tissues of rats in the (A)
castration, (B) BPH, (C) finasteride and (D) pollen groups. (E)
Quantitative image analysis of the immunohistochemical staining
expressed as optical densities across 10 fields for each rat
section. #P<0.05, vs. castration;
*P<0.05, vs. BPH. Castration, corn oil injection (sc)
+ PBS (p.o.); BPH, testosterone (sc) + PBS (p.o.); finasteride, 5
mg/kg finasteride (p.o.) + testosterone (sc); pollen, 88.7 mg/kg,
rape pollen SFE-CO2 (p.o.) + testosterone (sc); SFE,
supercritical fluid extract; BPH, benign prostatic hyperplasia;
PBS, phosphate-buffered saline. Magnification, ×100. |
Effect of rape pollen SFE-CO2
on COX-2 expression
As shown in Fig. 5,
COX-2 expression levels in the testosterone-induced BPH group
significantly increased compared with those in the castration
group. However, COX-2 levels in the finasteride- and pollen-treated
groups were significantly decreased compared with those in the
testosterone-induced BPH group.
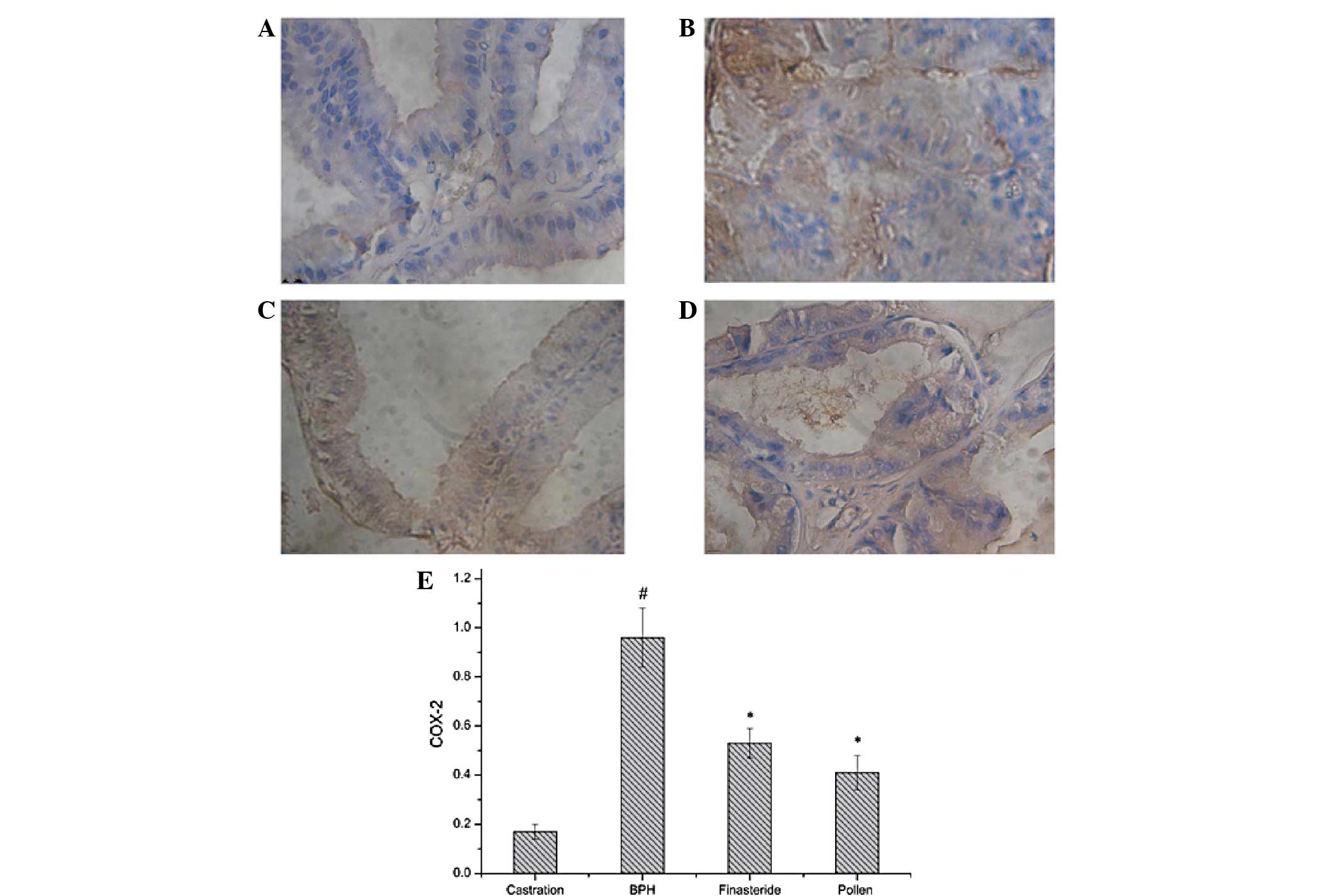 | Figure 5Immunohistochemical staining of COX-2
in the prostate tissues of rats in the (A) castration, (B) BPH, (C)
finasteride and (D) pollen groups. (E) Quantitative image analysis
of the immunohistochemical staining expressed as optical densities
across 10 fields for each rat section. #P<0.05, vs.
castration; *P<0.05, vs. BPH. Castration, corn oil
injection (sc) + PBS (p.o.); BPH, testosterone (sc) + PBS (p.o.);
finasteride, 5 mg/kg finasteride (p.o.) + testosterone (sc);
pollen, 88.7 mg/kg rape pollen SFE-CO2 (p.o.) +
testosterone (sc); SFE, supercritical fluid extract; BPH, benign
prostatic hyperplasia; PBS, phosphate-buffered saline; COX-2,
cyclooxygenase-2. Magnification, ×100. |
Discussion
In the present study, the effects of rape pollen
SFE-CO2 on prostate size and DHT and testosterone levels
were evaluated in the prostate tissue and serum of a
testosterone-induced BPH rat model. Testosterone-induced rats
exhibited increases in prostate size, DHT levels and 5α-reductase
and COX-2 expression levels when compared with the castration
group. In addition, prostatic hyperplasia was observed during
histopathological examinations. However, rape pollen
SFE-CO2-treated rats exhibited reductions in prostate
size, levels of DHT and testosterone in the serum and prostate and
expression levels of 5α-reductase and COX-2 in the prostate when
compared with testosterone-induced rats. Histopathological
examination also demonstrated that oral administration of rape
pollen SFE-CO2 attenuated testosterone-induced prostatic
hyperplasia.
Rats with BPH demonstrated significant increases in
prostatic index compared with the negative control animals.
However, pollen-treated animals exhibited significant reductions in
these measures when compared with the BPH animals. According to
previous studies, increased prostatic index is an important marker
indicating the development of BPH (17,18).
BPH involves epithelial and stromal hyperplasia of the prostate
(19,20), resulting in an increase in prostate
weight. When sufficiently large, the prostate constricts the
urethral canal to cause partial, or in certain cases, complete
obstruction (21). For these
reasons, a number of studies have investigated the inhibitory
effects of various substances on the development of BPH by
measuring the prostatic index (22). The results of the present study
indicate that rape pollen SFE-CO2 administration causes
a significant reduction in the prostatic index when compared with
the testosterone-induced BPH group. These results were consistent
with the histopathological examinations of the prostate tissues.
BPH animals experienced stromal proliferation and glandular
hyperplasia in the prostate, whereas animals treated with rape
pollen SFE-CO2 exhibited mild glandular hyperplasia.
These observations indicate that rape pollen SFE-CO2 is
an effective treatment for BPH.
The genesis of BPH depends on two factors:
Testicular androgen and the aging process (23). The most important androgen in the
prostate is DHT (24). DHT is
formed through the reduction of testosterone, catalyzed by the
enzyme 5α-reductase. This enzyme has two isoenzymes: 5α-reductase
type I and II (25). Dysregulation
of the reaction converting testosterone to DHT by 5α-reductase has
been reported to be a key step in the development of BPH. In
addition, elevated DHT levels correlate with the pathogenesis and
progression of androgen-dependent diseases, including prostate
cancer and BPH (26). BPH has been
successfully treated with 5α-reductase inhibitors that lower the
level of DHT available to the prostate tissue by blocking the
action of 5α-reductase that converts testosterone into DHT. A
number of studies have been conducted with the aim of reducing DHT
levels via the inhibition of 5α-reductase. Finasteride is a
5α-reductase inhibitor and an elective drug used for the treatment
of BPH. Finasteride reduces testosterone and DHT levels in the
serum and prostate, resulting in a reduction in prostate size and
ultimately providing relief from the lower urinary tract symptoms
associated with BPH (22).
However, finasteride also produces serious side-effects (27), which has led to a number of studies
investigating alternative materials for treating BPH with fewer
side-effects (28). Natural
products that appear to have limited adverse events are becoming
increasingly important in the treatment of BPH. Previous studies
have shown that numerous natural products, including saw palmetto
(10) Sphaeranthus indicus,
Pygeum africanum and Hypoxis rooperi, possess
anti-BPH potential (29). The
present study identified that finasteride reduced testosterone and
DHT levels in the serum and prostate, as well as the 5α-reductase
expression levels in the prostate. In addition, rape pollen
SFE-CO2 decreased the levels of testosterone and DHT in
the serum and prostate and also significantly decreased
5α-reductase I and II expression compared with that in the
testosterone-induced BPH group. These observations indicate that
rape pollen SFE-CO2 inhibits the development of BPH in
rats and these effects were closely associated with a reduction in
5α-reductase expression levels.
COX-2 is a proinflammatory inducible enzyme whose
production is triggered by mitogens, cytokines, reactive oxygen
species and growth factors in a variety of cell types. Increased
mRNA expression levels of COX-2 have been documented in BPH,
particularly in luminal epithelial cells (30). Several mechanisms have been
proposed to explain the role of COX-2 in prostate overgrowth.
Certain effects may result from COX-2-mediated increases in
prostaglandin (PG) synthesis, particularly PGE2 (31). However, COX-2 also upregulates
antiapoptotic protein Bcl-2 expression with a concomitant decrease
in prostate tissue apoptosis (32). Previous observations have indicated
that two COX-2 selective inhibitors, rofecoxib and celecoxib, are
effective as monotherapy or in combination with finasteride for the
management of lower urinary tract symptoms in human BPH (33,34).
The present study found that COX-2 levels in the pollen-treated
group significantly decreased compared with those in the
testosterone-induced BPH group. These observations indicate that
rape pollen SFE-CO2 inhibits the development of BPH in
rats and these effects were closely associated with a reduction in
COX-2 expression.
In conclusion, oral administration of rape pollen
SFE-CO2 in a BPH rat model significantly decreased the
prostatic index, as well as the DHT, 5α-reductase and COX-2
expression levels. These observations indicate that rape pollen
SFE-CO2 inhibits the development of BPH in rats and
these effects were closely associated with a reduction in the
levels of DHT, 5α-reductase and COX-2. The results of the present
study clearly indicate that rape pollen SFE-CO2 may be
useful in BPH treatment.
Acknowledgements
This study was supported by a grant from the State
Project For Essential Drug Research and Development (no.
2009ZX09301-007).
References
|
1
|
Glynn RJ, Campion EW, Bouchard GR and
Silbert JE: The development of benign prostatic hyperplasia among
volunteers in the Normative Aging Study. Am J Epidemiol. 121:78–90.
1985.PubMed/NCBI
|
|
2
|
Thorpe A and Neal D: Benign prostatic
hyperplasia. Lancet. 361:1359–1367. 2003. View Article : Google Scholar
|
|
3
|
Vikram A, Jena GB and Ramarao P: Increased
cell proliferation and contractility of prostate in insulin
resistant rats: linking hyperinsulinemia with benign prostate
hyperplasia. Prostate. 70:79–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Füllhase C, Chapple C, Cornu JN, et al:
Systematic review of combination drug therapy for non-neurogenic
male lower urinary tract symptoms. Eur Urol. 64:228–243.
2013.PubMed/NCBI
|
|
5
|
Alaiya AA, Al-Mohanna M, Aslam M, et al:
Proteomics-based signature for human benign prostate hyperplasia
and prostate adenocarcinoma. Int J Oncol. 38:1047–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McNicholas T and Swallow D: Benign
prostatic hyperplasia. Surgery (Oxford). 29:282–286. 2011.
View Article : Google Scholar
|
|
7
|
Sutcliffe S, Grubb RL III, Platz EA, et
al; Urologic Diseases in America Project. Non-steroidal
anti-inflammatory drug use and the risk of benign prostatic
hyperplasia-related outcomes and nocturia in the Prostate, Lung,
Colorectal, and Ovarian Cancer Screening Trial. BJU Int.
110:1050–1059. 2012. View Article : Google Scholar
|
|
8
|
McConnell JD: Benign prostatic
hyperplasia: Editorial comment. Curr Opin Urol. 8:1–3. 1998.
View Article : Google Scholar
|
|
9
|
Lin J, Zhou J, Xu W, Zhong X, Hong Z and
Peng J: Qianliening capsule treats benign prostatic hyperplasia via
suppression of the EGF/STAT3 signaling pathway. Exp Ther Med.
5:1293–1300. 2013.PubMed/NCBI
|
|
10
|
Wilt TJ, Ishani A, Stark G, MacDonald R,
Lau J and Mulrow C: Saw palmetto extracts for treatment of benign
prostatic hyperplasia: a systematic review. JAMA. 280:1604–1609.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilt TJ, Ishani A, Rutks I and MacDonald
R: Phytotherapy for benign prostatic hyperplasia. Public Health
Nutr. 3:459–472. 2000.PubMed/NCBI
|
|
12
|
Cheng N, Ren N, Gao H, Lei X, Zheng J and
Cao W: Antioxidant and hepatoprotective effects of Schisandra
chinensis pollen extract on CCl4-induced acute liver
damage in mice. Food Chem Toxicol. 55:234–240. 2013.PubMed/NCBI
|
|
13
|
McCartney HA and Lacey ME: Wind dispersal
of pollen from crops of oilseed rape (Brassica napus L.). J
Aerosol Sci. 22:467–477. 1991. View Article : Google Scholar
|
|
14
|
Grove MD, Spencer GF, Rohwedder WK, et al:
Brassinolide, a plant growth-promoting steroid isolated from
Brassica napus pollen. Nature. 281:216–217. 1979. View Article : Google Scholar
|
|
15
|
Hao XL, Zhou YM and Zhang XY: Construction
of fingerprint of rape pollen by using HPLC. Agricultural Science
& Technology-Hunan. 11(3): 107–109. 1382010.(In Chinese).
|
|
16
|
Veeresh Babu SV, Veeresh B, Patil AA and
Warke YB: Lauric acid and myristic acid prevent testosterone
induced prostatic hyperplasia in rats. Eur J Pharmacol.
626:262–265. 2010.PubMed/NCBI
|
|
17
|
Barry MJ, Fowler FJ Jr, O’Leary MP, et al:
The American Urological Association symptom index for benign
prostatic hyperplasia. The Measurement Committee of the American
Urological Association. J Urol. 148:1549–1557; discussion 1564.
1992.PubMed/NCBI
|
|
18
|
Yang A, Ren G, Tang L and Jiang W: Effects
of soy bean isoflavone on inhibition of benign prostatic
hyperplasia and the expressions of NO and NOS of rats. Wei Sheng
Yan Jiu. 38:172–174. 2009.(In Chinese).
|
|
19
|
Krieg M, Bartsch W, Thomsen M and Voigt
KD: Androgens and estrogens: their interaction with stroma and
epithelium of human benign prostatic hyperplasia and normal
prostate. J Steroid Biochem. 19:155–161. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chodak GW, Kranc DM, Puy LA, Takeda H,
Johnson K and Chang C: Nuclear localization of androgen receptor in
heterogeneous samples of normal, hyperplastic and neoplastic human
prostate. J Urol. 147:798–803. 1992.PubMed/NCBI
|
|
21
|
McConnell JD, Bruskewitz R, Walsh P, et
al; Finasteride Long-Term Efficacy and Safety Study Group. The
effect of finasteride on the risk of acute urinary retention and
the need for surgical treatment among men with benign prostatic
hyperplasia. N Engl J Med. 338:557–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gormley GJ, Stoner E, Bruskewitz RC, et
al; The Finasteride Study Group. The effect of finasteride in men
with benign prostatic hyperplasia. N Engl J Med. 327:1185–1191.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isaacs JT and Coffey DS: Etiology and
disease process of benign prostatic hyperplasia. Prostate Suppl.
2:33–50. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carson C III and Rittmaster R: The role of
dihydrotestosterone in benign prostatic hyperplasia. Urology. 61(4
Suppl 1): 2–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark RV, Hermann DJ, Cunningham GR,
Wilson TH, Morrill BB and Hobbs S: Marked suppression of
dihydrotestosterone in men with benign prostatic hyperplasia by
dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol
Metab. 89:2179–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bartsch G, Rittmaster RS and Klocker H:
Dihydrotestosterone and the concept of 5alpha-reductase inhibition
in human benign prostatic hyperplasia. Eur Urol. 37:367–380. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaplan SA, Chung DE, Lee RK, Scofield S
and Te AE: A 5-year retrospective analysis of 5α-reductase
inhibitors in men with benign prostatic hyperplasia: finasteride
has comparable urinary symptom efficacy and prostate volume
reduction, but less sexual side effects and breast complications
than dutasteride. Int J Clin Pract. 66:1052–1055. 2012.
|
|
28
|
Tacklind J, Macdonald R, Rutks I, Stanke
JU and Wilt TJ: Serenoa repens for benign prostatic
hyperplasia. Cochrane Database Syst Rev. 12:CD0014232012.PubMed/NCBI
|
|
29
|
Azimi H, Khakshur AA, Aghdasi I,
Fallah-Tafti M and Abdollahi M: A review of animal and human
studies for management of benign prostatic hyperplasia with natural
products: perspective of new pharmacological agents. Inflamm
Allergy Drug Targets. 11:207–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirschenbaum A, Klausner AP, Lee R, et al:
Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human
prostate. Urology. 56:671–676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SK, Kang JS, Jung da J, et al: Vitamin
C suppresses proliferation of the human melanoma cell SK-MEL-2
through the inhibition of cyclooxygenase-2 (COX-2) expression and
the modulation of insulin-like growth factor II (IGF-II)
production. J Cell Physiol. 216:180–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsujii M and DuBois RN: Alterations in
cellular adhesion and apoptosis in epithelial cells overexpressing
prostaglandin endoperoxide synthase 2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Silverio F, Bosman C, Salvatori M, et
al: Combination therapy with rofecoxib and finasteride in the
treatment of men with lower urinary tract symptoms (LUTS) and
benign prostatic hyperplasia (BPH). Eur Urol. 47:72–79.
2005.PubMed/NCBI
|
|
34
|
Falahatkar S, Mokhtari G, Pourreza F,
Asgari SA and Kamran AN: Celecoxib for treatment of nocturia caused
by benign prostatic hyperplasia: a prospective, randomized,
double-blind, placebo-controlled study. Urology. 72:813–816. 2008.
View Article : Google Scholar
|



















