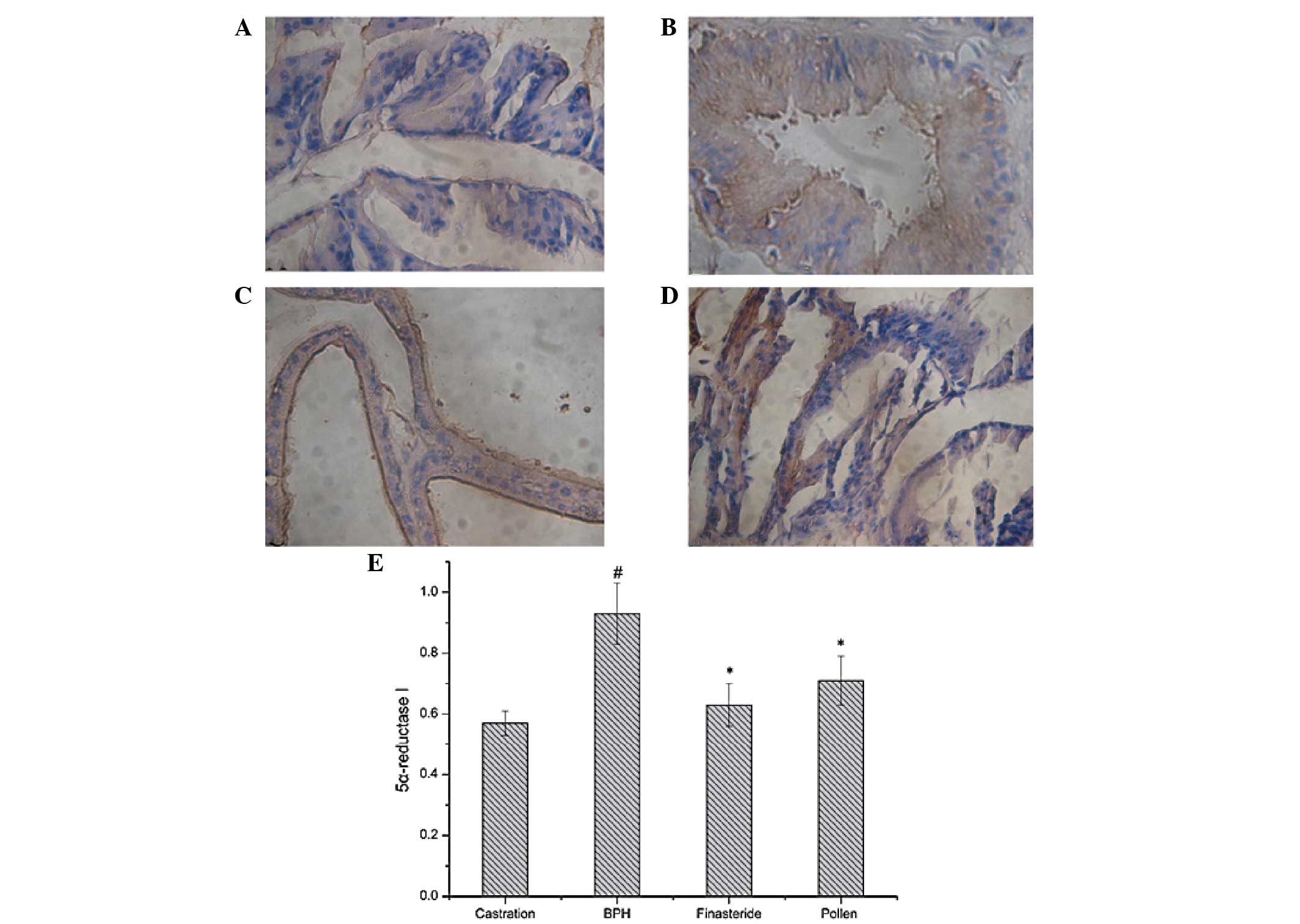
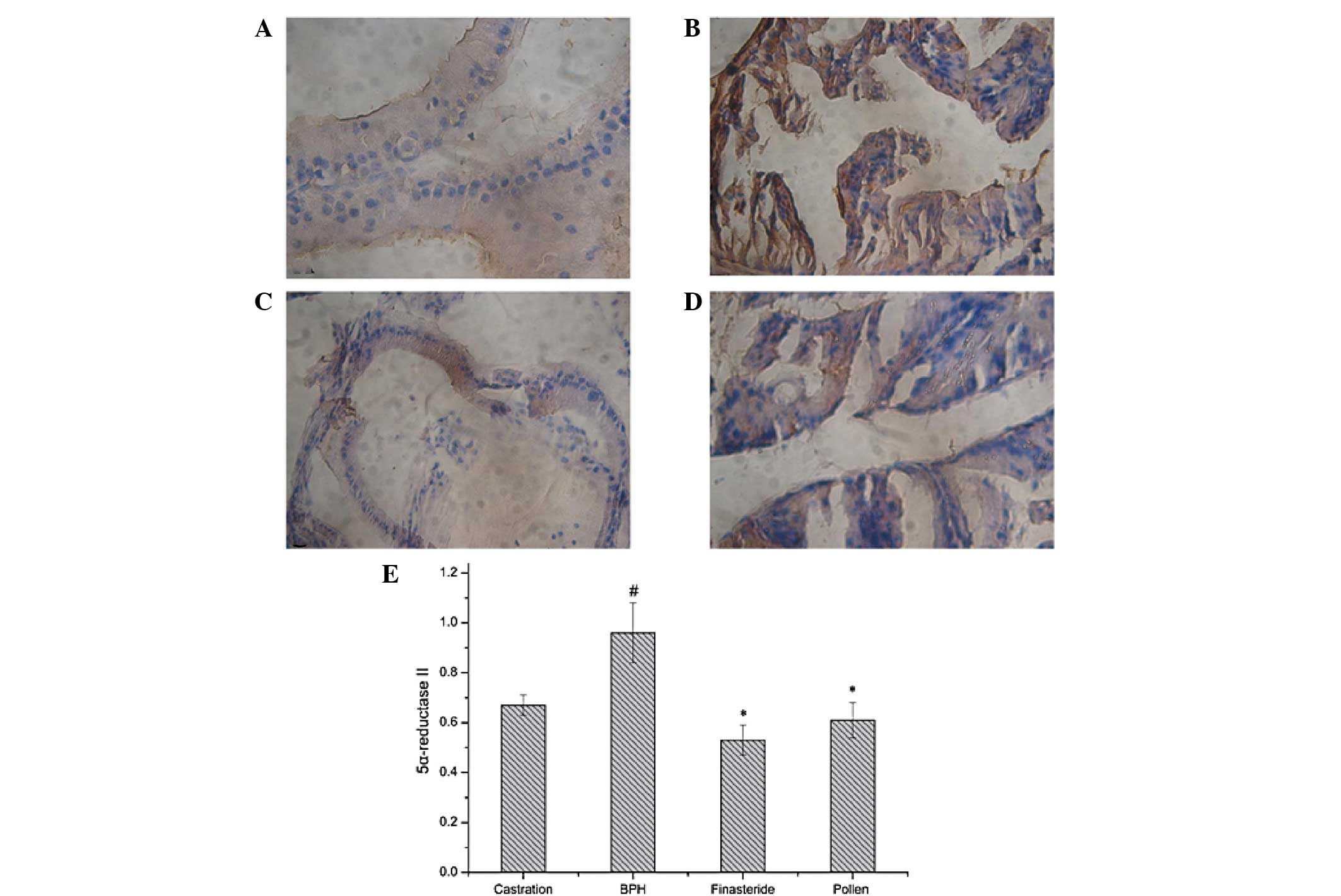
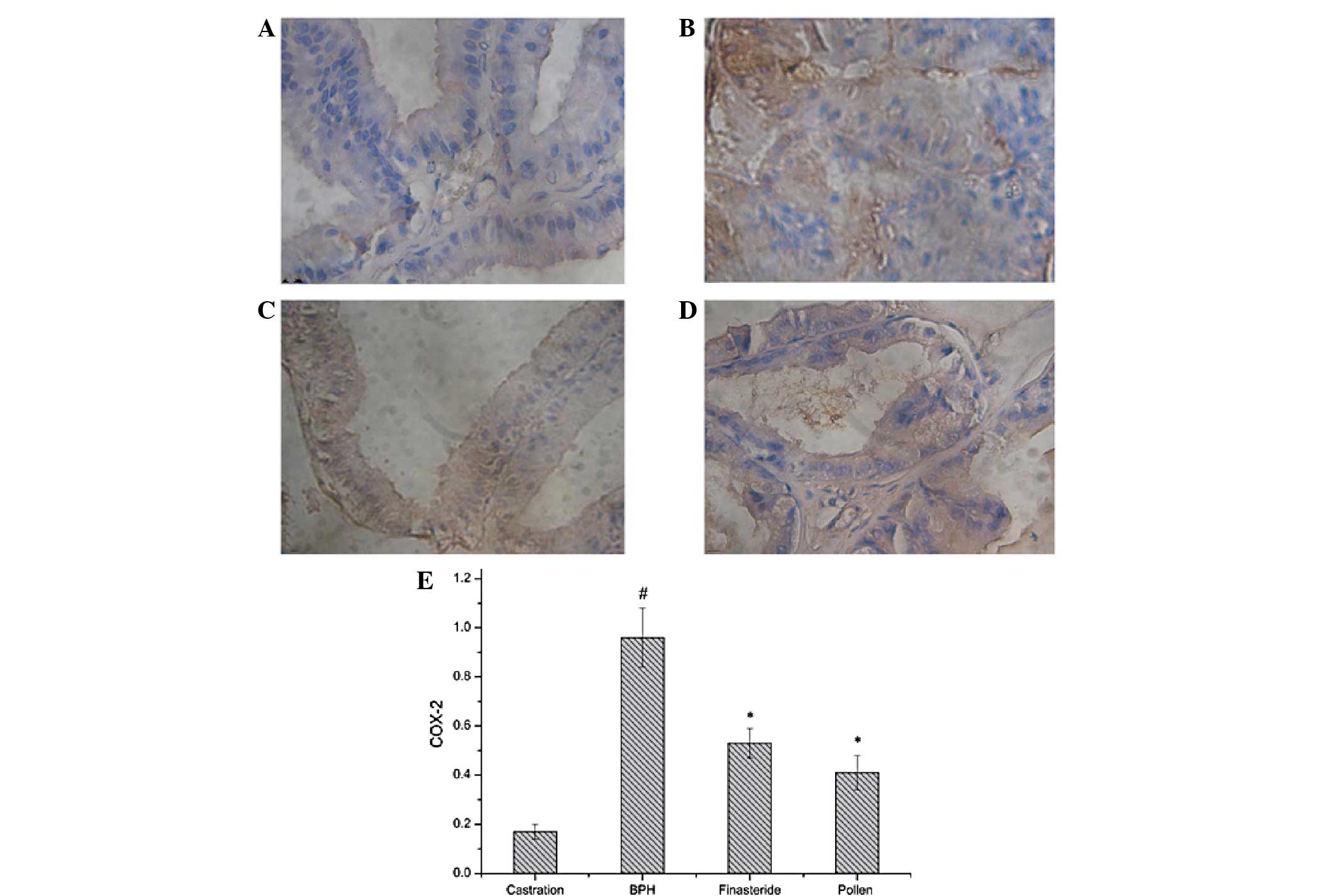
|
1
|
Glynn RJ, Campion EW, Bouchard GR and
Silbert JE: The development of benign prostatic hyperplasia among
volunteers in the Normative Aging Study. Am J Epidemiol. 121:78–90.
1985.PubMed/NCBI
|
|
2
|
Thorpe A and Neal D: Benign prostatic
hyperplasia. Lancet. 361:1359–1367. 2003. View Article : Google Scholar
|
|
3
|
Vikram A, Jena GB and Ramarao P: Increased
cell proliferation and contractility of prostate in insulin
resistant rats: linking hyperinsulinemia with benign prostate
hyperplasia. Prostate. 70:79–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Füllhase C, Chapple C, Cornu JN, et al:
Systematic review of combination drug therapy for non-neurogenic
male lower urinary tract symptoms. Eur Urol. 64:228–243.
2013.PubMed/NCBI
|
|
5
|
Alaiya AA, Al-Mohanna M, Aslam M, et al:
Proteomics-based signature for human benign prostate hyperplasia
and prostate adenocarcinoma. Int J Oncol. 38:1047–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McNicholas T and Swallow D: Benign
prostatic hyperplasia. Surgery (Oxford). 29:282–286. 2011.
View Article : Google Scholar
|
|
7
|
Sutcliffe S, Grubb RL III, Platz EA, et
al; Urologic Diseases in America Project. Non-steroidal
anti-inflammatory drug use and the risk of benign prostatic
hyperplasia-related outcomes and nocturia in the Prostate, Lung,
Colorectal, and Ovarian Cancer Screening Trial. BJU Int.
110:1050–1059. 2012. View Article : Google Scholar
|
|
8
|
McConnell JD: Benign prostatic
hyperplasia: Editorial comment. Curr Opin Urol. 8:1–3. 1998.
View Article : Google Scholar
|
|
9
|
Lin J, Zhou J, Xu W, Zhong X, Hong Z and
Peng J: Qianliening capsule treats benign prostatic hyperplasia via
suppression of the EGF/STAT3 signaling pathway. Exp Ther Med.
5:1293–1300. 2013.PubMed/NCBI
|
|
10
|
Wilt TJ, Ishani A, Stark G, MacDonald R,
Lau J and Mulrow C: Saw palmetto extracts for treatment of benign
prostatic hyperplasia: a systematic review. JAMA. 280:1604–1609.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilt TJ, Ishani A, Rutks I and MacDonald
R: Phytotherapy for benign prostatic hyperplasia. Public Health
Nutr. 3:459–472. 2000.PubMed/NCBI
|
|
12
|
Cheng N, Ren N, Gao H, Lei X, Zheng J and
Cao W: Antioxidant and hepatoprotective effects of Schisandra
chinensis pollen extract on CCl4-induced acute liver
damage in mice. Food Chem Toxicol. 55:234–240. 2013.PubMed/NCBI
|
|
13
|
McCartney HA and Lacey ME: Wind dispersal
of pollen from crops of oilseed rape (Brassica napus L.). J
Aerosol Sci. 22:467–477. 1991. View Article : Google Scholar
|
|
14
|
Grove MD, Spencer GF, Rohwedder WK, et al:
Brassinolide, a plant growth-promoting steroid isolated from
Brassica napus pollen. Nature. 281:216–217. 1979. View Article : Google Scholar
|
|
15
|
Hao XL, Zhou YM and Zhang XY: Construction
of fingerprint of rape pollen by using HPLC. Agricultural Science
& Technology-Hunan. 11(3): 107–109. 1382010.(In Chinese).
|
|
16
|
Veeresh Babu SV, Veeresh B, Patil AA and
Warke YB: Lauric acid and myristic acid prevent testosterone
induced prostatic hyperplasia in rats. Eur J Pharmacol.
626:262–265. 2010.PubMed/NCBI
|
|
17
|
Barry MJ, Fowler FJ Jr, O’Leary MP, et al:
The American Urological Association symptom index for benign
prostatic hyperplasia. The Measurement Committee of the American
Urological Association. J Urol. 148:1549–1557; discussion 1564.
1992.PubMed/NCBI
|
|
18
|
Yang A, Ren G, Tang L and Jiang W: Effects
of soy bean isoflavone on inhibition of benign prostatic
hyperplasia and the expressions of NO and NOS of rats. Wei Sheng
Yan Jiu. 38:172–174. 2009.(In Chinese).
|
|
19
|
Krieg M, Bartsch W, Thomsen M and Voigt
KD: Androgens and estrogens: their interaction with stroma and
epithelium of human benign prostatic hyperplasia and normal
prostate. J Steroid Biochem. 19:155–161. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chodak GW, Kranc DM, Puy LA, Takeda H,
Johnson K and Chang C: Nuclear localization of androgen receptor in
heterogeneous samples of normal, hyperplastic and neoplastic human
prostate. J Urol. 147:798–803. 1992.PubMed/NCBI
|
|
21
|
McConnell JD, Bruskewitz R, Walsh P, et
al; Finasteride Long-Term Efficacy and Safety Study Group. The
effect of finasteride on the risk of acute urinary retention and
the need for surgical treatment among men with benign prostatic
hyperplasia. N Engl J Med. 338:557–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gormley GJ, Stoner E, Bruskewitz RC, et
al; The Finasteride Study Group. The effect of finasteride in men
with benign prostatic hyperplasia. N Engl J Med. 327:1185–1191.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isaacs JT and Coffey DS: Etiology and
disease process of benign prostatic hyperplasia. Prostate Suppl.
2:33–50. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carson C III and Rittmaster R: The role of
dihydrotestosterone in benign prostatic hyperplasia. Urology. 61(4
Suppl 1): 2–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark RV, Hermann DJ, Cunningham GR,
Wilson TH, Morrill BB and Hobbs S: Marked suppression of
dihydrotestosterone in men with benign prostatic hyperplasia by
dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol
Metab. 89:2179–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bartsch G, Rittmaster RS and Klocker H:
Dihydrotestosterone and the concept of 5alpha-reductase inhibition
in human benign prostatic hyperplasia. Eur Urol. 37:367–380. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaplan SA, Chung DE, Lee RK, Scofield S
and Te AE: A 5-year retrospective analysis of 5α-reductase
inhibitors in men with benign prostatic hyperplasia: finasteride
has comparable urinary symptom efficacy and prostate volume
reduction, but less sexual side effects and breast complications
than dutasteride. Int J Clin Pract. 66:1052–1055. 2012.
|
|
28
|
Tacklind J, Macdonald R, Rutks I, Stanke
JU and Wilt TJ: Serenoa repens for benign prostatic
hyperplasia. Cochrane Database Syst Rev. 12:CD0014232012.PubMed/NCBI
|
|
29
|
Azimi H, Khakshur AA, Aghdasi I,
Fallah-Tafti M and Abdollahi M: A review of animal and human
studies for management of benign prostatic hyperplasia with natural
products: perspective of new pharmacological agents. Inflamm
Allergy Drug Targets. 11:207–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirschenbaum A, Klausner AP, Lee R, et al:
Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human
prostate. Urology. 56:671–676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SK, Kang JS, Jung da J, et al: Vitamin
C suppresses proliferation of the human melanoma cell SK-MEL-2
through the inhibition of cyclooxygenase-2 (COX-2) expression and
the modulation of insulin-like growth factor II (IGF-II)
production. J Cell Physiol. 216:180–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsujii M and DuBois RN: Alterations in
cellular adhesion and apoptosis in epithelial cells overexpressing
prostaglandin endoperoxide synthase 2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Silverio F, Bosman C, Salvatori M, et
al: Combination therapy with rofecoxib and finasteride in the
treatment of men with lower urinary tract symptoms (LUTS) and
benign prostatic hyperplasia (BPH). Eur Urol. 47:72–79.
2005.PubMed/NCBI
|
|
34
|
Falahatkar S, Mokhtari G, Pourreza F,
Asgari SA and Kamran AN: Celecoxib for treatment of nocturia caused
by benign prostatic hyperplasia: a prospective, randomized,
double-blind, placebo-controlled study. Urology. 72:813–816. 2008.
View Article : Google Scholar
|