Introduction
Depression is a psychiatric disorder that presents
as a reduction of confidence in oneself, the world and the future
(1). At present, depression is a
common mental disorder in the general population. Almost 20% of
individuals in the Western world succumb to a depressive episode
during their lifetime (2).
Depression is the leading cause of disability among individuals
worldwide and was one of the ten most common diseases globally in
2001 (3).
In a cross-sectional survey of a sample of 98,658
Chinese adults in 2010, 11.6% had diabetes mellitus (DM), of which
8.1% cases were newly detected (4). The world prevalence of DM among
adults (20–79 years old) was 6.4% in 2010, affecting 285 million
adults, and is predicted to increase to 7.7%, equating to 439
million adults, by 2030 according to theoretical calculations
(5).
There has been a significant, increasing trend in
the prevalence of depression among the diabetic population in
recent years in Taiwan (6).
Several meta-analysis studies have shown that the risks of elevated
levels of depression and of incident depression are increased in
individuals with type 2 DM compared with those in healthy subjects
(7,8). Furthermore, in patients with DM and
poor disease control, depression is an important risk factor for
poor patient adherence to medications, but not lack of treatment
intensification by physicians (9).
The coexistence of DM and depression is associated
with significant morbidity, mortality and increased healthcare cost
(10). Comorbid depression in
individuals with DM causes a serious threat to quality of life
(11). A study on the treatment of
depression in diabetic patients has revealed that classical
antidepressants, including monoamine oxidase inhibitors, induce
hypoglycemia and weight gain, whereas tricyclics lead to
hyperglycemia and carbohydrate cravings (12).
Therefore, there is an urgent requirement to
identify an agent with greater efficacy and fewer side-effects for
treating comorbid depression with DM.
In comorbid DM and depression, the majority of the
evidence supports the use of fluoxetine in the control of glucose
handling (13). However, selective
serotonin reuptake inhibitors may cause discontinuation or
withdrawal symptoms, including nausea, vomiting and diarrhea
(14), which are induced by the
inhibition of gastric motor activity (15). Metformin is the mainstay treatment
in the control (16) and
prevention (17) of DM and
associated comorbidities (18).
In traditional medicinal systems, numerous herbal
drugs may be combined to produce multi-herbal formulas that enhance
the effects and reduce the toxicity of the individual drugs. The
purpose of the present study was to demonstrate the hypoglycemic,
lipid-lowering and antidepressant effects of Zuogui Jiangtang Jieyu
formulation (ZGJTJY) in a model of unpredictable chronic mild
stress (UCMS) in rats with DM. The experimental protocol was in
accordance with guidelines of Hunan University of Traditional
Chinese Medicine and the Guide for the Care and Use of Laboratory
Animals (NIH publication no. 80-23, 1996) and was approved by the
Institutional Animal Care and Use Committee of Hunan University of
Traditional Chinese Medicine.
Material and methods
Traditional Chinese medicine
preparation
The ZGJTJY formulation consisted of 11 herbal
components: Astragalus membranaceus (18.0 g), Hypericum
perforatum (St. John’s wort; 3.0 g), rhizome of Curcuma
Longa (9.0 g), prepared Rehmannia root (15.0 g),
Cornus offinalis (12.0 g), Lycium barbarum L. (12.0
g), Cuscuta chinensis seed (9.0 g), Eucommia ulmoides
(9.0 g), Salvia miltiorrhiza (12.0 g), root bark of
Paeonia suffruticosa (6.0 g) and Achyranthes root
(9.0 g). It was provided by the pharmacy of The First Affiliated
Hospital, Hunan University of Traditional Chinese Medicine
(Changsha, China). The ZGJTJY was boiled, filtered and concentrated
to a 2.28-g/ml liquid at 80°C in a water bath and stored at 4°C in
a refrigerator. When used, it was diluted with distilled water and
administered by gavage.
Drug and materials
Metformin hydrochloride tablets were purchased from
Hunan Xiangya Pharmaceutical Co., Ltd. (Changsha, China; Lot:
1303106; 0.25 g/tablet); fluoxetine hydrochloride capsules were
obtained from Patheon France S.A.S (Bourgoin Jallieu, France; Lot:
0972A; 20 mg/tablet); and streptozotocin (STZ) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). The high-fat diet (HFD)
consisted of 58% fat, 25% protein and 17% carbohydrate, as a
percentage of the total kilocalories. The high-speed refrigerated
centrifuge was from Sigma-Aldrich (SIGMA 3K15, Sigma
Laborzentrifugen GmbH, Osterode am Harz, Germany), a microplate
reader was obtained from Thermo Fisher Scientific Inc. (Waltham,
MA, USA; MK3) and the open boxes were homemade.
Animals and drug administration
Specific pathogen-free, male Sprague Dawley rats
(weight, 200–220 g; license no. SCXK 2009-0004) were provided by
Hunan Slac Jingda Laboratory Animal Co., Ltd. (Changsha, China),
and all animals were housed with a 12-h light/dark cycle, in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (1996).
The rats were randomly divided into the blank
control, vehicle (model plus vehicle), positive control (model plus
metformin and fluoxetine), and high, medium and low dose ZGJTJY
(hZGJTJY, model plus high dose of ZGJTJY; mZGJTJY, model plus
medium dose of ZGJTJY; and lZGJTJY, model plus low dose of ZGJTJY,
respectively) groups, with 10 rats per group. The dosages of the
drugs were as follows: Metformin, 1.8 mg/kg; fluoxetine, 10.8
mg/kg; hZGJTJY, 2.28 g/ml; mZGJTJY, 1.14 g/ml; and lZGJTJY, 0.57
g/ml. The rats in the model group received the same volume of
distilled water. All rats were treated by gastric perfusion once
per day.
Model of UCMS in rats with DM
The experimental model of DM was induced with a
combination of low-dose STZ and a HFD. Following the onset of the
experiment, the rats were fed ad libitum with a HFD for two
weeks and then received 35 mg/kg STZ freshly dissolved in citrate
buffer (pH 4.5) intraperitoneally after fasting overnight (19). The rats with non-fasting plasma
glucose levels of ≥300 mg/dl were considered diabetic and selected
for further study.
The UCMS model was established according to the
methods of Willner with modifications (20). The stress procedure contained a
range of stressors, which consisted of: 24-h water deprivation, a
1-min tail pinch, 5-min thermal stimulation in a 45°C oven, 5-min
cold swimming at 4°C, a 24-h reversed light/dark cycle, 48-h food
deprivation, electric shock to the foot (10 mA current;
administered every other minute and lasting 10 sec per time for 30
times), shaking (once per second; lasting for 15 min), noise (85
dB) and strange smell. During a period of 28 days, one of the
stimuli was selected randomly and applied to the rats so that the
rats were not able to expect the stimulus. Every stimulus used 2 or
3 times in total for each rat within 28 days.
Open field test
An open field test was used to conduct scoring of
the rats in all groups. The open-field device was made of opaque
materials with a 80×80 cm square located on the bottom, which was
equally divided into 25 equilateral squares. Surrounding the base
there was a wall with a height of 40 cm. The rat was placed in the
central square and then the number of squares the rat crossed in 5
min was measured (only the squares that the rat entered on four
feet were included in the score of horizontal activity) and the
number of times the rat stood on hind limbs (the score of vertical
activity) was observed. Each rat was measured once for 5 min, which
was scored by two observers and the average value was recorded. The
sum of the horizontal and vertical activity scores was considered
to be indicative of the locomotor activity (LMA).
Morris water maze test
Spatial learning and memory were observed in the
Morris water maze using procedures similar to those described
previously (21). The Morris water
maze consisted of a circular fiberglass pool (200 cm in diameter)
filled with water (25±1°C) and made opaque with black non-toxic
paint. The pool was surrounded by light blue curtains and three
distal visual cues were fixed to the curtains. Four floor light
sources of equal power provided uniform illumination in the pool
and testing room. A charge-coupled device camera (kl-9511zh, Konlan
Company, Shuozhou, China) suspended above the pool center recorded
the swim paths of the animals and the video output was digitized by
an EthoVision XT tracking system (Noldus Information Technology,
Inc., Leesburg, VA, USA).
Four trials from each of the four quadrants were
conducted once a day for five days. The video analysis system
tracked, recorded and analyzed the swimming speed and the time
taken to locate the platform for each animal. Each trial lasted
either until the rat located the platform or for 60 sec, which was
recorded as the escape latency (EL) time, and the mean EL time of
the last four days as the outcome of learning. The platform was
removed for a 60-sec probe trial on the final day, and the time
spent swimming in the platform quadrant was recorded as the space
exploration time (SET).
Detection of plasma glucose and serum
lipid levels
Following the final behavioral test, a single touch
glucometer (OneTouch Ultra 2; LifeScan, High Wycombe, UK) was used
to determine the glucose levels in plasma collected from the tail
vein of the rats. Subsequently, the rats were anesthetized, and
blood samples were collected by the abdominal aortic method in
tubes containing EDTA and centrifuged at 2,500 × g for 15 min at
4°C. The serum was stored at −70°C until analysis. The serum levels
of glycosylated hemoglobin (HbA1c), total cholesterol (TC),
triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and
low-density lipoprotein cholesterol (LDL-C) were determined using
enzymatic kits (Nanjing Jiancheng, Nanjing, China). All serum
samples were measured with a RT-1904C Semi-auto Chemistry Analyzer
(Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, China)
Statistical analysis
All results are presented as the mean ± standard
error of the mean. Variance analysis was used make comparisons
among the groups. P<0.05 was considered to indicate a
statistically significant difference. A test for homogeneity was
used to examine the data and if the data was homogeneous, a one-way
analysis of variance (ANOVA) was conducted directly on the data.
Between the two groups, the least significant difference method was
used to compare any differences. Otherwise, the parameters were
changed first and then the test for homogeneity was used again.
ANOVA was only conducted on the data which became homogeneous
following the change of parameters. All statistical analyses were
performed using SPSS software for Windows, version 18.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Open field test
As shown in Fig. 1,
the total scores of the open field test were significantly lower in
the vehicle group than those in the blank control group
(P<0.05). Compared with those in the vehicle group, hZGJTJY
increased the LMA levels of the UCMS-DM model rats (P<0.05).
Morris water maze test
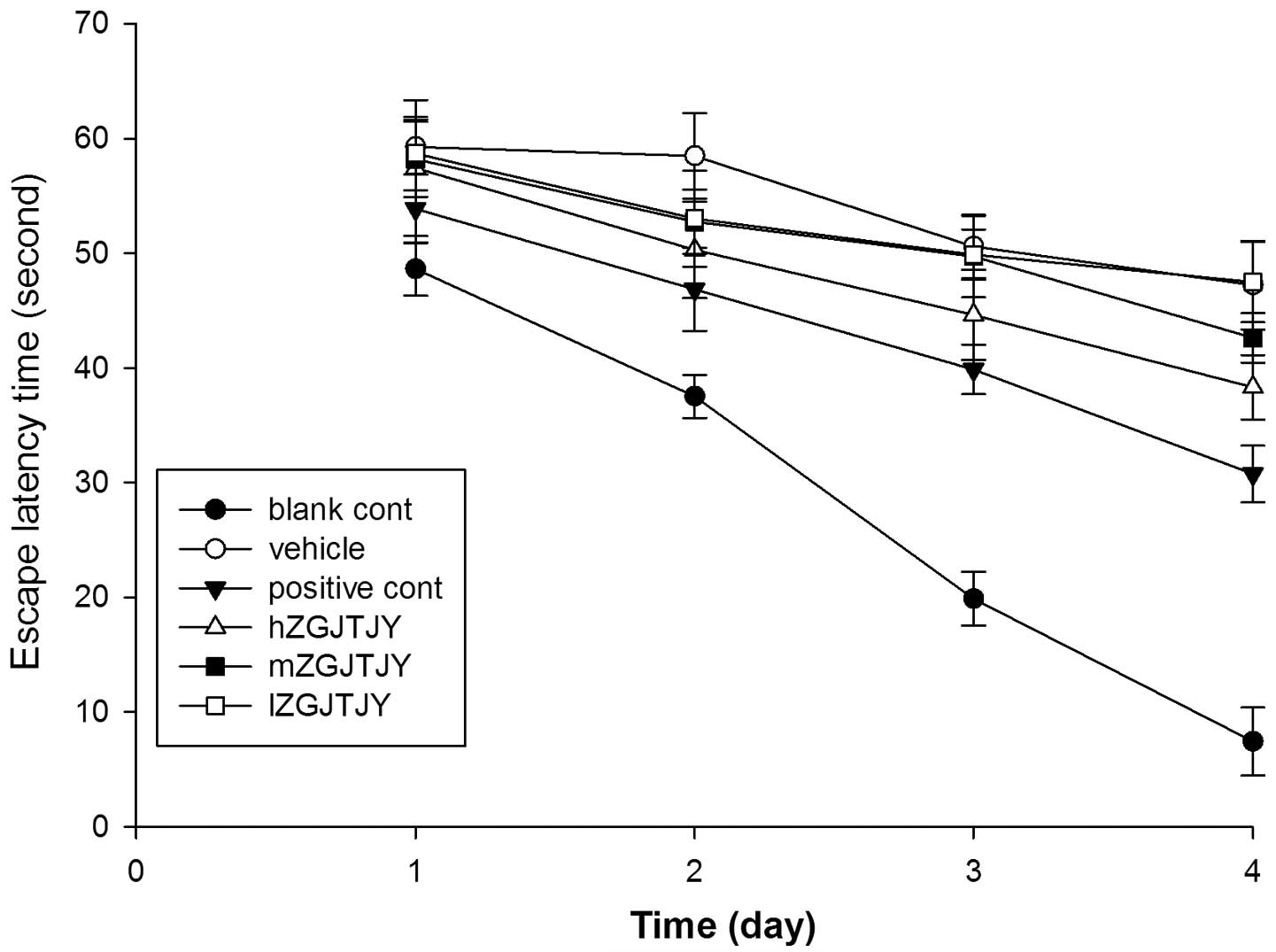
The EL times in the Morris water maze test (Fig. 2) were significantly longer in the
vehicle group than those in the blank control group on days two,
three and four (P<0.05, P<0.01 and P<0.01, respectively).
hZGJTJY markedly decreased the EL times of the model group on day 4
(P<0.05).
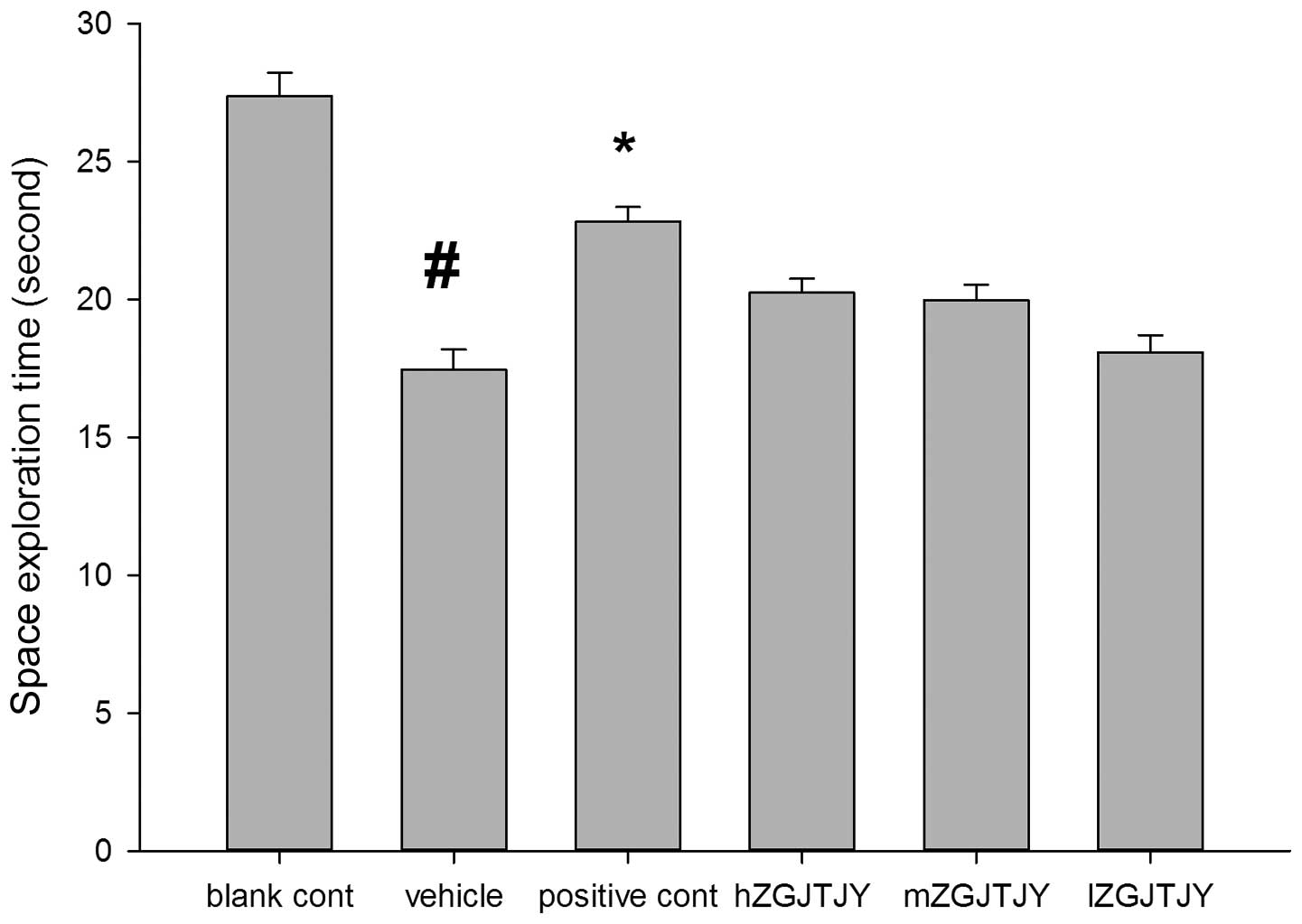
The SETs in the Morris water maze test (Fig. 3) were shorter in the vehicle group
than those in the blank control group (P<0.05). Compared with
those in the model group, hZGJTJY increased the SETs.
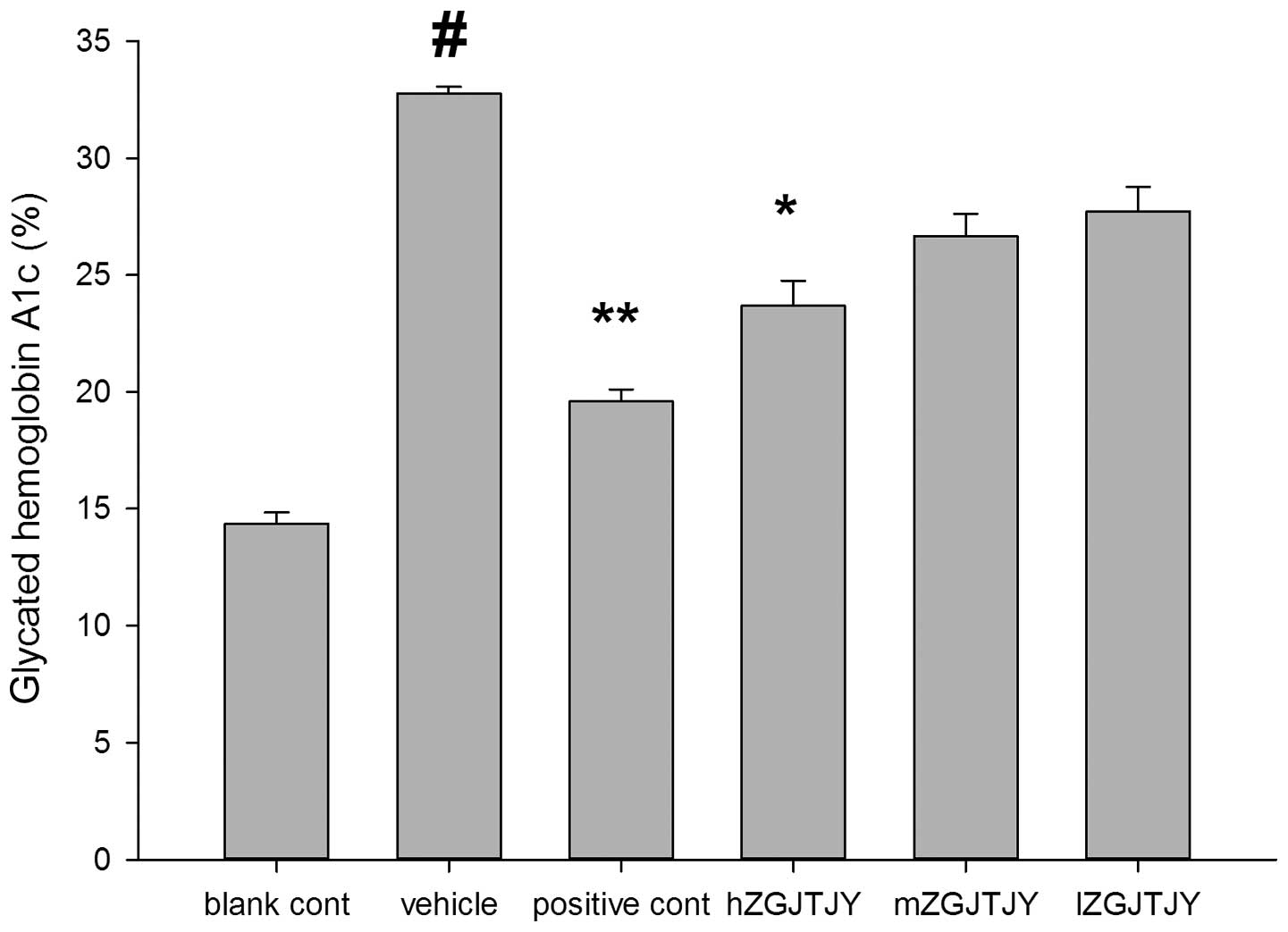
Blood glucose and HbA1c levels
The blood glucose (Fig.
4) and HbA1c (Fig. 5) levels
were higher in the vehicle group than those in the blank control
group (P<0.05). hZGJTJY reduced the blood glucose and HbA1c
levels (P<0.05), while mZGJTJY significantly lowered the blood
glucose levels of the model group (P<0.05).
Lipid analysis
The TC, TG and LDL-C levels were significantly
higher, while the HDL-C levels were significantly lower in the
vehicle group than those in the blank control group. hZGJTJY
reduced the TC, TG and LDL-C levels (P<0.05), and elevated the
HDL-C levels (P<0.01) compared with those in the model group.
mZGJTJY also significantly increased the HDL-C levels compared with
those in the model group (P<0.05; Table I).
 | Table ISerum lipid content in all groups. |
Table I
Serum lipid content in all groups.
| Group | TC | TG | HDL-C | LDL-C |
|---|
| Blank control | 0.87±0.04 | 0.39±0.06 | 1.19±0.18 | 0.78±0.16 |
| Vehicle | 2.64±0.12a | 1.73±0.08a | 0.36±0.04a | 1.42±0.07a |
| Positive control | 1.45±0.07b | 0.87±0.04b | 0.89±0.08c | 0.82±0.03b |
| hZGJTJY | 1.85±0.02b | 1.25±0.07b | 0.74±0.06c | 0.94±0.06b |
| mZGJTJY | 2.27±0.06 | 1.57±0.12 | 0.51±0.09b | 1.21±0.02 |
| lZGJTJY | 2.36±0.15 | 1.59±0.05 | 0.49±0.03 | 1.29±0.12 |
Discussion
The comorbidity of depression with chronic physical
diseases, including arthritis and DM, is well recognized in
developed countries (22–24).
Comorbid depression accompanied with DM is common
clinically, but the establishment of a mammalian model of it is
challenging. At the initial stages of the present study, PubMed and
other websites were searched and no experimental designs similar to
that of the present study were found. The present study established
a model of depression accompanied by DM, which comprised a
combination of the two patterns of animal model. The model proposed
for the first time in the present study intensifies the depression
status in DM, compared with the real depression status of a patient
with DM.
A previously described model of type 2 DM was
induced with the combination of a HFD and low-dose STZ (19). That model simulated the human
syndrome of depression with DM and was identified as suitable for
testing antidiabetic agents for the treatment of type 2 DM. The
chronic stress-induced depression model is an effective model for
studying depression and has been widely used in basic research and
drug screening for depression (25,26).
The model simulates the core symptoms of depression: Loss of
interest, anhedonia, and a reduction in exploratory ability and
sexual behavior. Helplessness and anhedonia are the core symptoms
of depression and the majority of the current models only mimic
anhedonia. The currently available chronic mild stress model is
possibly the most valid and widely used animal model of
depression.
In summary, the present study attempted to establish
a model of depression accompanied with DM for the first time, and
demonstrated that a high dose of ZGJTJY increased the LMA levels in
the open field test, the EL times of the model on day four of the
Morris water maze test and the SETs in the Morris water maze test.
In addition it increased HDL-C levels, and reduced the blood
glucose, HbA1c, TC, TGs and LDL-C levels compared with those in the
model group. If the action of ZGJTJY is positive in clinic, further
clinical research would enhance the development of the new
drug.
Acknowledgements
This study was supported in part by the Natural
Science Foundation of Hunan province (no. 13JJ5030) and the
National Natural Science Foundation of China (no. 81373578).
Abbreviations:
|
ZGJTJY
|
Zuogui Jiangtang Jieyu formulation
|
|
UCMS
|
unpredictable chronic mild stress
|
|
DM
|
diabetes mellitus
|
|
STZ
|
streptozotocin
|
References
|
1
|
Lavergne F and Jay TM: A new strategy for
antidepressant prescription. Front Neurosci. 4:1922010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujitsuka N, Asakawa A, Hayashi M, et al:
Selective serotonin reuptake inhibitors modify physiological
gastrointestinal motor activities via 5-HT2c receptor and acyl
ghrelin. Biol Psychiatry. 65:748–759. 2009. View Article : Google Scholar
|
|
3
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: systematic analysis of population health data.
Lancet. 367:1747–1757. 2006. View Article : Google Scholar
|
|
4
|
Ning G and Bloomgarden Z: Diabetes in
China: prevalence, diagnosis, and control. J Diabetes. 5:3722013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CH, Lee YY, Liu CC, Chen HF, Ko MC and
Li CY: Urbanization and prevalence of depression in diabetes.
Public Health. 126:104–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali S, Stone MA, Peters JL, Davies MJ and
Khunti K: The prevalence of co-morbid depression in adults with
Type 2 diabetes: a systematic review and meta-analysis. Diabet Med.
23:1165–1173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barnard KD, Skinner TC and Peveler R: The
prevalence of co-morbid depression in adults with Type 1 diabetes:
systematic literature review. Diabet Med. 23:445–448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katon W, Russo J, Lin EH, et al: Diabetes
and poor disease control: is comorbid depression associated with
poor medication adherence or lack of treatment intensification?
Psychosom Med. 71:965–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egede LE and Ellis C: Diabetes and
depression: global perspectives. Diabetes Res Clin Pract.
87:302–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schram MT, Baan CA and Pouwer F:
Depression and quality of life in patients with diabetes: a
systematic review from the European depression in diabetes (EDID)
research consortium. Curr Diabetes Rev. 5:112–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erenmemisoglu A, Ozdogan UK, Saraymen R
and Tutus A: Effect of some antidepressants on glycaemia and
insulin levels of normoglycaemic and alloxan-induced hyperglycaemic
mice. J Pharm Pharmacol. 51:741–743. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goodnick PJ: Use of antidepressants in
treatment of comorbid diabetes mellitus and depression as well as
in diabetic neuropathy. Ann Clin Psychiatry. 13:31–41. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Lee SY, Kim JH, et al: Depressive
mood and quality of life in functional gastrointestinal disorders:
differences between functional dyspepsia, irritable bowel syndrome
and overlap syndrome. Gen Hosp Psychiatry. 32:499–502. 2010.
View Article : Google Scholar
|
|
15
|
Kim HS, Rhee PL, Park J, et al:
Gender-related differences in visceral perception in health and
irritable bowel syndrome. J Gastroenterol Hepatol. 21:468–473.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang HS: Study on relative problems of
acupuncture and moxibustion for treatment of simple obesity.
Zhongguo Zhen Jiu. 28:522–524. 2008.(In Chinese).
|
|
17
|
Salpeter SR, Buckley NS, Kahn JA and
Salpeter EE: Meta-analysis: Metformin treatment in persons at risk
for diabetes mellitus. Am J Med. 121:149–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Selvin E, Bolen S, Yeh HC, et al:
Cardiovascular outcomes in trials of oral diabetes medications: a
systematic review. Arch Intern Med. 168:2070–2080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: a model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Willner P: Validity, reliability and
utility of the chronic mild stress model of depression: a 10-year
review and evaluation. Psychopharmacology (Berl). 134:319–329.
1997.PubMed/NCBI
|
|
21
|
Wong TP, Howland JG, Robillard JM, et al:
Hippocampal long-term depression mediates acute stress-induced
spatial memory retrieval impairment. Proc Natl Acad Sci USA.
104:11471–11476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cassano P and Fava M: Depression and
public health: an overview. J Psychosom Res. 53:849–857. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cassileth BR, Lusk EJ, Strouse TB, et al:
Psychosocial status in chronic illness. A comparative analysis of
six diagnostic groups. N Engl J Med. 311:506–511. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noël PH, Williams JW Jr, Unützer J, et al:
Depression and comorbid illness in elderly primary care patients:
impact on multiple domains of health status and well-being. Ann Fam
Med. 2:555–562. 2004.PubMed/NCBI
|
|
25
|
Xu Y, Barish PA, Pan J, Ogle WO and
O’Donnell JM: Animal models of depression and neuroplasticity:
assessing drug action in relation to behavior and neurogenesis.
Methods Mol Biol. 829:103–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan HC, Cao X, Das M, Zhu XH and Gao TM:
Behavioral animal models of depression. Neurosci Bull. 26:327–337.
2010. View Article : Google Scholar : PubMed/NCBI
|