Introduction
Systemic lupus erythematosus (SLE) is a
multifactorial autoimmune disease. The assortment of autoantibodies
produced is broad and, as a consequence, the manifestations of the
disease are diverse (1). SLE is
characterized by arthritis, cutaneous rash, vasculitis, involvement
of the central nervous system (CNS) and renal and cardiopulmonary
manifestations (2). Reactive
oxygen species (ROS) have been considered as risk and enhancer
factors for autoimmune diseases (3). Several previous studies have
indicated that the chronic immune activation present in the disease
is caused by the depletion of intracellular glutathione (GSH)
through oxygen-derived free radical production, known as oxidative
stress (4–6).
Several clinical conditions, particularly
inflammatory and/or immuno-mediated disorders, have been associated
not only with oxidative stress in terms of increased ROS formation,
but also with impaired antioxidant status mainly in terms of
reduced GSH levels and lowered cellular redox potential (7). GSH protects against oxidative stress
and detoxifies xenobiotics; thus, is involved in the maintenance of
homeostasis. Therefore, reductions in GSH levels are associated
with aging and the pathogenesis of a variety of diseases,
autoimmune or not, including SLE, rheumatoid arthritis (RA),
autoimmune thyroiditis, muscular dystrophy, amyotrophic lateral
sclerosis, acquired immunodeficiency syndrome (AIDS), Alzheimer’s,
alcoholic liver disease, cataract genesis, respiratory distress
syndrome and Werner syndrome (8–11).
Altered GSH concentrations may play an important role in a number
of autoimmune pathological conditions, prevalently elicited,
determined and maintained by inflammatory/immune responses mediated
by oxidative stress reactions (12).
In several diseases, including SLE, abnormal T-cell
activation and death occurs, which is crucially dependent on the
controlled production of ROS and adenosine triphosphate (ATP) in
the mitochondria (13). ROS exert
their prooxidant activity by inducing tissue damage and the
dissociation of iron ions or iron-containing compounds (heme) from
a protein-bound state. In addition, the exposure of specific
antibodies to heme, transition metal ions or ROS may induce the
appearance of new antigen-binding specificities for various
autoantigens (14). ROS generation
also provides oxidants for thiol oxidation or peroxynitrite
formation, which may be a basis for antibody modification (15).
A number of studies have shown that excessive ROS
production damages macromolecules, including DNA and proteins, and
modulates the expression of a variety of inflammatory molecules,
exacerbating inflammation and tissue damage in SLE (16,17).
Oxidative damage mediated by ROS results in the generation of
deleterious by-products, including aldehydic products, leading to
the formation of highly immunogenic adducts with proteins. Thus,
pathogenic antibodies are induced in a variety of diseases,
including SLE and RA (18).
GSH is synthesized by two consecutive ATP-dependent
enzymatic reactions. Glutamate cysteine ligase (GCL) catalyzes the
first and rate-limiting step of GSH synthesis. GCL is a
heterodimeric enzyme, consisting of a catalytic subunit, (GCLc) and
a modulatory subunit (GCLm) that are encoded by two distinct genes
(19). GCLc constitutes all the
enzymatic activity, but catalytic efficiency is increased
substantially by covalent interaction with GCLm (20,21).
Although there is an association between SLE and
GSH, GCL activity levels in immune cells from SLE patients remain
unclear. Thus, the aim of the present study was to determine the
changes in GCL activity in SLE. Thioredoxin (TRX) levels have been
shown to increase in response to oxidative stress in experimental
(22) and human studies (23). Thus, TRX concentrations were also
examined in SLE patients and controls.
The SLE disease activity index (SLEDAI) is a
validated model of global assessments from experienced clinicians
on disease activity in lupus. It represents the consensus of a
group of experts in the field of lupus study (24). Higher SLEDAI scores indicate a
greater severity of disease (25).
In addition, erythrocyte sedimentation rates (ESR) and
autoantibodies are indicators of the degree of inflammation and may
be used to monitor disease activity. Therefore, the associations
between GSH levels and GCL activity with demographic
characteristics, clinical manifestations and laboratory parameters
in peripheral blood mononuclear cells (PBMCs) were analyzed in the
present study.
Materials and methods
Patients and controls
A total of 30 SLE patients of Northern Han Chinese
descent, diagnosed according to the criteria of the American
College of Rheumatology, were enrolled in the study. Patients were
selected from individuals attending outpatient meetings at the
Department of Internal Medicine at Qingdao Municipal Hospital
(Qingdao, China). In addition, 30 healthy controls were recruited
that were ethnicity-, gender- and age-matched with the patients.
The patients enrolled were not smokers or alcoholics, and were not
associated with any other autoimmune disease. Blood samples from
the patients and healthy controls were collected with informed
consent from the patients and approval from the Ethics Committee of
Qingdao Municipal Hospital. Demographic, laboratory and clinical
characteristics of the groups are listed in Table I.
 | Table IDemographic characteristics, clinical
features and laboratory measurements of the subjects (n=30 per
group). |
Table I
Demographic characteristics, clinical
features and laboratory measurements of the subjects (n=30 per
group).
|
Characteristics | SLE patients | Healthy
controls |
|---|
| Demographic
characteristics |
| Female, n (%) | 24 (80) | 23 (76.7) |
| Male, n (%) | 6 (20) | 7 (23.3) |
| Age, years | 34.7 (21–57) | 35.5 (22–60) |
| Clinical
features |
| LN, n (%) | 18 (60) | - |
| Arthritis, n
(%) | 18 (60) | - |
| Serositis, n
(%) | 9 (30) | - |
| CNS disease, n
(%) | 2 (6.7) | - |
| SLEDAI | 13.16 (2–39) | - |
| Laboratory
measurements |
| C3, g/l | 1.10
(0.87–1.7) | |
| C4, g/l | 0.073
(0.07–0.48) | - |
| IgG, g/l | 16.94
(6.8–34.7) | - |
| IgA, g/l | 1.49
(0.68–3.35) | - |
| IgM, g/l | 1.38
(0.32–2.12) | - |
| ANA, n (%) | 30 (100) | - |
| Anti-dsDNA, n
(%) | 21 (70) | - |
| Anti-Sm, n
(%) | 9 (30) | - |
| CRP, mg/l | 47.2 (3–101) | - |
| ESR, mm/h | 54 (8–119) | 9 (4–20) |
Preparation of PBMCs
Peripheral blood samples (2 ml), anticoagulated with
sodium citrate, were collected from the controls and patients prior
to the administration of any immunosuppressive drug. PBMCs were
separated by density gradient centrifugation using the Ficoll Paque
system (HengXin chemical reagent Co., Ltd., Shanghai, China),
according to the manufacturer’s instructions.
Laboratory measurements
For the patients with SLE, serum levels of C3, C4,
IgG, IgA, IgM and C-reactive protein (CRP) were analyzed using an
automatic nephelometric immunoassay analyzer (Siemens, Munich,
Germany). Autoantibodies, including antinuclear (ANA), anti-dsDNA
and anti-Smith (Sm) antibodies, were detected using immunoblotting
kits, according to the manufacturer’s instructions (EUROIMMUN AG,
Lübeck, Germany).
SLEDAI is a global score reflecting all aspects of
disease activity and is a validated model for the assessment of
disease activity in SLE (24).
Disease activity was determined using the SLEDAI score. ESRs were
analyzed using an automatic analyzer (Monitor-J+ analyzer, Vital
Diagnostics Srl, Forli, Italy) in all the subjects.
Measurement of GSH and oxidized
glutathione (GSSG) concentration in PBMCs
GSH concentration was measured using a GSH
colorimetric assay kit (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer’s instructions.
PBMCs were prepared by density gradient centrifugation. The
concentration was adjusted to 1×107 cells/ml and the
cells were washed in phosphate-buffered saline (PBS). This was
followed by centrifugation at 10,000 × g at 4°C for 20 min. Cells
in the sediment were collected and mixed with protein removal
solution M (v/v, 1/3). The mixtures were vortexed fully and
freeze-thawed twice using liquid nitrogen and a water bath. After
placing on ice for 5 min, the mixtures were centrifuged at 10,000 ×
g at 4°C for 10 min. The supernatant was used for GSH detection.
For the GSSG assay, GSH was removed with GSH removal liquid,
following the manufacturer’s instructions.
Analysis of TRX levels
PBMCs in PBS were adjusted to a concentration of
1×107 cells/ml and freeze-thawed four times using liquid
nitrogen and a water bath. The cells were then centrifuged at 5,000
× g at 4°C for 5 min. The supernatant was used for TRX detection.
TRX levels were analyzed using a human TRX ELISA assay kit (Uscn
Life Science, Inc., Wuhan, China). Procedures were conducted
according to the manufacturer’s instructions.
GCL activity assay
GCL activity was determined by a fluorescence assay
as described by Chen et al (26). The concentration of PBMCs was
adjusted to 5×107 cells/ml in TES/SB buffer (w/v, 1/4)
consisting of 20 mM Tris, 1 mM EDTA, 250 mM sucrose, 20 mM sodium
borate and 2 mM serine. The cells were sonicated at 100 W for 60
sec and then centrifuged at 10,000 × g at 4°C for 10 min. The
supernatants were collected and centrifuged again at 15,000 × g at
4°C for 20 min. The supernatants were collected and the protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology), with bovine serum
albumin used as the standard.
For the GCL activity assay, aliquots of 30 μl
supernatant were mixed with 30 μl GCL reaction cocktail (400 mM
Tris, 40 mM ATP, 40 mM L-glutamic acid, 2 mM EDTA, 20 mM sodium
borate, 2 mM serine and 40 mM MgCl2). Following
incubation at 37°C for 5 min, 30 μl cysteine solution (30 mM;
dissolved in TES/SB buffer) was added and the mixtures were
incubated at 37°C for 13 min. The enzymatic reaction in the
mixtures was stopped by precipitating proteins with 200 mM
5-sulfosalicylic acid (SSA). After placing on ice for 20 min, the
mixtures were centrifuged at 2,000 × g at 4°C for 10 min. Following
centrifugation, 20-μl samples of each supernatant containing
γ-glutamylcysteine (γ-GC) were added to a 96-well plate designed
for fluorescence detection. For each assay, 20 μl γ-GC standards,
containing 5 μl GCL reaction cocktail [5 μl SSA (200 mM), 5 μl
H2O and 5 μl γ-GC standard solution (0, 20, 40, 60, 80,
100, 120 and 140 μM in TES/SB buffer)], was added to each well of
the same 96-well plate to generate a standard curve. Next, 180 μl
2,3-naphthalenedicarboxyaldehyde (NDA) was added to each well.
Following incubation in the dark at room temperature for 30 min,
the formation of NDA-γ-GC was measured (472 nm excitation/528 nm
emission) using a fluorescent plate reader (GENios Plus; Tecan,
Männedorf Switzerland). The production of γ-GC in each sample was
calculated using the standard curve. Values were expressed in mM
per min per mg of protein.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± SD. The differences between the subject
groups were analyzed using the independent Student’s t-test, while
correlation analysis was performed using Spearman’s rank test.
P<0.05 was considered to indicate a statistically significant
difference. Figures were constructed using GraphPad Prism software
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA).
Results
Laboratory measurements of patients with
SLE
Demographic characteristics, clinical manifestations
and laboratory measurements of the patients with SLE are presented
in Table I. Lupus nephritis (LN)
is the major indicator of morbidity and mortality in SLE and was
identified in 18 of the 30 patients. Arthritis, serositis and CNS
disease were identified in 18, 9 and 2 patients, respectively.
A positive result for ANA, anti-dsDNA and anti-Sm
autoantibodies was found in 30, 21 and 9 SLE patients,
respectively. For the SLE patients, the mean ESR value was 54 mm/h,
ranging between 8 and 119 mm/h. In addition, the mean CRP value was
47.2 mg/l with a range between 3 and 101 mg/l. The mean SLEDAI
value was 13.16, ranging between 2 and 39.
Levels of GSH and GSSG in PBMCs from
patients with SLE and healthy controls
In order to explore the role of oxidative stress in
SLE, GSH and GSSG concentrations and the redox state (GSH/GSSG),
were examined in PBMCs from 30 patients with SLE and 30 gender- and
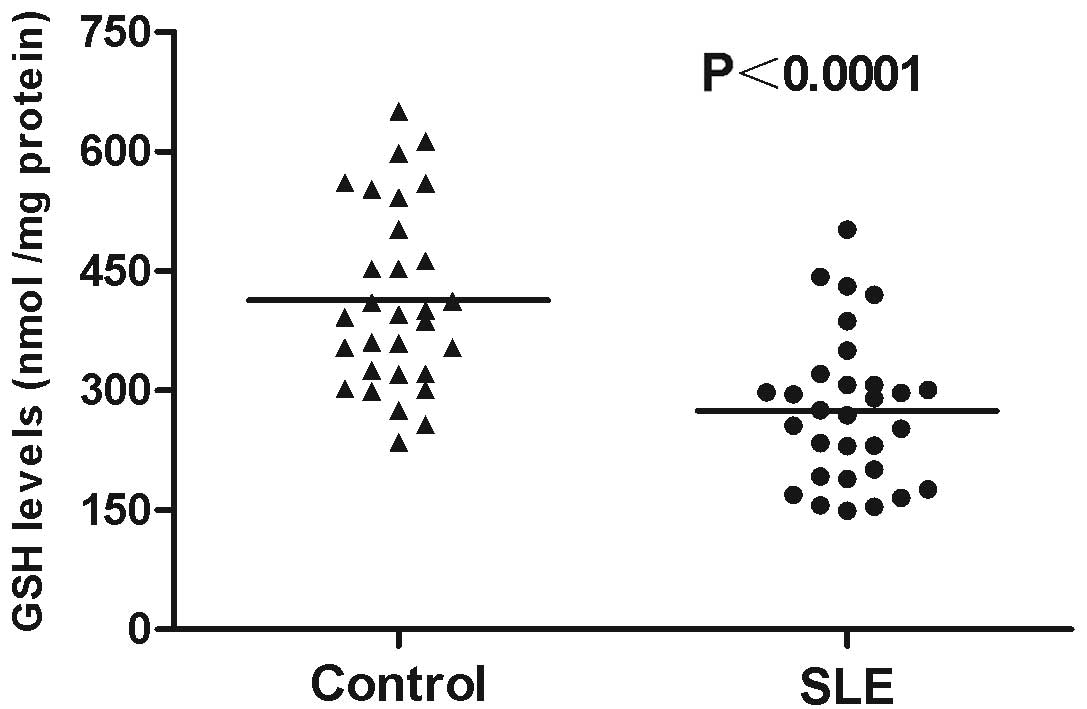
age-matched healthy controls. GSH levels considerably decreased in
PBMCs from the patients with SLE (274.90±17.08 nmol/mg protein)
compared with those in the healthy controls (413.63±20.79 nmol/mg
protein; P<0.0001; Fig. 1). By
contrast, GSSG levels significantly increased (124.95±4.27 nmol/mg
protein; P<0.01) in patients with SLE compared with those in the
healthy controls (68.94±1.89 nmol/mg protein; Table II). The reduction in GSH and
increase in GSSG concentrations resulted in a decreased redox state
(GSH/GSSG) in patients with SLE (2.27±0.43; P<0.001) compared
with that in the healthy controls (6.11±1.07; Table II). Furthermore, the levels of the
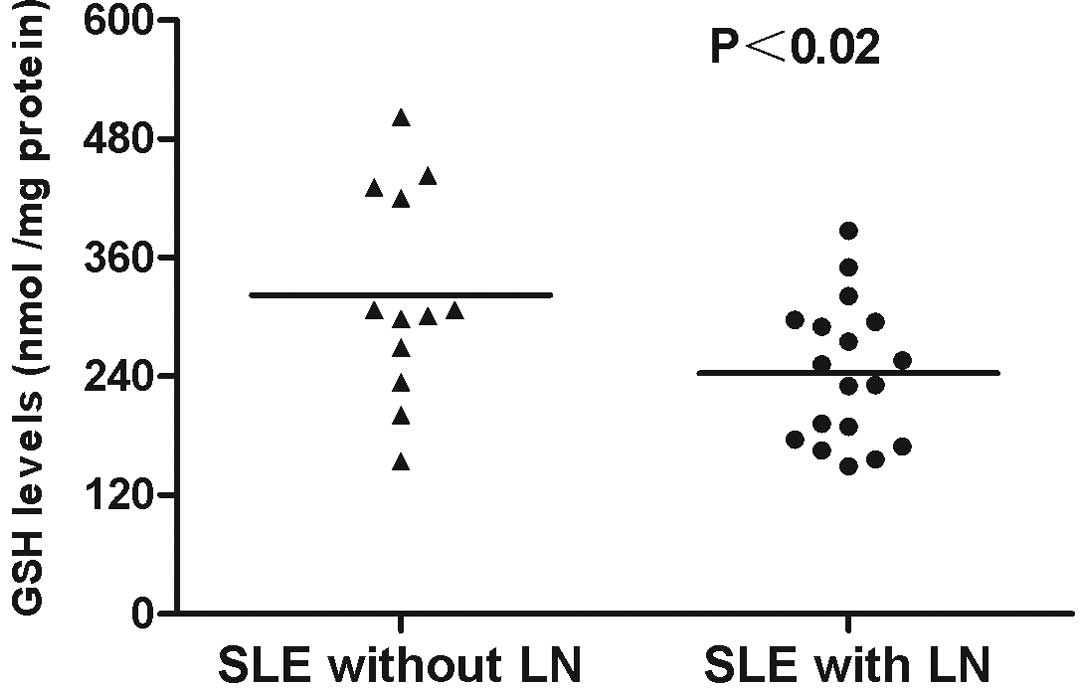
antioxidant GSH were markedly decreased in patients with SLE and LN
(243.33±16.73 nmol/mg protein) compared with those in SLE patients
without LN (322.25±30.58 nmol/mg protein; P<0.02; Fig. 2). The level of GSSG significantly
increased (121.06±8.32 nmol/mg protein; P<0.05) and the redox
state markedly decreased (2.01±0.58; P<0.05) in patients with
SLE and LN compared with those in SLE patients without LN (Table II).
 | Table IIOxidant and antioxidant parameters in
PBMCs from patients with SLE and healthy controls. |
Table II
Oxidant and antioxidant parameters in
PBMCs from patients with SLE and healthy controls.
| Parameters | Controls | SLE patients | SLE patients with
LN | SLE patients
without LN |
|---|
| GSH, nmol/mg
protein | 413.63±20.79 |
274.90±17.08c | 243.33±16.73 |
322.25±30.58a |
| GSSG, nmol/mg
protein | 68.94±1.89 | 124.95±4.27b | 121.06±8.32 | 147.15±7.51a |
| GSH/GSSG | 6.11±1.07 | 2.27±0.43c | 2.01±0.58 | 2.29±0.51a |
| TRX, ng/ml | 14.6±7.2 | 27.2±9.7b | 34.2±5.6 | 25.7±6.3a |
TRX levels in PBMCs from patients with
SLE and healthy controls
In order to further investigate the status of
oxidative stress in SLE, TRX concentrations in the PBMCs from
patients with SLE were examined. The average TRX concentration in
the patients with SLE was 27.2±9.7 ng/ml, which was significantly
increased compared with that in the healthy controls (14.6±7.2;
P<0.01; Table II). In
addition, the concentration of TRX significantly increased
(34.2±5.6; P<0.05) in patients with SLE and LN when compared
with the concentration in SLE patients without LN (Table II).
Changes in the enzymatic activity levels
of GCL in PBMCs from patients with SLE and healthy controls
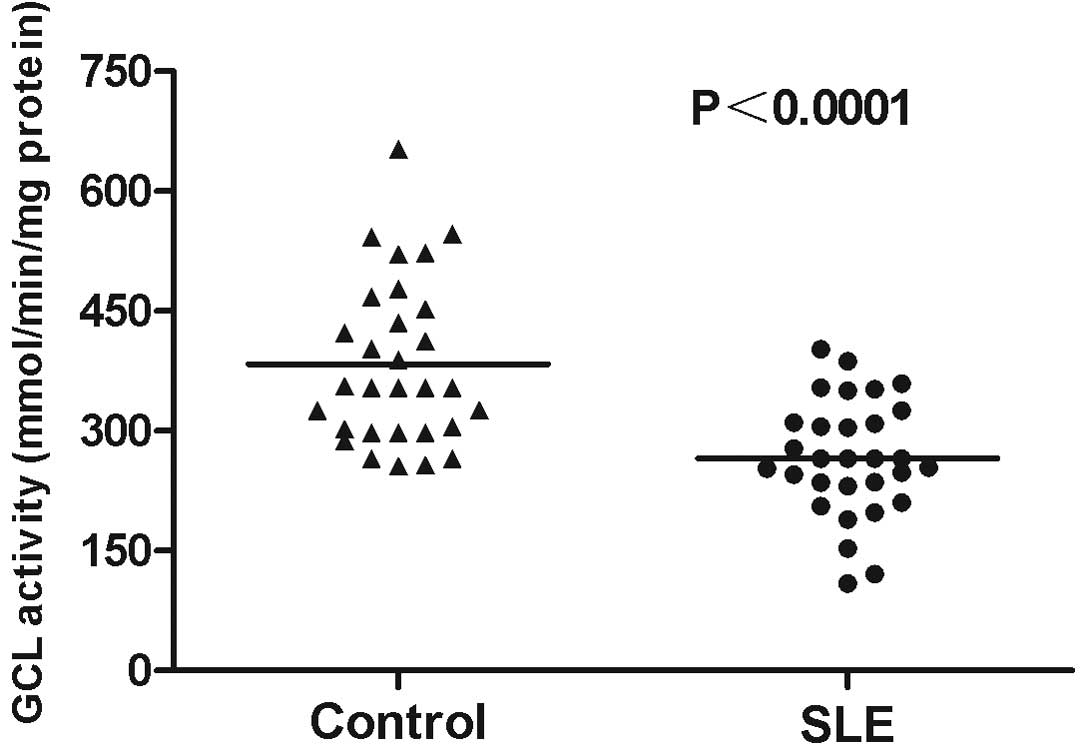
GCL activity levels in PBMCs from the 30 SLE
patients were analyzed. The average GCL activity level in the
patients with SLE was 266.10±13.31 mmol/min/mg protein, which was
significantly reduced compared with that in the healthy controls
(383.27±18.68; P<0.0001; Fig.
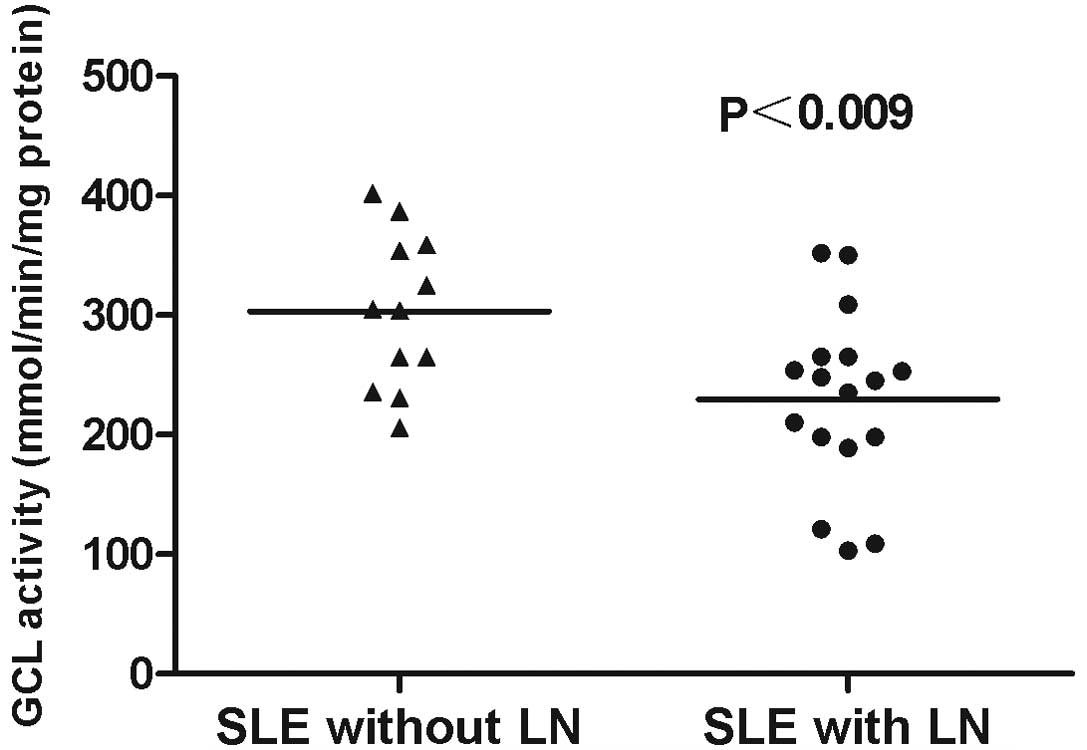
3). In addition, GCL activity levels in SLE patients with LN
(229.64±17.82) were significantly lower when compared with those in
the SLE patients without LN (303.25±18.47; P<0.009; Fig. 4).
Correlation analysis between GSH levels
and characteristics or laboratory parameters in patients with
SLE
Associations between GSH levels in PBMCs and
demographic characteristics, clinical manifestations and laboratory
parameters were analyzed. The results demonstrated that the levels
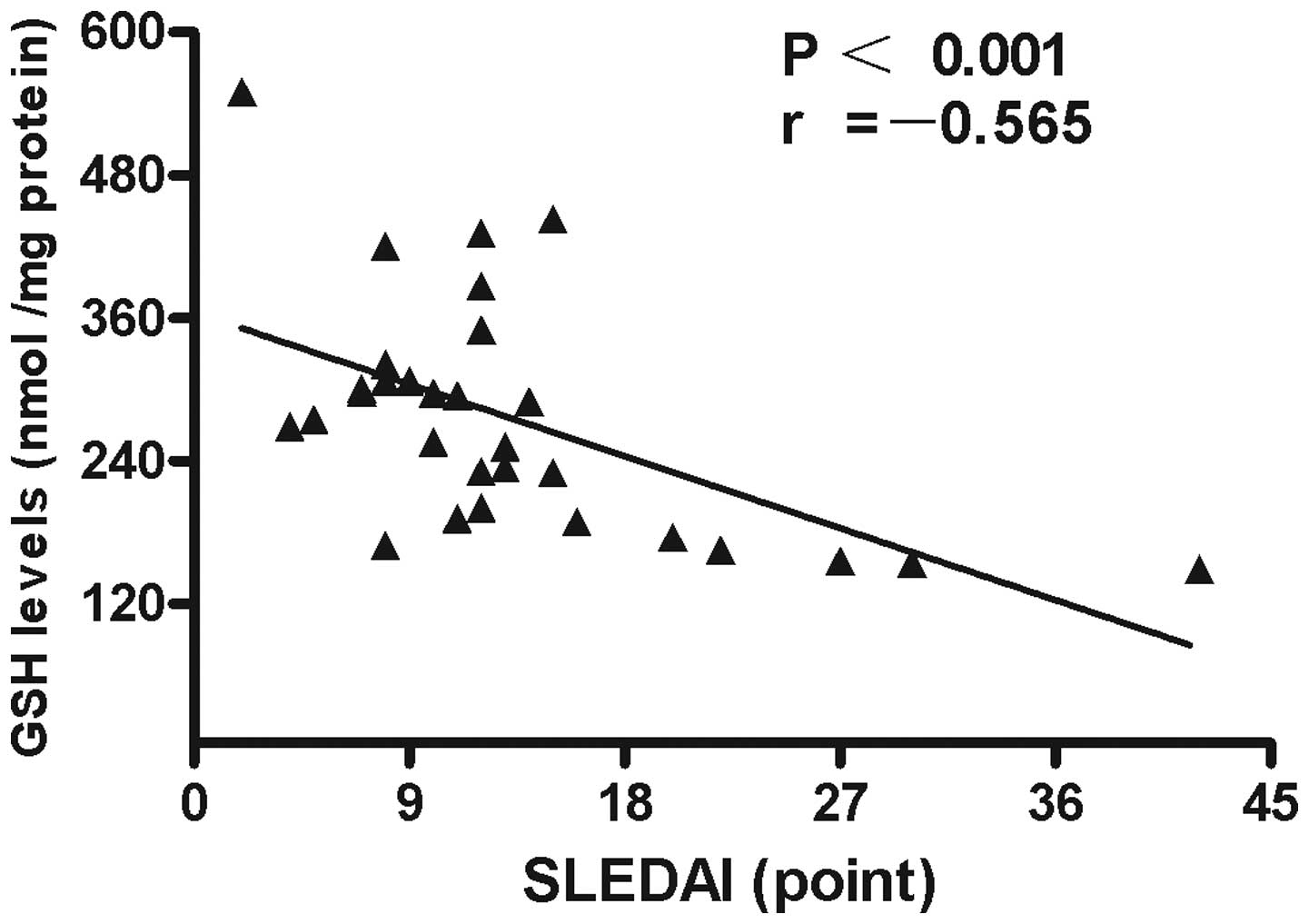
of GSH in the PBMCs negatively correlated with SLEDAI values
(r=−0.565; P<0.001; Fig. 5). No
statistically significant associations were identified between GSH
levels and other characteristics, clinical manifestations or
laboratory parameters in the patients with SLE.
Correlation analysis between GCL activity
and characteristics or laboratory parameters in patients with
SLE
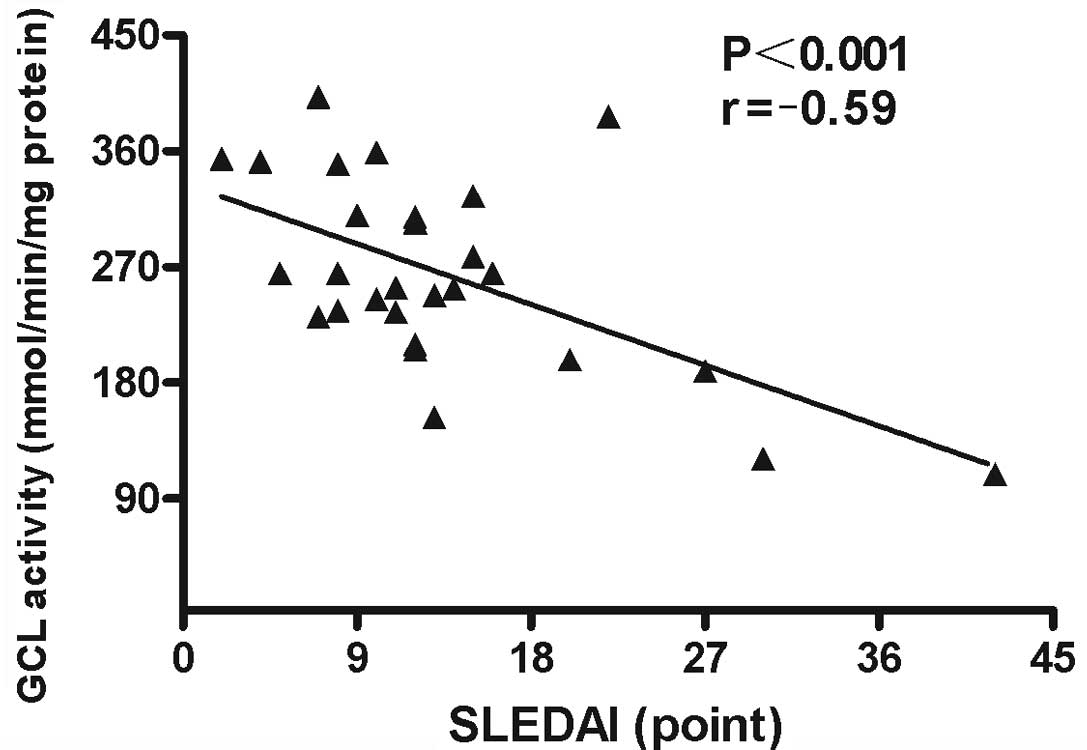
Associations between GCL activity levels in PBMCs
and demographic characteristics, clinical manifestations and
laboratory parameters were analyzed. The results revealed that GCL
activity levels in PBMCs negatively correlated with SLEDAI scores
(r=−0.59; P<0.001; Fig. 6) and
ESR (r=−0.505; P<0.001; Fig. 7)
in patients with SLE. No statistically significant correlations
were identified between GCL activity levels and other
characteristics, clinical manifestations or laboratory parameters
in the patients with SLE.
Discussion
Oxidative stress is hypothesized to play a major
role in the initiation and progression of autoimmune disease by
excessive free radical formation (27). The inability of the antioxidant
defense system to cope with oxidative stress is considered to be a
possible cause in SLE. The inflammatory nature of the disease
indicates that excessive ROS production and an imbalanced redox
system may contribute to the immune dysfunction, autoantigen
production and perturbation of programmed cell death in SLE
(28).
In the present study, GCL activity levels in SLE
patients were analyzed, and negative correlations between GCL
activity and SLEDAI or ESR were identified. The results clearly
indicate that GSH levels and GCL enzymatic activity levels decrease
significantly in the PBMCs of patients with SLE, which may be a
hallmark of oxidative stress in SLE and coupled together as a cause
and consequence in the severity of the disease. The results
indicate that GCL activity levels may be involved in disease
activity and pathogenesis of SLE.
GCL catalyzes the first and rate-limiting step of
GSH synthesis, in which glutamate ligates with cysteine to form
γ-GC. This rapidly reacts with glycine to form GSH via the action
of GSH synthase (29). The present
study demonstrated a good correlation between GSH levels and GCL
activity, the primary determinant of the rate of GSH synthesis
(30). These observations indicate
that a reduction in GCL activity results in a reduction in GSH
levels in SLE patients. Consistent with the reduction in GSH
levels, GCL activity was inadequate in the PBMCs of patients with
SLE. The deficiency of GCL activity and reduction in GSH
concentration may contribute to the pathogenesis of SLE through
several mechanisms. One possible mechanism is that insufficient GSH
content may affect caspase activity, transcription factor
activation, Bcl-2 expression and function, thiol-redox signaling
and phosphatidylserine externalization, which are early processes
in apoptosis (28,31). A negative association of GSH levels
with T-lymphocyte and CD4+ and CD8+
lymphocyte subset apoptosis, and intracellular activated caspase-3
may support the role of GSH in the alteration of apoptosis of T
lymphocytes in the SLE disease state (1). These results indicate that GSH is
involved in the depletion of CD4+ T lymphocytes in
patients with SLE (1). The
reduction in cellular GSH levels has been attributed mainly to GSH
oxidation, promoted by increased production of ROS in the cells
(32). It has been reported that
GSH depletion in antigen-presenting cells inhibits the production
of Th1-related cytokines, including interferon-γ and
interleukin-12, and supports the Th2-mediated humoral immune
response (33). Since GSH has a
significant effect on the ability of the immune system to activate
the appropriate Th response, altering the levels of GSH may have
significant implications in Th1/Th2-related diseases, including SLE
(26). Consequently, insufficient
levels of GCL activity and GSH may result in SLE disease,
indicating a critical and cell-specific function in the etiology of
SLE.
Adequate concentrations of GSH are required for a
variety of functions, including the protection of the cell from
oxidative damage, the quenching of oxidant species, lymphocyte
activation, natural killer cell activation and lymphocyte-mediated
cytotoxicity (34,35). A reduction in the level of
intracellular GSH correlates with the severity of disease,
particularly in patients with LN (36,37).
The inverse correlation between GCL activity, GSH
and SLE disease activity and severity, indicated by SLEDAI scores
and ESRs, indicates that insufficient levels of GCL activity and
GSH may contribute to the severity of disease. Higher SLEDAI scores
indicate more severe disease activity (25). Thus, the negative correlation
between GCL activity, GSH and SLEDAI scores in patients with SLE
indicates that the lower the levels of GCL activity and GSH, the
more severe the disease. In addition, ESR is an indicator of the
degree of inflammation and is used to monitor disease activity.
Since GCL activity was shown to negatively correlate with the ESR
in patients with SLE, GCL activity levels may be an index of
disease activity. In addition, there was a significant difference
in GCL activity and GSH levels between SLE patients with and
without LN. Therefore, correlation analysis between GCL activity
and SLEDAI, ESR and LN further indicates a potential role of GCL
activity and GSH in the pathogenesis of SLE.
TRX is elevated in patients with increased oxidative
stress, including AIDS (38) and
RA (39). In the present study,
increased TRX levels were observed in patients with SLE, which
further demonstrates the change in redox state in SLE patients.
In conclusion, GCL enzymatic activity is
downregulated and inversely correlates with specific disease
parameters in SLE patients. The results indicate that a reduction
in GCL activity levels correlates with SLE disease activity and
severity. The results support the hypothesis that oxidative stress
is a therapeutic target for pharmacological agents in SLE. However,
further mechanistic in vitro and in vivo studies are
required to investigate how the interplay between GSH and
pathogenesis may lead to the intolerance and aggressiveness of SLE
disease activity. Further studies should be directed to evaluate
the role of GSH in the pathogenesis of SLE.
References
|
1
|
Shah D, Aggarwal A, Bhatnagar A, et al:
Association between T lymphocyte sub-sets apoptosis and peripheral
blood mononuclear cells oxidative stress in systemic lupus
erythematosus. Free Radic Res. 45:559–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordon C: Long-term complications of
systemic lupus erythematosus. Rheumatology (Oxford). 41:1095–1100.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mansour RB, Lassoued S, Gargouri B, et al:
Increased levels of autoantibodies against catalase and superoxide
dismutase associated with oxidative stress in patients with
rheumatoid arthritis and systemic lupus erythematosus. Scand J
Rheumatol. 37:103–108. 2008. View Article : Google Scholar
|
|
4
|
Kurien BT and Scofield RH: Free radical
mediated peroxidative damage in systemic lupus erythematosus. Life
Sci. 73:1655–1666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munoz LE, Gaipl US and Herrmann M:
Predictive value of anti-dsDNA autoantibodies: importance of the
assay. Autoimmun Rev. 7:594–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Ayene R, Ward KM, et al: Glucose
deprivation increases nuclear DNA repair protein Ku and resistance
to radiation induced oxidative stress in human cancer cells. Cell
Biochem Funct. 27:93–101. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Townsend DM, Tew KD and Tapiero H: The
importance of glutathione in human disease. Biomed Pharmacother.
57:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gambhir JK, Lali P and Jain AK:
Correlation between blood antioxidant levels and lipid peroxidation
in rheumatoid arthritis. Clin Biochem. 30:351–355. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pastore A, Federici G, Bertini E and
Piemonte F: Analysis of glutathione: implication in redox and
detoxification. Clin Chim Acta. 333:19–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burek CL and Rose NR: Autoimmune
thyroiditis and ROS. Autoimmun Rev. 7:530–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Griffiths HR: Is the generation of
neo-antigenic determinants by free radicals central to the
development of autoimmune rheumatoid disease? Autoimmun Rev.
7:544–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perricone C, De Carolis C and Perricone R:
Glutathione: a key player in autoimmunity. Autoimmun Rev.
8:697–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandez D and Perl A: Metabolic control
of T cell activation and death in SLE. Autoimmun Rev. 8:184–189.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimitrov JD, Vassilev TL, Andre S, et al:
Functional variability of antibodies upon oxidative processes.
Autoimmun Rev. 7:574–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crane FL and Low H: Reactive oxygen
species generation at the plasma membrane for antibody control.
Autoimmun Rev. 7:518–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grisham MB: Reactive oxygen species in
immune responses. Free Radic Biol Med. 36:1479–1480. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan SZ, Gheita TA, Kenawy SA, et al:
Oxidative stress in systemic lupus erythematosus and rheumatoid
arthritis patients: relationship to disease manifestations and
activity. Int J Rheum Dis. 14:325–331. 2011.PubMed/NCBI
|
|
18
|
Kurien BT and Scofield RH: Autoimmunity
and oxidatively modified autoantigens. Autoimmun Rev. 7:567–573.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang CS, Chang LS, Anderson ME and
Meister A: Catalytic and regulatory properties of the heavy subunit
of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem.
268:19675–19680. 1993.PubMed/NCBI
|
|
20
|
McConnachie LA, Mohar I, Hudson FN, et al:
Glutamate cysteine ligase modifier subunit deficiency and gender as
determinants of acetaminophen-induced hepatotoxicity in mice.
Toxicol Sci. 99:628–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dalton TP, Dieter MZ, Yang Y, et al:
Knockout of the mouse glutamate cysteine ligase catalytic subunit
(Gclc) gene: Embryonic lethal when homozygous, and proposed model
for moderate glutathione deficiency when heterozygous. Biochem
Biophys Res Commun. 279:324–329. 2000. View Article : Google Scholar
|
|
22
|
Kasuno K, Nakamura H, Ono T, et al:
Protective roles of thioredoxin, a redox-regulating protein, in
renal ischemia/reperfusion injury. Kidney Int. 64:1273–1282. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maurice MM, Nakamura H, Gringhuis S, et
al: Expression of the thioredoxin-thioredoxin reductase system in
the inflamed joints of patients with rheumatoid arthritis.
Arthritis Rheum. 42:2430–2439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bombardier C, Gladman DD, Urowitz MB, et
al: Derivation of the SLEDAI. A disease activity index for lupus
patients The Committee on Prognosis Studies in SLE. Arthritis
Rheum. 35:630–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hawker G, Gabriel S, Bombardier C, et al:
A reliability study of SLEDAI: a disease activity index for
systemic lupus erythematosus. J Rheumatol. 20:657–660.
1993.PubMed/NCBI
|
|
26
|
Chen CN, Brown-Borg HM, Rakoczy SG, et al:
Aging impairs the expression of the catalytic subunit of glutamate
cysteine ligase in soleus muscle under stress. J Gerontol A Biol
Sci Med Sci. 65:129–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shah D, Sah S and Nath SK: Interaction
between glutathione and apoptosis in systemic lupus erythematosus.
Autoimmun Rev. 12:741–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ortona E, Margutti P, Matarrese P, et al:
Redox state, cell death and autoimmune diseases: a gender
perspective. Autoimmun Rev. 7:579–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ziegler DM: Role of reversible
oxidation-reduction of enzyme thiols-disulfides in metabolic
regulation. Annu Rev Biochem. 54:305–329. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maher P: The effects of stress and aging
on glutathione metabolism. Ageing Res Rev. 4:288–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kasahara Y, Iwai K, Yachie A, et al:
Involvement of reactive oxygen intermediates in spontaneous and
CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood.
89:1748–1753. 1997.PubMed/NCBI
|
|
32
|
Hammond CL, Madejczyk MS and Ballatori N:
Activation of plasma membrane reduced glutathione transport in
death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol.
195:12–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Messina JP and Lawrence DA: Cell cycle
progression of glutathione-depleted human peripheral blood
mononuclear cells is inhibited at S phase. J Immunol.
143:1974–1981. 1989.PubMed/NCBI
|
|
34
|
Franco R, Panayiotidis MI and Cidlowski
JA: Glutathione depletion is necessary for apoptosis in lymphoid
cells independent of reactive oxygen species formation. J Biol
Chem. 282:30452–30465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ballatori N, Krance SM, Notenboom S, et
al: Glutathione dysregulation and the etiology and progression of
human diseases. Biol Chem. 390:191–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shah D, Kiran R, Wanchu A and Bhatnagar A:
Oxidative stress in systemic lupus erythematosus: relationship to
Th1 cytokine and disease activity. Immunol Lett. 129:7–12. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Túri S, Németh I, Torkos A, et al:
Oxidative stress and antioxidant defense mechanism in glomerular
diseases. Free Radic Biol Med. 22:161–168. 1997.PubMed/NCBI
|
|
38
|
Nakamura H, De Rosa SC, Yodoi J, et al:
Chronic elevation of plasma thioredoxin: inhibition of chemotaxis
and curtailment of life expectancy in AIDS. Proc Natl Acad Sci USA.
98:2688–2693. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jikimoto T, Nishikubo Y, Koshiba M, et al:
Thioredoxin as a biomarker for oxidative stress in patients with
rheumatoid arthritis. Mol Immunol. 38:765–772. 2002. View Article : Google Scholar : PubMed/NCBI
|