Introduction
The swim bladder is an organ that is important for
balance and polysaccharides account for as much as 10% of its
weight. The large yellow croaker (Larimichthys crocea) is
one of the main commercial fish in the coastal waters of China, and
its swim bladder is rich in protein, microelements and vitamins. It
is used in traditional Chinese medicine since it is considered to
have curative effects against a number of different conditions,
including amnesia, insomnia, dizziness, anepithymia and weakness
after giving birth (1). A previous
study has also suggested that the large yellow croaker swim bladder
may serve to remove free radicals and protect against certain types
of cancer (2). Polysaccharides are
important functional materials. It has been shown that
polysaccharides present in the swim bladder may accelerate wound
healing, as well as prevent infection and thrombus formation
(3). In addition, in vivo
studies have demonstrated that polysaccharides from Lentinus
edodes and spirulina seaweed serve to prevent and cure injury
(4,5).
Gastric injury is damage to the stomach. It may
occur due to chemical injury and may involve injury to the gastric
mucosa. Ethanol promotes the rapid formation of lesions in the
stomach due to an inflammatory reaction (6). Ethanol-induced gastric injury is
associated with the loss of epithelial cells, mucosal edema and
subepithelial hemorrhage (7).
Therefore, in the present study HCl/ethanol was used for the
chemical induction of gastric injury.
In the present study, the preventive effect of
polysaccharides from the large yellow croaker swim bladder (PLYCSB)
on gastric injury was investigated. The serum levels of
inflammatory-associated cytokines were used to evaluate the
preventive effect of PLYCSB on HCl/ethanol-induced gastric injury
in ICR mice. In addition, gastric tissue histology was used to
determine the preventive effects in vivo. Furthermore, the
mRNA and protein expression levels of superoxide dismutase (SOD),
glutathione peroxidase (GSH-Px), nitric oxide (NO) and
malondialdehyde (MDA) in the tissues were analyzed in order to
determine the preventive effect of PLYCSB.
Materials and methods
PLYCSB preparation
Wild Yellow Sea Larimichthys crocea were
purchased in Shandong, China. The swim bladders from the
Larimichthys crocea (1 kg) were freeze-dried and then
crushed. Petroleum ether (3 l) was added to the swim bladder and
reflux extraction was performed twice (1 h each time) at 60°C to
remove the protein, and the residue was gathered following
filtration. A total of 3 l absolute ethanol was then added and
reflux extraction was performed for a further 3 h, and the residue
without protein was filtered and gathered. Finally, 3 l water was
added, the residue was extracted at 60°C for 2 h and the filtered
liquid was collected. Crude polysaccharides from the large yellow
croaker swim bladder were obtained following evaporation (8).
Animals
Seven-week-old male ICR mice (n=50) were purchased
from the Experimental Animal Center of Chongqing Medical University
(Chongqing, China). The mice were maintained at 23±1°C with a
relative humidity of 50±5% and a 12-h light/dark cycle. The mice
had unlimited access to a standard mouse chow diet and water.
Gastric injury experiment
The mice were divided into five groups (n=10 in each
group). The normal group mice received no treatment during the
experimental period. The control group mice received no treatment
for the first 4 weeks. The PLYCSB group mice were orally
administered either 25 or 50 mg/kg PLYCSB every day for 4 weeks.
The mice of the omeprazole group (a drug cure comparator group)
received a 25 mg/kg oral dose of omeprazole daily for 4 weeks.
Then, after fasting for 24 h, the control and treatment groups were
administered 1 ml HCl/ethanol (60% in 150 mM HCl) via esophageal
intubation. After 1 h the mice were sacrificed using ether
anesthesia. The stomachs were removed and 10 ml formalin (1%) was
injected for 10 min to inflate the stomach and to fix the tissue
walls, as well as to open the greater curvature (9). The
of hemorrhagic lesions developed in the
stomach was measured using a digital camera (D550; Canon, Tokyo,
Japan) with a square grid
The images were analyzed using ImageJ software
(National Institutes of Health, Bethesda, MD, USA) using the
following formula: Gastric injury inhibitory rate (%) = (gastric
injury area of control mice - gastric injury area of treated
mice)/gastric injury area of control mice. The gastric secretion
volume from each mouse was measured using a 10-ml measuring
cylinder. The pH of the gastric juice was measured using a pH meter
(SevenEasy pH meter; Mettler Toledo, Schwerzenbach, Switzerland)
after being diluted 10-fold with distilled water. The experimental
protocol was approved by the Animal Ethics Committee of Chongqing
Medical University.
Analysis of inflammation-associated
cytokines in serum by enzyme-linked immunosorbent assay
(ELISA)
For the serum cytokine assay, blood from the
inferior vena cava was collected in a tube and centrifuged at 730 ×
g at 4°C for 10 min. The serum was aspirated and assayed as
described below. The concentrations of inflammation-associated
cytokines, interleukin (IL)-1β, IL-4, IL-6 and IL-8, in serum were
measured using an ELISA in accordance with the manufacturer’s
instructions (Biolegend, San Diego, CA, USA). Briefly, biotinylated
antibody reagent was added to 96-well plates. The supernatants from
the homogenized serum were then added and the plates were incubated
at 37°C in CO2 for 2 h. The plates were washed with
phosphate-buffered saline (PBS), streptavidin-horseradish
peroxidase (HRP) solution was added and the plate was then
incubated for a further 30 min at room temperature. The absorbance
was measured at 450 nm using a microplate reader (iMark; Bio-Rad,
Hercules, CA, USA) (10).
Determination of MOT (motilin), SS
(somatostatin), SP (substance P) and VIP (vasoactive intestinal
peptide) levels in the serum
Blood was collected from the mice in a tube and
centrifuged at 730 × g at 4°C for 10 min. The MOT, SS, SP and VIP
levels in the serum were then determined using commercially
available kits (Beijing Pu’er Weiye Bio-Technology Co., Ltd.,
Beijing, China).
Determination of SOD, GSH-Px, NO and MDA
levels
The gastric tissue was homogenized using a
high-speed tissue homogenizer (T10; IKA-Werke GmbH & Co. KG,
Staufen, Germany) at 730 × g at 4°C for 10 min. The SOD, GSH-Px, NO
and MDA levels were then determined using commercially available
kits (Nanjing Juli Institute of Biomedical Engineering, Nanjing,
China).
Analysis of the expression of
inflammation-associated genes in gastric tissue using PCR
Total RNA from the gastric tissue cells was isolated
using TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) in accordance with the manufacturer’s
instructions. The RNA was digested with RNase-free DNase (Roche,
Basel, Switzerland) for 15 min at 37°C and purified using an RNeasy
kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s
instructions. Total RNA (2 μg) was incubated at 37°C for l h with
avian myeloblastosis virus reverse transcriptase (GE Healthcare,
Little Chalfont, United Kingdom) with random hexanucleotides to
form cDNA, in accordance with the manufacturer’s instructions. The
following primers were used to specifically amplify the genes of
interest: inducible nitric oxide synthase (iNOS) forward, 5′-AGA
GAG ATC GGG TTC ACA-3′ and reverse, 5′-CAC AGA ACT GAG GGT ACA-3′;
cyclooxygenase-2 (COX-2) forward, 5′-TTA AAA TGA GAT TGT CCG AA-3′
and reverse, 5′-AGA TCA CCT CTG CCT GAG TA-3′; tumor necrosis
factor-α (TNF-α) forward, 5′-GAC CCT CAG ACT CAG ATC ATC CTT CT-3′
and reverse, 5′-ACG CTG GCT CAG CCA CTC-3′; and IL-1β forward,
5′-CTC CAT GAG CTT TGT ACA AGG-3′ and reverse, 5′-TGC TGA TGT ACC
AGT TGG GG-3′. The internal control gene of GAPDH was amplified
using the following primers: forward, 5′-CGG AGT CAA CGG ATT TGG
TC-3′ and reverse, 5′-AGC CTT CTC CAT GGT CGT GA-3′. Amplification
was performed in a thermal cycler (Eppendorf, Hamburg, Germany).
The PCR products were separated on a 1.0% agarose gel and
visualized using ethidium bromide staining (11).
Protein extraction and western blot
analysis of the gastric tissue
The total protein was obtained from the gastric
tissue using Radio-Immunoprecipitation Assay buffer as previously
described (11). The protein
concentrations were determined using a Bio-Rad protein assay kit.
For the western blot analysis, aliquots of the lysate containing
30–50 μg protein were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
electrotransferred onto a nitrocellulose membrane (Schleicher and
Schuell, Keene, NH, USA). The membranes were then subjected to
immunoblot analysis and the proteins were visualized using an
enhanced chemiluminescence (ECL) method (GE Healthcare). The cell
lysates were separated using 12% SDS-PAGE, transferred onto a
polyvinylidene fluoride membrane (GE Healthcare), blocked with 5%
skimmed milk and then hybridized with primary antibodies (diluted
1:1,000). The antibodies against TNF-α, IL-1β, iNOS and COX-2 were
obtained from Santa Cruz Biotechnology. The membranes were then
incubated with the HRP-conjugated secondary antibodies (Santa Cruz
Biotechnology) for 1 h at room temperature. The blots were washed 3
times with PBS-T and then developed using an
electrochemiluminescence (ECL) reagent (Amersham Life Science,
Arlington Heights, IL, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between the mean values between the groups were
analyzed using a one-way analysis of variance with Duncan’s
multiple range test. P<0.05 was considered to indicate a
statistically significant difference. SAS version 9.1 (SAS
Institute Inc., Cary, NC, USA) was used to conduct the statistical
analyses.
Results
Gastric injury levels
Administration of PLYCSB to mice prior to the
induction of gastritis was found to reduce gastric injury. The mice
in the control group demonstrated a gastric injury area of
19.25±3.86 mm2. Treatment with 25 and 50 mg/kg PLYCSB
resulted in a gastric injury inhibition index of 38.34 and 66.49%,
respectively (Table I and Fig. 1). In particular, the greater level
of protection against gastric injury was achieved with the higher
dose of PLYCSB. The protective effect of 50 mg/kg PLYCSB was
comparable with that observed for omeprazole (79.84%), which was
used as the positive drug control.
 | Table IPrevention of HCl/ethanol-induced
gastric injury in ICR mice by treatment with PLYCSB. |
Table I
Prevention of HCl/ethanol-induced
gastric injury in ICR mice by treatment with PLYCSB.
| Rate of gastric
injury inhibition |
|---|
|
|
|---|
| Group | Gastric injury
(mm2) | Inhibitory rate
(%) |
|---|
| Normal | 0.00±0.00e | 100.00 |
| Control | 19.25±3.86a | 0.00 |
| PLYCSB (25
mg/kg) | 11.87±2.66b | 38.34 |
| PLYCSB (50
mg/kg) | 6.45±1.32c | 66.49 |
| Omeprazole (25
mg/kg) | 3.88±0.98d | 79.84 |
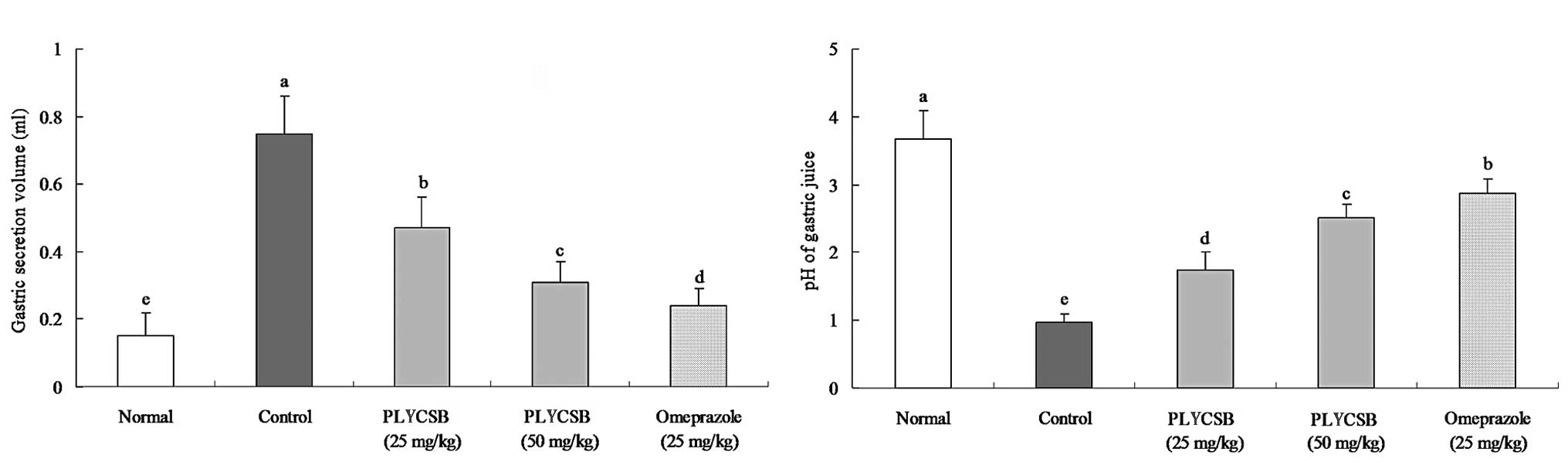
Gastric secretion volume and pH of the
gastric juice
The gastric secretion volume of normal mice was the
lowest (0.15±0.07 ml) among all the groups (Fig. 2A). The volume was increased in the
control mice (0.75±0.11 ml); however, the increase was attenuated
following treatment with 25 mg/kg PLYCSB (0.47±0.09 ml), 50 mg/kg
PLYCSB (0.31±0.06 ml) and omeprazole (0.24±0.05 ml). The pH values
of the gastric juices were 3.68±0.42, 0.98±0.12, 1.74±0.27,
2.51±0.21 and 2.86±0.22 for the normal, control, 25 mg/kg PLYCSB,
50 mg/kg PLYCSB and omeprazole groups, respectively (Fig. 2B). These results demonstrate that
there is a reduction in gastric secretion and an increase in
gastric pH in mice treated with PLYCSB compared with those in the
control mice.
Effect of PLYCSB on serum levels of the
cytokines IL-1β, IL-4, IL-6 and IL-8
The levels of IL-1β, IL-6 and IL-8 were lowest in
the normal mice; however, in control mice these levels were
significantly increased (Table
II). The levels of IL-1β, IL-6 and IL-8 in mice treated with 25
and 50 mg/kg PLYCSB were lower compared with those in control mice.
Furthermore, the mice treated with 50 mg/kg PLYCSB showed
expression levels of IL-1β, IL-6 and IL-8 that were comparable with
those in mice treated with omeprazole, and the expression levels
were only slightly higher compared with those in normal mice. The
level of IL-4 in all groups showed the opposite trend compared with
that for the levels of IL-1β, IL-6 and IL-8. In the present study
it was shown that the levels of IL-1β, IL-6 and IL-8 in the
HCl/ethanol-induced gastric injury mice were markedly decreased,
whilst the level of IL-4 was markedly increased following treatment
with PLYCSB.
 | Table IISerum IL-1β, IL-4, IL-6 and IL-8
cytokine levels of PLYCSB-treated mice with HCl/ethanol-induced
gastric injury. |
Table II
Serum IL-1β, IL-4, IL-6 and IL-8
cytokine levels of PLYCSB-treated mice with HCl/ethanol-induced
gastric injury.
| Group | IL-1β (pg/ml) | IL-4 (pg/ml) | IL-6 (pg/ml) | IL-8 (pg/ml) |
|---|
| Normal | 44.82±3.62e | 25.12±1.15a | 32.71±3.82e | 45.52±2.85e |
| Control | 120.65±17.56a | 10.65±1.34e | 97.25±9.70a | 91.34±1.92a |
| PLYCSB (25
mg/kg) | 90.66±7.78b | 15.24±0.92d | 69.88±5.57b | 73.18±2.02b |
| PLYCSB (50
mg/kg) | 71.97±3.58c | 18.98±0.61c | 50.53±3.35c | 62.15±1.71c |
| Omeprazole (25
mg/kg) | 61.30±5.22d | 21.12±0.82b | 43.91±2.12d | 57.63±1.32d |
Effect of PLYCSB on serum levels of MOT,
SS, SP and VIP
The levels of MOT and SP were highest in the control
mice, whilst the levels of SS and VIP were lowest in the control
mice amongst all groups (Table
III). In the PLYCSB-treated mice, the levels of MOT, SP, SS and
VIP were significantly different from those in the control mice.
The high dose of PLYCSB (50 mg/kg PLYCSB) and omeprazole treatment
significantly attenuated the HCl/ethanol-induced changes in these
levels, with results comparable with those in the normal mice.
 | Table IIISerum MOT, SS, SP and VIP levels of
PLYCSB-treated mice with HCl/ethanol-induced gastric injury. |
Table III
Serum MOT, SS, SP and VIP levels of
PLYCSB-treated mice with HCl/ethanol-induced gastric injury.
| Group | MOT (μg/l) | SS (μg/l) | SP (μg/l) | VIP (μg/l) |
|---|
| Normal | 41.3±2.1e | 115.6±10.7a | 60.6±2.1e | 97.7±3.4a |
| Control | 93.6±4.1a | 57.6±4.2e | 118.3±2.6a | 43.8±1.8b |
| PLYCSB (25
mg/kg) | 67.3±5.7b | 75.2±5.1d | 83.6±2.2b | 60.3±1.9d |
| PLYCSB (50
mg/kg) | 56.1±3.2c | 90.3±2.5c | 72.6±2.8c | 78.6±2.0c |
| Omeprazole (25
mg/kg) | 51.2±2.0d | 98.3±6.6b | 67.3±2.4d | 84.6±1.8e |
SOD, GSH-Px, NO and MDA levels in gastric
tissue
The activities of SOD and GSH-Px in the gastric
tissue of normal mice were higher compared with those in the other
groups (Table IV). These
activities were significantly decreased in the control mice. Mice
treated with 50 mg/kg PLYCSB and omeprazole showed SOD activities
similar to those in normal mice; however, the activity in mice
treated with 25 mg/kg PLYCSB was decreased compared with that in 50
mg/kg PLYCSB-treated mice. The activity of GSH-Px in each group
followed the same trend as the activity of SOD. The NO levels in
each group of mice decreased in the order normal, omeprazole, 50
mg/kg PLYCSB, 25 mg/kg PLYCSB and control. The MDA levels of these
groups showed the opposite trend from that observed for the NO
levels.
 | Table IVTissue SOD, GSH-Px, NO and MDA levels
in PLYCSB-treated mice with HCl/ethanol-induced gastric injury. |
Table IV
Tissue SOD, GSH-Px, NO and MDA levels
in PLYCSB-treated mice with HCl/ethanol-induced gastric injury.
| Group | SOD (kU/l) | GSH-Px
(mmol/l) | NO (μmol/l) | MDA (μmol/l) |
|---|
| Normal |
336.12±38.92a | 3.92±0.37a | 13.74±1.55a | 12.87±1.79e |
| Control |
222.35±35.11e | 2.11±0.28e | 2.35±0.45e | 63.11±4.56a |
| PLYCSB (25
mg/kg) |
262.91±27.79d | 2.79±0.30d | 6.11±1.08d | 41.36±3.87b |
| PLYCSB (50
mg/kg) |
291.30±17.28c | 3.41±0.22c | 10.36±1.05c | 28.71±1.56c |
| Omeprazole (25
mg/kg) |
308.76±22.35b | 3.61±0.12b | 11.85±0.42b | 19.78±3.54d |
Effect of PLYCSB on the expression of
inflammation-associated genes iNOS, COX-2, TNF-α and IL-1β
The present study investigated whether the
anti-inflammatory actions of PLYCSB were associated with an
inhibition of inflammation-associated genes, specifically iNOS,
COX-2, TNF-α and IL-1β. As shown in Fig. 3, the mRNA and protein expression
levels of iNOS, COX-2, TNF-α and IL-1β were reduced in the gastric
tissues treated with PLYCSB and omeprazole compared with those in
the control tissues. PLYCSB and omeprazole significantly modulated
the expression of genes associated with inflammation. Additionally,
the mRNA and protein expression levels of these genes were
decreased in the presence of the PLYCSB in a dose-dependent manner.
These findings indicate that PLYCSB may help prevent gastric injury
by increasing anti-inflammatory activities. In combination, these
results showed that PLYCSB has a strong anti-inflammatory effect on
gastric injury.
Discussion
The swim bladder has been historically used in
traditional Chinese medicine. The swim bladder has previously been
shown to ameliorate different pathological conditions associated
with inflammation, and it also been demonstrated to strengthen
platelet function, capillary vessels and clotting factors (12). Swim bladders are mainly composed of
polysaccharides; however, few studies have investigated the
function of the polysaccharides from the swim bladder. In the
present study, the preventive effect of PLYCSB on gastric injury
was investigated for the first time, to the best of our knowledge.
The results demonstrated that PLYCSB conferred the same level of
protection against gastric injury as omeprazole, a drug used to
treat gastritis.
A highly acidic environment in the stomach is an
important marker of gastric injury. Gastric injury causes an
increase in gastric secretion and acid output, resulting in a
significantly decreased gastric pH (13). Mice treated with 50 mg/kg PLYCSB
had decreased gastric secretion and a higher gastric pH compared
with that in the control and low dose PLYCSB groups. This may
explain why 50 mg/kg PLYCSB demonstrated a greater protective
effect against gastric injury, compared with that of 25 mg/kg
PLYCSB. In the present study, PLYCSB was found to exhibit a
preventive effect on gastric injury by decreasing the levels of
stomach acid.
The levels of serum cytokines, including IL-6 and
IL-12, in patients with inflammatory diseases are elevated compared
with those in healthy individuals (14). Cytokine receptors and the
inflammatory cytokines IL-6 and IL-12 have a pathogenic role in
gastric disease, and decreased expression levels of these indicate
a preventive effect against gastric injury (15,16).
IL-6 is an interleukin that functions as a proinflammatory and
anti-inflammatory cytokine (17).
T cells and macrophages secrete IL-6 to stimulate an immune
response, particularly during tissue damage, which leads to
inflammation. IL-6 is also involved in fighting infection (18). IL-4 has an important role in
inflammation and wound repair and an increase in IL-4 secretion may
aid the treatment of inflammatory disease (19). IL-8 is also associated with
inflammation. Oxidative stress increases IL-8 secretion, which then
causes the recruitment of inflammatory cells and induces an
increase in oxidative stress mediators, making IL-8 a significant
factor in localized inflammation (20).
MOT and SP are excitatory gastrointestinal hormones.
The levels of MOT and SP increase following stimulation by gastric
injury (21). Once MOT is
stimulated, it causes the surplus secretion of gastric acid. An
increase in gastric acid causes the inner part of the stomach to
become too acidic, thereby compounding gastric injury (22). The results from the present study
show that the levels of MOT and SP increase following treatment
with HCl/ethanol. By contrast, SS and VIP are inhibitory
gastrointestinal hormones, which are capable of inhibiting the
secretion of gastric acid (21).
It has been previously demonstrated that damaging the gastric
mucosa results in the surplus secretion of gastric fluids and a
reduction of the pH to a value lower than the normal value
(23). The levels of SS and VIP
were observed to increase following treatment with a high dose of
PLYCSB compared with the levels in the control mice, which is
likely to result in a reduction in gastric secretion volume and an
increase in the pH of gastric juice. In the present study, the
gastric secretion and pH of the gastric juice were in accordance
with this.
Following gastric injury, the gastric tissue may be
partially oxidized due to damage. SOD and GSH-Px are important
antioxidants that reduce peroxide in the gastric tissue into less
harmful or harmless substances, which is important in the recovery
of gastric injury (24).
Ethanol-induced gastric mucosal damage may involve the generation
of oxygen-derived radicals, independent of the xanthine oxidase
system. By acting as oxygen radical scavengers, SOD and GSH-Px
provide significant gastroprotection (25). Tissue injury is caused by an
imbalance between the damage of the gastric tissue and protective
factors. NO serves to protect the gastric mucosa and keep the blood
flowing smoothly. NO levels decrease significantly in patients
suffering from gastric injury. In addition, NO has been
demonstrated to be an effective component to guard against gastric
injury (26). MDA is a marker of
oxidative stress and it is generated in large amounts in the
damaged areas of gastric tissue. Therefore, MDA can be used as an
indicator of gastric injury (27).
The results from the present study demonstrated that a higher
concentration of PLYCSB decreases the degree of gastric injury.
iNOS, COX-2, TNF-α and IL-1β genes in the tissue may
be used as biomarkers to monitor visceral damage. Following
inflammatory stimuli, COX-2 and iNOS have been shown to induce
deleterious effects in the stomach (28). iNOS and COX 2 in order to boost
inflammatory responses in early stages of tissues injury (29). Inflammatory processes are mediated
by multiple molecular mechanisms. Two of the most important are
those associated with the production of iNOS and COX-2. The time
courses by which inflammatory stimuli elicit iNOS and COX-2 protein
synthesis are similar, which indicates that the two systems may
interact with each other (30).
The expression levels of TNF-α and IL-1β in patients with
inflammatory diseases are higher compared with those in healthy
individuals, and lower expression levels of TNF-α and IL-1β have
been found to be indicative of improved anti-inflammatory effects
(23). In the present study, it
was demonstrated that PLYCSB significantly suppressed the
expression of the inflammatory genes iNOS, COX-2, TNF-α and IL-1β.
The levels of the protein products of these genes were also
reduced.
In conclusion, the preventive effect of PLYCSB
against gastric injury was evaluated in the present study using
various in vivo experimental methods, including the use of a
serum cytokine assay to analyze the levels of IL-1β, IL-4, IL-6 and
IL-8; analysis of the serum levels of MOT, SS, SP and VIP;
investigating the tissue levels of SOD, GSH-Px, NO and MDA, and
using PCR and western blot analysis to determine the expression
levels of inflammatory-associated genes and proteins, specifically
iNOS, COX-2, TNF-α and IL-1β. Observation of the stomachs of mice
in the different treatment groups revealed that PLYCSB had a
preventive effect against HCl/ethanol-induced gastric injury,
indicating that PLYCSB represents a potentially useful agent for
the treatment or prevention of drug-induced gastric injury in
vivo.
Acknowledgements
This study was supported by the Program for
Chongqing Innovative Research Team in University (KJTD201325).
References
|
1
|
Jian JC and Wu ZH: Effects of traditional
Chinese medicine on nonspecific immunity and disease resistance of
large yellow croaker, Pseudosciaena crocea (Richardson).
Aquaculture. 218:1–9. 2003. View Article : Google Scholar
|
|
2
|
Li C and Yao CL: Molecular and expression
characterizations of interleukin-8 gene in large yellow croaker
(Larimichthys crocea). Fish Shellfish Immunol. 34:799–809.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu S and Yu B: Peptides from variegated
carp (Aristichthys nobilis) swim bladder: Fermentation
production and assessment of antioxidant properties. Food Sci.
30:332–334. 2009.(In Chinese).
|
|
4
|
Yu ZH, Yin LH, Yang Q and Liu Y: Effect of
Lentinus edodes polysaccharide on oxidative stress, immunity
activity and oral ulceration of rats stimulated by phenol.
Carbohydr Polym. 75:115–118. 2009.
|
|
5
|
Laurienzo P: Marine polysaccharides in
pharmaceutical applications: an overview. Mar Drugs. 8:2435–2465.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabo S, Trier JS, Brown A and Schnoor J:
Early vascular injury and increased vascular permeability in
gastric mucosal injury caused by ethanol in the rat.
Gastroenterology. 88:228–236. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Medeiros JV, Gadelha GG, Lima SJ, Garcia
JA, Soares PM, Santos AA, Brito GA, Ribeiro RA and Souza MH: Role
of the NO/cGMP/K(ATP) pathway in the protective effects of
sildenafil against ethanol-induced gastric damage in rats. Br J
Pharmacol. 153:721–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan C and Yan X: Study on extraction of
Lycium barbarum polysaccharides by different methods and
their antioxidant effects in vitro. Food Sci. 29:183–187. 2008.(In
Chinese).
|
|
9
|
Ramirez RO and Roa CC Jr: The
gastroprotective effect of tannins extracted from duhat
(Syzygium cumini Skeels) bark on HCl/ethanol induced gastric
mucosal injury in Sprague-Dawley rats. Clin Hemorheol Microcirc.
29:253–261. 2003.PubMed/NCBI
|
|
10
|
Wang Q, Zhao X, Qian Y and Wang R: In
vitro antioxidative activity of yellow tea and its in vivo
preventive effect on gastric injury. Exp Ther Med. 6:423–426.
2013.PubMed/NCBI
|
|
11
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao H, Tian XL and Liu X: Study on
molecular identification and pharmacology of hemostasis action for
isinglass. Zhongguo Shipin Xuebao. 9(4): 170–176. 2009.(In
Chinese).
|
|
13
|
Ligumsky M, Sestieri M, Okon E and
Ginsburg I: Antioxidants inhibit ethanol-induced gastric injury in
the rat. Role of manganese, glycine, and carotene. Scand J
Gastroenterol. 30:854–860. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gratacós J, Collado A, Filella X, Sanmartí
R, Cañete J, Llena J, Molina R, Ballesta A and Muñoz-Gómez J: Serum
cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing
spondylitis: a close correlation between serum IL-6 and disease
activity and severity. Br J Rheumatol. 33:927–931. 1994.PubMed/NCBI
|
|
15
|
Fox JG, Beck P, Dangler CA, Whary MT, Wang
TC, Shi NH and Nagler-Anderson C: Concurrent enteric helminth
infection modulates inflammation and gastric immune responses and
reduces helicobacter-induced gastric atrophy. Nat Med. 6:536–542.
2000. View Article : Google Scholar
|
|
16
|
D’Elios MM, Manghetti M, De Carli M, Costa
F, Baldari CT, Burroni D, Telford JL, Romagnani S and Del Prete G:
T helper 1 effector cells specific for Helicobacter pylori
in the gastric antrum of patients with peptic ulcer disease. J
Immunol. 158:962–967. 1997.
|
|
17
|
Ferguson-Smith AC, Chen YF, Newman MS, May
LT, Sehgal PB and Ruddle FH: Regional localization of the
interferon-beta 2/B-cell stimulatory factor 2/hepatocyte
stimulating factor gene to human chromosome 7p15-p21. Genomics.
2:203–208. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Poll T, Keogh CV, Guirao X,
Buurman WA, Kopf M and Lowry SF: Interleukin-6 gene-deficient mice
show impaired defense against pneumococcal pneumonia. J Infect Dis.
176:439–444. 1997.PubMed/NCBI
|
|
19
|
Maeda S and Yanagihara Y: Inflammatory
cytokines (IL-4, IL-5 and IL-13). Nippon Rinsho. 59:1894–1899.
2001.(In Japanese).
|
|
20
|
Vlahopoulos S, Boldogh I, Casola A and
Brasier AR: Nuclear factor-kappaB-dependent induction of
interleukin-8 gene expression by tumor necrosis factor alpha:
evidence for an antioxidant sensitive activating pathway distinct
from nuclear translocation. Blood. 94:1878–1889. 1999.
|
|
21
|
Wang HY, Liu YM, Li HY, Feng QJ, Guo JY
and Niu X: Effects of oils in Alpinia officinarum Hance on
serum motilin, somatostatin, substance P, vasoactive intestinal
peptide in gastrelcosis mice model. Chinese J Exp Tradit Med
Formulae. 17(4): 105–107. 2011.(In Chinese).
|
|
22
|
Zhang SR, Shao JY and Yu YW: The
protective effects of furazolidone and some commonly used antiulcer
drugs on several gastric ulcer models in rats. Yao Xue Xue Bao.
19:5–11. 1984.(In Chinese).
|
|
23
|
Zhao X, Wang Q, Qian Y and Song JL: Ilex
kudingcha C.J. Tseng (Kudingcha) prevents HCl/ethanol-induced
gastric injury in Sprague-Dawley rats. Mol Med Rep. 7:1613–1616.
2013.PubMed/NCBI
|
|
24
|
Qu CY, Li DG, Wang YQ and Chen MM: Effect
of chitosan on the serum levels of MDA, SOD, and GSH-Px in rats
with gastric ulcer. Shanghai Med Pharm J. 29:219–222. 2008.(In
Chinese).
|
|
25
|
Ligumsky M, Sestieri M, Okon E and
Ginsburg I: Antioxidants inhibit ethanol-induced gastric injury in
the rat: Role of manganese, glycine, and carotene. Scand J
Gastroenterol. 30:854–860. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng HH and AXR: Change of serum nitrogen
monoxidum and nitrogen monoxidum synthase levels in patients with
peptic ulcer in high altitude region. Lab Med. 25:427–428. 2010.(In
Chinese).
|
|
27
|
Wang G, Tu ZL, Chen L, Yuan SH and Yang
GY: Mechanism of antiulcer effects of Jinguolan. Herald Med.
28:42–45. 2009.(In Chinese).
|
|
28
|
Li HL, Sun BZ and Ma FC: Expression of
COX-2, iNOS, p53 and Ki-67 in gastric mucosa-associated lymphoid
tissue lymphoma. World J Gastroenterol. 10:1862–1866.
2004.PubMed/NCBI
|
|
29
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Gene Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SF, Huri DA and Snyder SH: Inducible
nitric oxide synthase binds, S-nitrosylates, and activates
cyclooxygenase-2. Science. 310:1966–1970. 2005. View Article : Google Scholar : PubMed/NCBI
|