Introduction
Congestive heart failure (CHF) is often accompanied
by high mortality rates. Therefore, accurate risk stratification is
critically important in identifying high-risk patients who may
benefit from advanced treatment (1–4). A
number of clinical variables and biological markers have been
applied over the last decade in predictive models of survival for
patients with CHF (5,6), including inflammatory cytokines
(7,8) high-sensitivity C-reactive protein
(CRP) (9) natriuretic peptides
(10,11) neurohormones (12) and oxidative stress (13), all of which are useful for
diagnosis and prognosis. However, these biomarkers are very
expensive to analyze and cost effectiveness must be considered when
including markers in predictive models.
Anemia is a common feature of patients with CHF and
it has important implications for the prognosis and treatment of
CHF (14–17). A cause-and-effect relationship
between anemia and CHF has not been demonstrated. Red blood cell
distribution width (RDW) is a measure of the size variability in
the red blood cell population and it is calculated as the standard
deviation (SD) of the distribution of RDW divided by the mean
corpuscular volume. Several studies have confirmed that RDW
predicts mortality in acute coronary syndrome (18,19)
and heart failure (20–22). However, the predictive value of RDW
for in-hospital, and long-term outcomes for acute CHF and acute CHF
predictive value relative to hemoglobin (Hgb) and anemia remain
unknown (23).
The present study therefore investigated whether RDW
is useful for risk stratification in patients hospitalized with
acute exacerbation of CHF and analyzed the prognostic value of RDW
compared with that of Hgb levels and anemia status.
Materials and methods
Patients
Patients with acute onset of CHF were consecutively
enrolled in an observational study between January 2007 and
December 2009. All patients were hospitalized at the cardiac care
unit (CCU) of Juntendo University Hospital (Tokyo, Japan).
Demographic and clinical information, as well as age, gender, New
York Heart Association (NYHA) functional class, history of
cigarette smoking, ischemic heart disease, hypertension,
hyperlipidemia, atrial fibrillation, diabetes mellitus, chronic
renal failure, hemodialysis and medications taken upon admission
were obtained from medical records. Body mass index was calculated
from height and weight data. Cardiac structure and function was
assessed by echocardiography using a hematology analyzer (HF-3000,
Chengdu, China).
The patients were followed up by telephone, personal
interviews and by reviewing electronic medical records. The
endpoint for short-term outcomes was in-hospital mortality.
Mortality from all causes and readmission to hospital with the
onset of CHF were long-term endpoints. The end of the follow-up was
June 2010. The current study was performed according to the ethics
policies of Juntendo University Hospital and was approved by the
internal review board. Informed consent was obtained from the
patient.
Laboratory measurements
Blood samples were obtained immediately upon
admission to the CCU and analyzed as quickly as possible in the
clinical chemistry laboratory. Anemia was defined as Hgb <12
g/dl in women and <13 g/dl in men, according to the World Health
Organization (WHO) criteria. Renal function was evaluated as the
estimated glomerular filtration rate (eGFR), calculated according
to the simplified modification of diet in renal disease (MDRD)
equation: eGFR (ml/min/1.73 m2) = 186 × [serum
creatinine (Scr)]−1.154 × (age)−0.203 ×
(0.742 if female) where Scr is the serum creatinine level in
mg/dl.
Statistical analysis
Discrete variables were presented as frequency
counts and ratios (%). Continuous variables were analyzed based on
a normal distribution using the Shapiro-Wilk test. They were
expressed as mean ± SD when normally distributed, or as medians
with inter-quartile ranges (IQRs) if not. Proportions and means or
medians were compared using the χ2 test, the two-tailed
independent Student’s t-test, one-way analysis of variance (ANOVA)
and the Wilcoxon or Kruskal-Wallis one-way ANOVA test. Univariable
correlations were examined using the Pearson’s correlation
coefficient in the context of normality, whereas correlations among
non-normally distributed variables were examined using the
Spearman’s rho test. Variables with a retention P-value of 0.10 in
the univariate analysis were entered into a multivariable linear
regression model. Variables with non-normal distributions were
log-transformed prior to being entered into this model.
Independent predictors of short- and long-term
outcomes were identified using the logistic regression and Cox
proportional hazard models, respectively. Kaplan-Meier accumulated
survival curves were constructed and log-rank values were
calculated to assess their statistical significance.
All data were analyzed using JMP 8.0 (SAS Institute,
Inc., Cary, NC, USA) and SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The current study comprised 521 patients with acute
onset CHF from a wide range of age groups [72 (64, 80) years]. The
median (IQR) values of RDW and mean level of Hgb overall were 14.5
(13.6, 15.9)% and 11.7±2.5 g/dl, respectively. Based on the WHO
criteria, 324 (62.2%) patients were diagnosed with anemia. Among
the patients, 192 (36.9%), 200 (38.4%) and 129 (24.8%) were
classified as NYHA functional classes II, III and IV, respectively.
Table I shows the patient
characteristics upon admission, grouped according to quartiles of
RDW. Patients with a higher RDW significantly differed from the
others in terms of age, Hgb, anemia, NYHA classification, left
ventricular ejection fraction (LVEF), B-type natriuretic peptide
(BNP), lipid profiles [specifically, total cholesterol (TC), low
density lipoprotein cholesterol (LDL-C), high density lipoprotein
cholesterol (HDL-C) and triglycerides (TG)] blood urea nitrogen
(BUN), eGFR, CRP and loop diuretics at presentation.
 | Table IBaseline clinical characteristics of
patients at time of admission, grouped according to red blood cell
distribution width. |
Table I
Baseline clinical characteristics of
patients at time of admission, grouped according to red blood cell
distribution width.
| RDW | |
|---|
|
| |
|---|
| Characteristic | Quartile 1
(<13.6%, n=142) | Quartile 2
(13.6–14.5%, n=123) | Quartile 3
(14.5–15.9%, n=128) | Quartile 4
(>15.9%, n=128) | P-value |
|---|
| Demographics |
| Age (years), median
(IQR) | 68 (62, 77) | 74 (63, 81) | 74 (66, 82) | 73 (65, 80) | 0.0012 |
| Male | 102 (71.8%) | 79 (64.2%) | 79 (61.7%) | 87 (68.0%) | 0.31 |
| BMI
(kg/m2), median (IQR) | 21.2 (18.7,
24.7) | 20.4 (18.4,
23.1) | 20.5 (18.1,
23.5) | 20.2 (17.8,
23.2) | 0.39 |
| Current smoker | 8 (5.6%) | 3 (2.4%) | 6 (4.7) | 6 (4.4%) | 0.60 |
| History of CRF | 7 (4.9%) | 11 (8.9%) | 12 (9.4%) | 17 (13.3) | 0.11 |
| Hemodialysis at
presentation | 3 (2.1%) | 7 (5.7%) | 7 (5.5%) | 9 (7.0%) | 0.23 |
| History of
hypertension | 53 (37.3%) | 56 (45.5%) | 50 (39.1%) | 42 (32.8%) | 0.22 |
| History of
hyperlipidemia | 18 (12.7%) | 15 (12.2%) | 12 (9.4%) | 20 (15.6%) | 0.51 |
| History of atrial
fibrillation | 18 (12.7%) | 21 (17.1%) | 27 (21.1%) | 25 (19.5%) | 0.27 |
| History of diabetes
mellitus | 14 (9.9%) | 17 (13.8%) | 22 (17.2%) | 22 (17.2%) | 0.24 |
| Primary disease | | | | | 0.20 |
| Ischemic heart
disease | 48 (33.8%) | 41 (33.3%) | 45 (35.2%) | 45 (35.2%) | |
| Hypertensive heart
disease | 36 (25.4%) | 38 (30.9%) | 26 (20.3%) | 31 (24.2%) | |
| Valvular heart
disease and congenital heart disease | 14 (9.9%) | 14 (11.4%) | 25 (19.5%) | 25 (19.5%) | |
| Dilated
cardiomyopathy | 17 (12.0%) | 11 (8.9%) | 10 (7.8%) | 14 (10.9%) | |
| Hypertrophic
cardiomyopathy | 8 (5.6%) | 7 (5.7%) | 5 (3.9%) | 6 (4.7%) | |
| Anemia |
| Hemoglobin (g/dl),
mean±SD | 12.9±2.3 | 12.3±2.1 | 11.1±2.4 | 10.4±2.4 | <0.0001 |
| Anemia (World
Health Organization) | 60 (42.3%) | 64 (52.0%) | 91 (71.1%) | 109 (85.2%) | <0.0001 |
| Cardiac
function |
| NYHA | | | | | 0.0049 |
| Class II | 71 (50.0%) | 42 (34.2%) | 41 (32.0%) | 37 (29.1%) | |
| Class III | 46 (32.4%) | 54 (43.9%) | 51 (39.8%) | 49 (38.6%) | |
| Class IV | 25 (17.6%) | 27 (22.0%) | 36 (28.1%) | 41 (32.3%) | |
| LVEF (%), median
(IQR) | 50 (35, 58) | 44 (35, 58) | 43 (34, 54) | 35 (28, 52) | 0.0009 |
| BNP (pg/ml),
median (IQR) | 444.0 (213.0,
1056.7) | 822.6 (471.9,
1448.3) | 834.1 (377.8,
1722.0) | 917.6 (436.2,
1690.2) | <0.0001 |
| Lipid and glucose
profiles |
| TC (mg/dl), median
(IQR) | 176 (150, 206) | 164 (146, 196) | 158 (135, 185) | 157 (131, 188) | 0.0060 |
| LDL-C (mg/dl),
median (IQR) | 104 (74, 131) | 101 (84, 123) | 88 (73, 107) | 87 (69, 112) | 0.0038 |
| HDL-C (mg/dl),
median (IQR) | 43 (36, 52) | 45 (37, 51) | 42 (35, 54) | 38 (30, 45) | 0.0005 |
| TG (mg/dl), median
(IQR) | 91 (71, 134) | 78 (60, 112) | 82 (57, 115) | 86 (63, 114) | 0.05 |
| HbA1c (%), median
(IQR) | 5.5 (5.1, 6.3) | 5.5 (5.2, 6.1) | 5.6 (5.3, 6.2) | 5.6 (5.0, 6.4) | 0.85 |
| Renal function |
| BUN (mg/dl),
median (IQR) | 19 (15, 27) | 26 (17, 35) | 29 (20, 43) | 32 (21, 50) | <0.0001 |
| eGFR, median
(IQR) | 85.0 (62.0,
99.2) | 65.5 (33.0,
87.0) | 53.6 (25.9,
80.5) | 52.4 (26.9,
79.1) | <0.0001 |
| Inflammation
indices |
| CRP (mg/dl),
median (IQR) | 0.6 (0.2, 3.5) | 0.8 (0.2, 2.4) | 1.0 (0.3, 3.8) | 1.1 (0.4, 4.4) | 0.03 |
| Medications |
| Digoxin at
presentation | 9 (6.4%) | 8 (6.6%) | 10 (7.8%) | 16 (12.5%) | 0.27 |
| Thiazide at
presentation | 2 (1.4%) | 2 (1.6%) | 0 (0%) | 4 (3.1%) | 0.25 |
| Loop diuretic at
presentation | 13 (9.2%) | 15 (12.2%) | 25 (19.5%) | 26 (20.3%) | 0.022 |
| Aldosterone
antagonist at presentation | 11 (7.8%) | 9 (7.3%) | 13 (10.2%) | 13 (10.2%) | 0.77 |
| β-blocker at
presentation | 23 (16.2%) | 14 (11.4%) | 24 (18.8%) | 22 (17.2%) | 0.40 |
| ACE-I or ARB at
presentation | 22 (15.5%) | 20 (16.3%) | 22 (17.2%) | 26 (20.3%) | 0.75 |
| Statins at
presentation | 15 (10.6%) | 10 (8.2%) | 10 (7.8%) | 11 (8.6%) | 0.86 |
| Aspirin at
presentation | 23 (16.2%) | 18 (14.6%) | 30 (23.4% | 24 (18.8) | 0.29 |
Predictors of RDW values
Univariate analysis showed that age, history of CRF,
hemodialysis at presentation, history of diabetes mellitus, Hgb,
anemia, NYHA classification, LVEF, BNP, TC, LDL-C, HDL-C, TG, BUN,
eGFR and loop diuretics at presentation were significantly
correlated with RDW (P<0.05, data not shown). However, further
multivariate analysis revealed Hgb, BNP, eGFR and HDL-C as
independent predictors of RDW (Table
II).
 | Table IIMultiple linear regression assessing
independent predictors of red blood cell distribution width. |
Table II
Multiple linear regression assessing
independent predictors of red blood cell distribution width.
| Predictor | Estimated
coefficient | P-value |
|---|
| Hgb | −0.27 | <0.0001 |
| Log BNP | 0.39 | 0.0013 |
| Log eGFR | −0.55 | 0.0096 |
| Log HDL-C | −0.85 | 0.047 |
| NYHA (IV-III) | 0.53 | 0.059 |
| Log BUN | 0.40 | 0.18 |
| Log LDL-C | −0.80 | 0.18 |
| Loop diuretic at
presentation | 0.39 | 0.24 |
| Log TC | 0.86 | 0.35 |
| NYHA (III-II) | 0.20 | 0.44 |
| Hemodialysis | −0.25 | 0.50 |
| Log TG | −0.14 | 0.59 |
| Log LVEF | −0.14 | 0.60 |
| DM | −0.087 | 0.61 |
| CRF | 0.096 | 0.76 |
| Log Age | 0.18 | 0.79 |
Prediction value of in-hospital mortality
and long-term outcome
Thirty-three (6.3%) patients succumbed in hospital.
After a median follow-up of 24 months (range, 6–42 months), 315
(64.5%) patients reached the endpoint of mortality or
re-hospitalization.
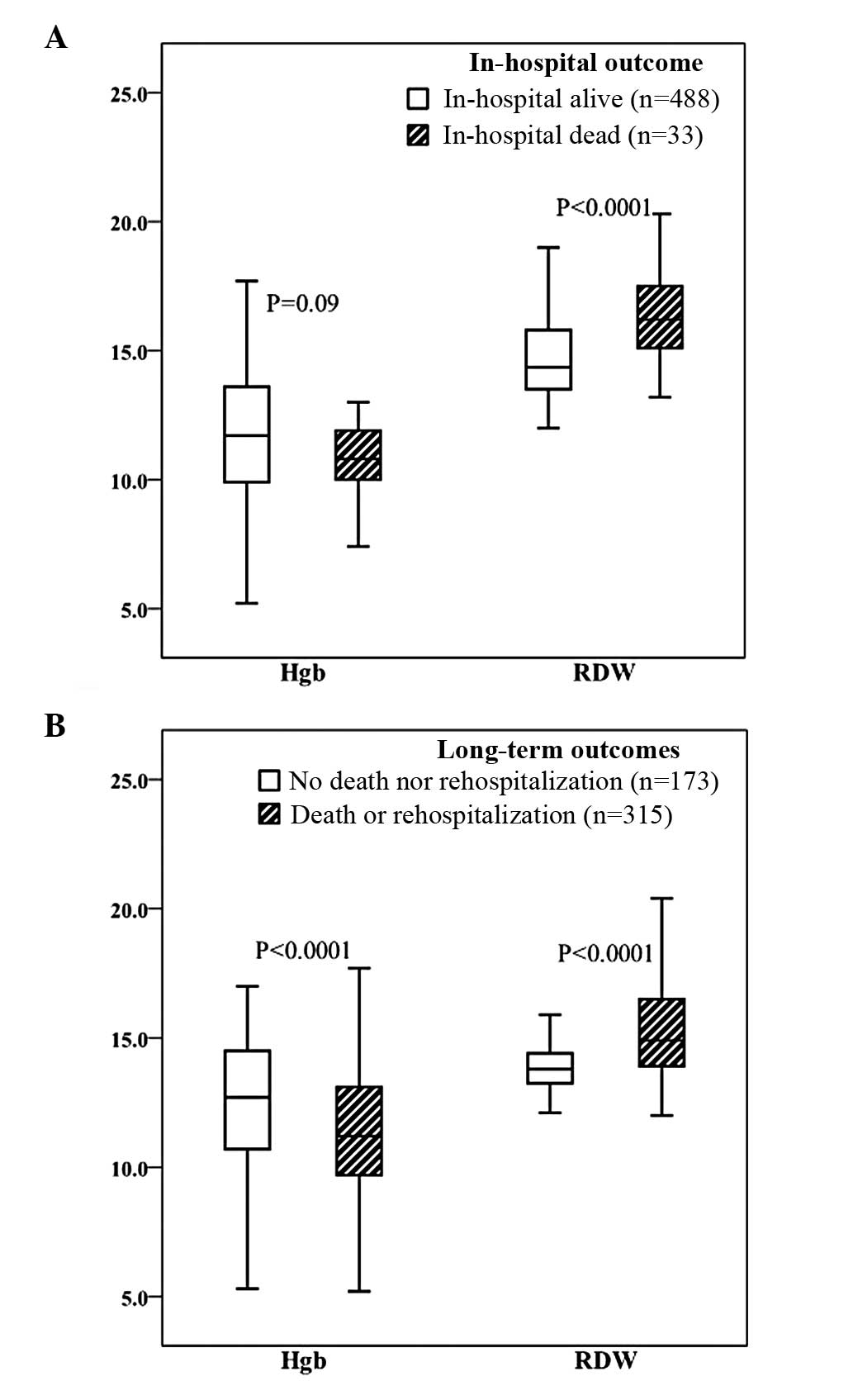
The RDW values were significantly higher in the
group that succumbed while in hospital (DIH) compared with the
group that remained alive while in hospital (AIH) [16.2 (15.1,
17.6)% vs. 14.4 (13.5, 15.8)%, P<0.0001)]. However, Hgb did not
significantly differ between the two groups (11.0±1.8 vs. 11.8±2.6
g/dl, P>0.05; Fig. 1A).
Logistic regression analysis showed that in-hospital mortality was
significantly associated with RDW, NYHA (IV-III), eGFR and CRP
(P<0.05), but not with Hgb (Table
III).
 | Table IIILogistic regression analysis for
in-hospital mortality. |
Table III
Logistic regression analysis for
in-hospital mortality.
| Predictor | Estimated
Coefficient | P-value |
|---|
| Log RDW | 5.21 | 0.044 |
| Hgb | 0.28 | 0.10 |
| Log age | 2.18 | 0.19 |
| Gender (male) | 0.61 | 0.090 |
| Log BMI | −3.81 | 0.06 |
| NYHA (III-II) | 0.76 | 0.57 |
| NYHA (IV-III) | 2.56 | 0.0037 |
| Log LVEF | −0.30 | 0.69 |
| Log BNP | 0.16 | 0.70 |
| Log eGFR | −2.08 | 0.042 |
| Log BUN | 0.72 | 0.26 |
| Log CRP | 0.75 | 0.0044 |
| Log UA | −0.89 | 0.23 |
Throughout the median 24 months of follow-up, both
Hgb and RDW significantly differed between the two groups (Fig. 1B). The mean levels of Hgb in
patients that reached an endpoint or did not reach an endpoint were
11.4±2.5 and 12.5±2.4 g/dl, respectively (P<0.0001); the median
(IQR) values of RDW were 14.9 (13.9, 16.5)% and 13.8 (13.3, 14.4)%
respectively (P<0.0001).
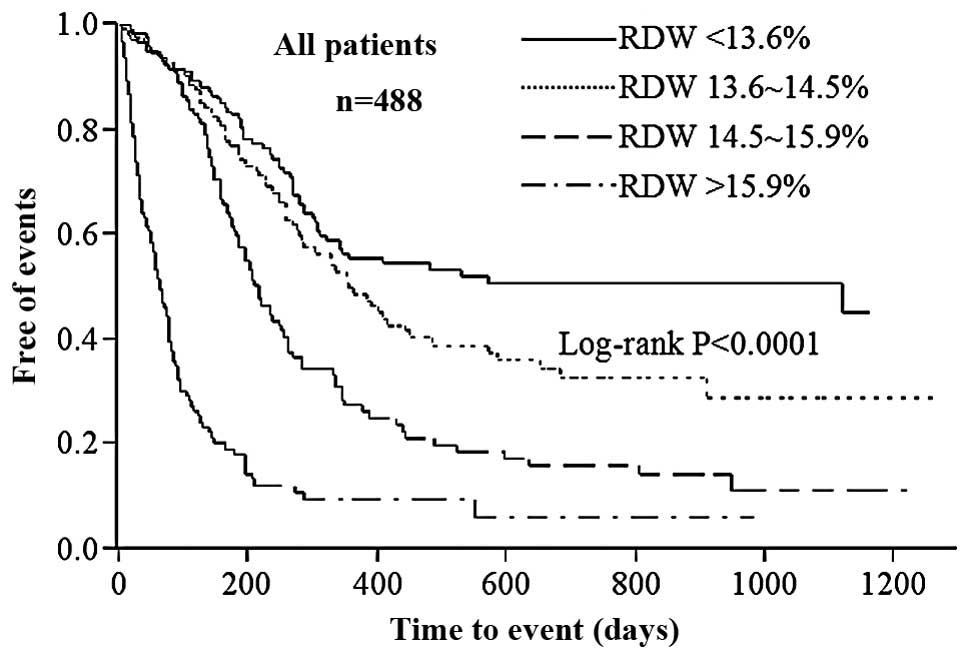
Kaplan-Meier survival curves for mortality from all
causes and readmission to hospital demonstrated that rates of
endpoints were significantly higher among patients with a higher
RDW (Fig. 2; Log-rank
P<0.0001). The graded increased probability of endpoints with
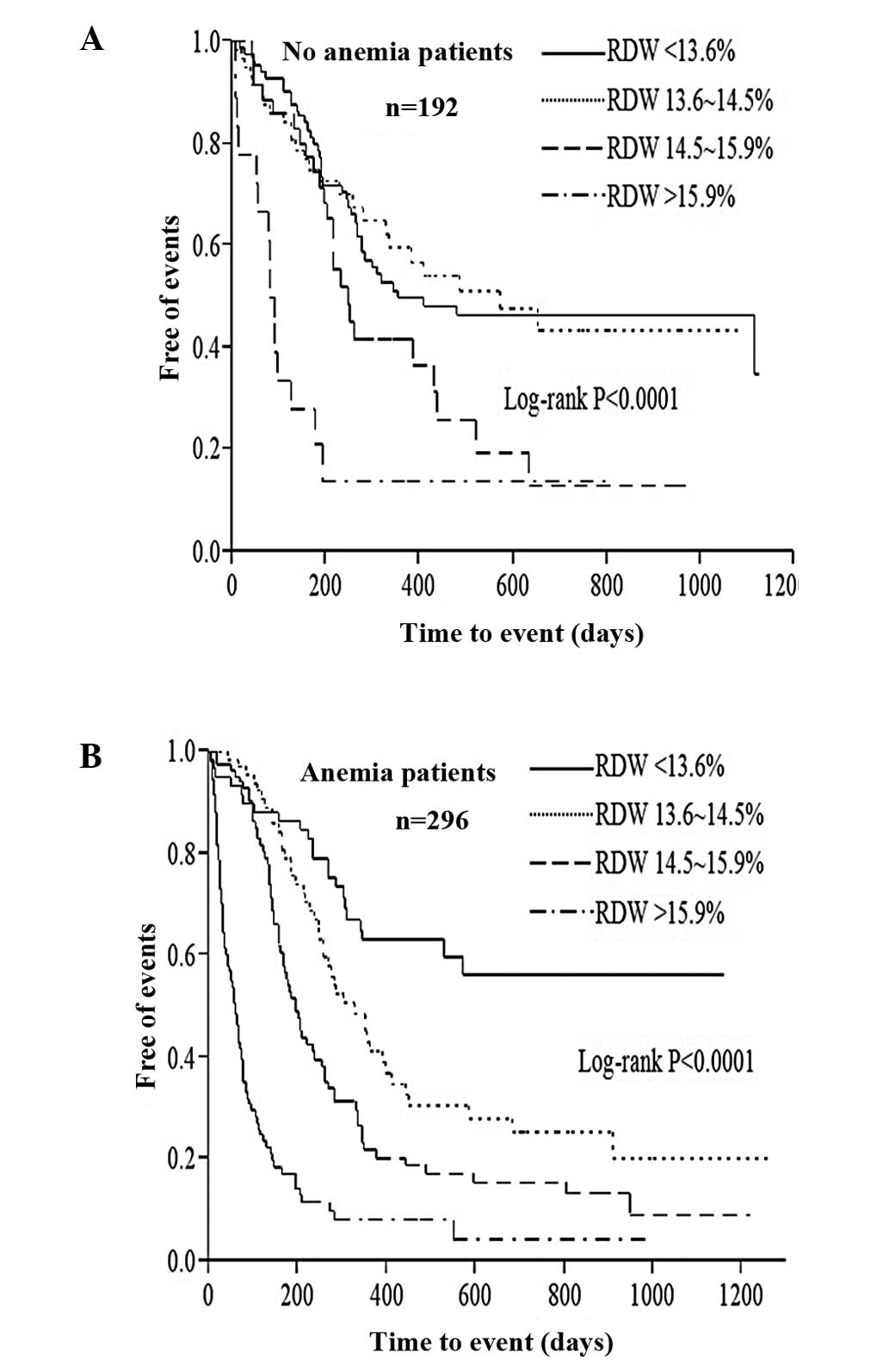
increasing RDW quartiles during follow-up persisted regardless of
the presence of anemia (Fig. 3)
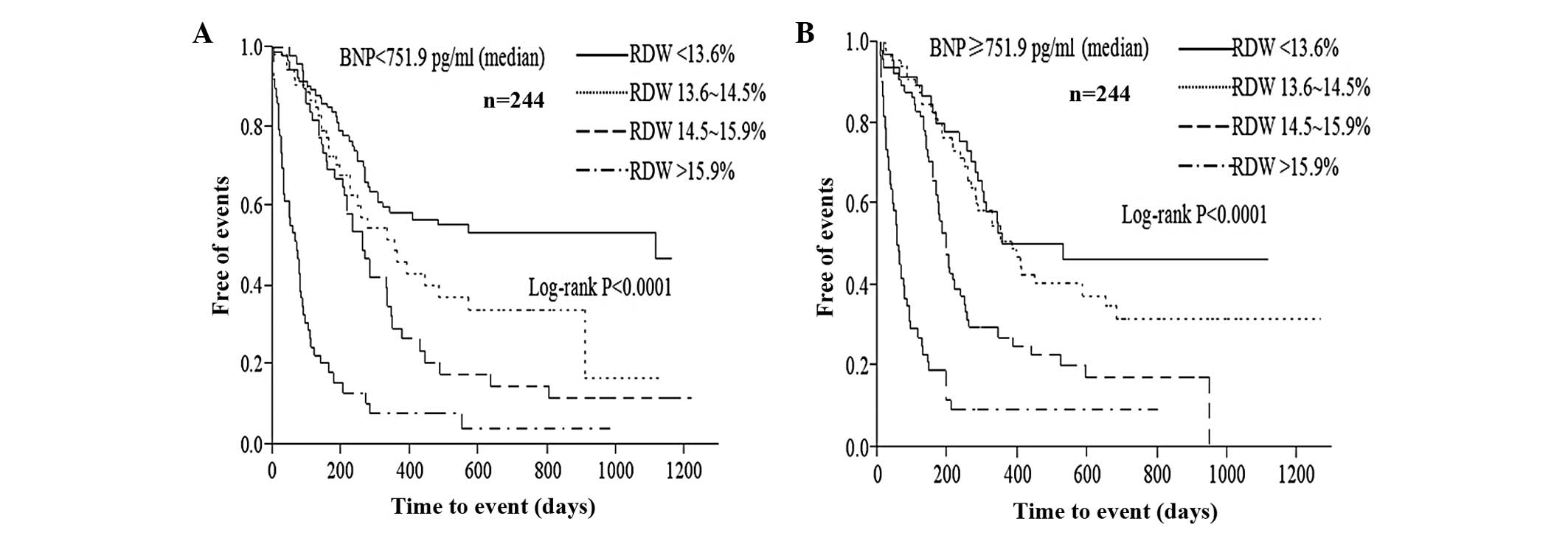
and variations in BNP level (Fig.
4). The univariate Cox proportional hazard model analysis
(Table IV) associated Hgb [per SD
increase, hazard ratio (HR), 0.72; 95% CI, 0.64–0.81; P<0.0001)
and RDW (per SD increase, HR, 2.25; 95% CI, 2.02–2.49; P<0.0001]
with higher risks of reaching endpoints. After adjustment in the
multivariate Cox proportional hazard model analysis (Table IV), RDW remained a significant
risk factor (per SD increase, HR, 2.19; 95% CI, 1.92–2.50;
P<0.0001), whereas Hgb did not remain a predictive value (per SD
increase, HR, 1.01; 95% CI, 0.96–1.13; P=0.86). In this final
adjusted model, the other independent predictors included LVEF (per
SD increase, HR, 0.81; 95% CI, 0.71–0.92; P=0.0016), age (10 years
increase, HR, 1.19; 95% CI, 1.07–1.34; P=0.0017) and NYHA classes
III and IV (HR, 1.52; 95% CI, 1.15–2.03; P=0.0029).
 | Table IVCox proportional hazard models for
long-term outcomes. |
Table IV
Cox proportional hazard models for
long-term outcomes.
| Univariate,
unadjusted | Multivariate,
adjusted |
|---|
|
|
|
|---|
| Predictor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| RDW (per SD
increase) | 2.25
(2.02–2.49) | <0.0001 | 2.19
(1.92–2.50) | <0.0001 |
| Hgb (per SD
increase) | 0.72
(0.64–0.81) | <0.0001 | 1.01
(0.96–1.13) | 0.86 |
| LVEF (per SD
increase) | 0.71
(0.63–0.79) | <0.0001 | 0.81
(0.71–0.92) | 0.0016 |
| NYHA (III/IV) | 2.01
(1.58–2.58) | <0.0001 | 1.52
(1.15–2.03) | 0.0029 |
| eGFR (per SD
increase) | 0.78
(0.69–0.87) | <0.0001 | 0.92
(0.78–1.07) | 0.29 |
| Age (10 years
increase) | 1.19
(1.08–1.30) | 0.0002 | 1.19
(1.07–1.34) | 0.0017 |
| BUN (per SD
increase) | 1.20
(1.09–1.31) | 0.0004 | 0.99
(0.85–1.14) | 0.92 |
| HDL-C (per SD
increase) | 0.87
(0.76–0.98) | 0.0223 | 1.04
(0.96–1.13) | 0.94 |
| Log (CRP) | 1.07
(1.00–1.15) | 0.0448 | 1.04
(0.96–1.12) | 0.34 |
| History of diabetes
mellitus | 1.30
(0.96–1.72) | 0.084 | 0.93
(0.66–1.29) | 0.68 |
Discussion
The results of the present study revealed that RDW
may act as a powerful independent predictive factor for short- and
long-term outcomes in patients with acute exacerbation of CHF and
that this prognostic value remains significant even after
adjustment for other known prognostic factors. The correlation
between RDW and the long-term outcomes of patients with acute CHF
provides prognostic data upon which to base risk factors and
persists regardless of the levels of Hgb or BNP. Other prognostic
markers identified in the current study were age, LVEF and NYHA
class, all of which were consistent with the findings of published
models on heart failure.
The RDW is a measurement of the size variation among
circulating red cells and is calculated as part of the routine
complete blood count (24). The
RDW, along with mean cell volume (MCV), is useful in the
differential diagnosis of the causes of anemia (25). The normal range for RDW is between
11.5% and 14.5%, and higher RDW values represent a greater
variability in cell size (24,25).
The median RDW value in the present study was 14.5%, indicating
that half of the patients had an RDW value that was above the upper
limit of the normal range.
Anemia has convincingly served as a powerful
predictor of re-hospitalization rates and survival in CHF (14,26,27).
Several mechanisms have been proposed to explain the association
between anemia and outcomes of heart failure, including nutritional
deficiencies, impaired renal function, inadequate production of
erythropoietin, inflammatory stress (such as circulating cytokines
and chemokines that predict a higher mortality risk in the
population) and the impact of comorbidities (4,17,28).
In addition, the hemodynamic changes accompanying severe anemia,
including increased preload, reduced peripheral vascular resistance
and increased cardiac output, may ultimately lead to a detrimental
increase in left ventricular mass (15,27).
Any or all of these mechanisms may also influence RDW.
The RDW values become elevated under conditions of
increased red cell destruction or ineffective red cell production
(24). Increased RDW may represent
a nutritional deficiency (iron, vitamin B12 or folic acid), bone
marrow depression or chronic inflammation (24,25).
Inflammatory cytokines are predictors of the prognosis of heart
failure. Proinflammatory cytokines inhibit erythropoietin-induced
erythrocyte maturation, which is partly reflected by an increase in
RDW (29). Therefore, RDW may
serve as an integrative marker of multiple pathological processes
that are involved in heart failure, including the above conditions,
which are often found in patients with heart failure (30) This may explain why RDW values
correlate with disease severity and are associated with prognosis.
The current study identified Hgb, BNP, eGFR and HDL-C as
independent predictors of RDW; eGFR reflected renal function and
HDL-C reflected nutritional status. However, a significant
relationship was not identified between RDW and inflammatory
indices (CRP and UA) in the multivariate regression model.
The most notable finding of the present study was
that after multivariate adjustment of all the patient data, RDW,
but not Hgb, was predictive of both in-hospital and long-term
outcomes. This indicates that the pathophysiology leading to
increased RDW may affect outcomes in chronically ill patients,
irrespective of anemia status. The finding may be explained as
follows. As noted above, many variables are associated with anemia,
such as inflammatory factors, nutritional deficiencies, renal
dysfunction and inadequate erythropoietin production. The
relationships among these variables and outcomes may be more
directly reflected through their impact on RDW, than on Hgb. This
was demonstrated in the present study as RDW predicted mortality in
patients with and without anemia.
Secondly, RDW may be an earlier marker of prognosis
than Hgb as it reflects the early steps in the complex processes
that lead to anemia. At this point red blood cell production is
ineffective and red blood cell destruction increases, but Hgb
remains within the normal range. In addition, all the patients in
the current study had the onset of acute CHF. Hemodynamic changes
during this acute phase, such as fluid retention, interfere with
the accurate measurement of Hgb, which may generate misinformation
about the status of anemia. Under these circumstances, RDW is
likely to be a more appropriate marker of baseline anemia
status.
Another notable finding of the present study was
that after stratifying levels of BNP, RDW remained associated with
long-term outcomes. Although BNP is considered to be a powerful
predictor of CHF outcomes (31), a
single biomarker is insufficient to assess the outcomes of the
entire study population due to disease heterogeneity. A recent
multiple-marker approach has been suggested for risk stratification
of heart failure (32–34). The current study found that a
graded increased probability of endpoints with increased RDW during
follow-up in groups with low and high BNP indicated that the
predictive value of RDW was independent of that of BNP. Due to its
widespread availability and cost-effectiveness, the inclusion of
RDW in multiple marker models of CHF risk stratification is
recommended.
The RDW is a readily available and inexpensive test
for patients with CHF. Results are reported together with a
complete differential blood count and no extra cost is incurred.
The RDW has a better prognostic value than Hgb for both short- and
long-term outcomes in patients with decompensated CHF and the
prognostic value in long-term outcomes remains significant
regardless of anemia or BNP levels. Thus, RDW appears to carry
prognostic information concerning states other than anemia. In the
future, the inclusion of RDW in a combined model for the risk
stratification of patients with acute exacerbation of CHF is
recommended. Further study is required to clarify the detailed
mechanisms of the effect of elevated RDW in CHF.
References
|
1
|
Levy D, Kenchaiah S, Larson MG, et al:
Long-term trends in the incidence of and survival with heart
failure. N Engl J Med. 347:1397–1402. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mueller C, Laule-Kilian K, Christ A, et
al: Inflammation and long-term mortality in acute congestive heart
failure. Am Heart J. 151:845–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roger VL, Weston SA, Redfield MM, et al:
Trends in heart failure incidence and survival in a community-based
population. JAMA. 292:344–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowie MR, Wood DA, Coats AJ, et al:
Survival of patients with a new diagnosis of heart failure: a
population based study. Heart. 83:505–510. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue Y, Chan J, Sakariya S and Maisel A:
Biomarker-guided treatment of congestive heart failure. Congest
Heart Fail. 16(Suppl 1): S62–S67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allen LA: Use of multiple biomarkers in
heart failure. Curr Cardiol Rep. 12:230–236. 2010. View Article : Google Scholar
|
|
7
|
Vistnes M, Christensen G and Omland T:
Multiple cytokine biomarkers in heart failure. Expert Rev Mol
Diagn. 10:147–157. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bozkurt B, Mann DL and Deswal A:
Biomarkers of inflammation in heart failure. Heart Fail Rev.
15:331–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Araújo JP, Lourenço P, Azevedo A, et al:
Prognostic value of high-sensitivity C-reactive protein in heart
failure: a systematic review. J Card Fail. 15:256–266.
2009.PubMed/NCBI
|
|
10
|
Rehman SU and Januzzi JL Jr: Natriuretic
peptides for guiding heart failure therapy. Compr Ther. 34:32–40.
2008.PubMed/NCBI
|
|
11
|
Adams KF Jr, Felker GM, Fraij G, et al:
Biomarker guided therapy for heart failure: focus on natriuretic
peptides. Heart Fail Rev. 15:351–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Odedra K and Ferro A: Neurohormones and
heart failure: the importance of aldosterone. Int J Clin Pract.
60:835–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trachtenberg BH and Hare JM: Biomarkers of
oxidative stress in heart failure. Heart Fail Clin. 5:561–577.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belziti CA: Prevalence of anemia in heart
failure and its effects on prognosis. Expert Rev Cardiovasc Ther.
7:131–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salisbury AC and Kosiborod M: Outcomes
associated with anemia in patients with heart failure. Heart Fail
Clin. 6:359–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He SW and Wang LX: The impact of anemia on
the prognosis of chronic heart failure: a meta-analysis and
systemic review. Congest Heart Fail. 15:123–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghali JK: Anemia and heart failure. Curr
Opin Cardiol. 24:172–178. 2009. View Article : Google Scholar
|
|
18
|
Dabbah S, Hammerman H, Markiewicz W and
Aronson D: Relation between red cell distribution width and
clinical outcomes after acute myocardial infarction. Am J Cardiol.
105:312–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nabais S, Losa N, Gaspar A, et al:
Association between red blood cell distribution width and outcomes
at six months in patients with acute coronary syndromes. Rev Port
Cardiol. 28:905–924. 2009.PubMed/NCBI
|
|
20
|
Förhécz Z, Gombos T, Borgulya G, et al:
Red cell distribution width: a powerful prognostic marker in heart
failure. Eur J Heart Fail. 12:4152010.PubMed/NCBI
|
|
21
|
Al-Najjar Y, Goode KM, Zhang J, et al: Red
cell distribution width: an inexpensive and powerful prognostic
marker in heart failure. Eur J Heart Fail. 11:1155–1162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Kimmenade RR, Mohammed AA,
Uthamalingam S, et al: Red blood cell distribution width and 1-year
mortality in acute heart failure. Eur J Heart Fail. 12:129–136.
2010.PubMed/NCBI
|
|
23
|
Pascual-Figal DA, Bonaque JC, Redondo B,
Caro C, Manzano-Fernandez S, Sánchez-Mas J, Garrido IP and Valdes
M: Red blood cell distribution width predicts long-term outcome
regardless of anaemia status in acute heart failure patients. Eur J
Heart Fail. 11:840–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evans TC and Jehle D: The red blood cell
distribution width. J Emerg Med. 9:71–74. 1991. View Article : Google Scholar
|
|
25
|
Viswanath D, Hegde R, Murthy V, et al: Red
cell distribution width in the diagnosis of iron deficiency anemia.
Indian J Pediatr. 68:1117–1119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crosato M, Steinborn W and Anker SD:
Anemia in chronic congestive heart failure: frequency, prognosis,
and treatment. Heart Fail Monit. 4:2–6. 2003.PubMed/NCBI
|
|
27
|
Terrovitis JV, Anastasiou-Nana M, Kaldara
E, et al: Anemia in heart failure: pathophysiologic insights and
treatment options. Future Cardiol. 5:71–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Felker GM, Adams KF Jr, Gattis WA and
O’Connor CM: Anemia as a risk factor and therapeutic target in
heart failure. J Am Coll Cardiol. 44:959–966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pierce CN and Larson DF: Inflammatory
cytokine inhibition of erythropoiesis in patients implanted with a
mechanical circulatory assist device. Perfusion. 20:83–90. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Felker GM, Allen LA, Pocock SJ, et al;
CHARM Investigators. Red cell distribution width as a novel
prognostic marker in heart failure: data from the CHARM Program and
the Duke Databank. J Am Coll Cardiol. 50:40–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maisel A: B-type natriuretic peptide
levels: diagnostic and prognostic in congestive heart failure:
what’s next? Circulation. 105:2328–2331. 2002.
|
|
32
|
Ikonomidis I, Michalakeas CA, Lekakis J,
et al: Multimarker approach in cardiovascular risk prediction. Dis
Markers. 26:273–285. 2009. View Article : Google Scholar
|
|
33
|
Velagaleti RS, Gona P, Larson MG, et al:
Multimarker approach for the prediction of heart failure incidence
in the community. Circulation. 122:1700–1706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin WH, Chen JW, Feng AN, et al:
Multimarker approach to risk stratification among patients with
advanced chronic heart failure. Clin Cardiol. 30:397–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|